Abstract
Given their roles in immune regulation, the expression of the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) 1 and 2 isoforms was investigated in human naïve (CD45RA+) and memory (CD45RO+) CD4+ T cells. Stimulation of both types of cells via the CD3/CD28 pathway resulted in high expression of both PPARγ receptors as measured by real-time PCR. Treatment with the PPARγ agonist, ciglitazone, increased PPARγ1 expression but decreased PPARγ2 expression in stimulated naïve and memory cells. Furthermore, when present, the magnitude of both PPARγ receptors expression was lower in naïve cells, perhaps suggesting a lower regulatory control of these cells. Similar profiles of selected proinflammatory cytokines were expressed by the two cell types following stimulation. The induction of PPARγ1 and suppression of PPARγ2 expressions in naïve and memory CD4+ T cells in the presence of ciglitazone suggest that the PPARγ subtypes may have different roles in the regulation of T-cell function.
1. Introduction
Peripheral CD4+ T cells can be divided into two broad functional groups based on their expression of distinct isoforms of the CD45 surface molecule, CD45RA representing naïve CD4+ T cells and CD45RO representing memory CD4+ T cells [1]. Memory CD4+ T cells require a shorter lag time to proliferate when they are stimulated by antigens and are less dependent on costimulation than are naïve CD4+ T cells [2]. On the other hand, naïve CD4+ T cells have been reported to be the source of autoreactive lymphocytes in multiple sclerosis [3, 4], suggesting a differential regulatory mechanism for these cells.
The peroxisome proliferator-activated receptors (PPARs) are ligand-activated receptors that belong to the nuclear receptor superfamily [5]. Three isoforms of PPARs have been identified and are encoded by separate genes, namely, PPARα, γ, and β/δ [6, 7]. PPARγ is predominantly expressed in adipose tissue, colon, spleen, adrenal gland, and monocytes/macrophage [6, 7]. This isoform is further divided into four subtypes: PPARγ1, γ2, γ3, and γ4 due to alternative promoter use and RNA splicing [8]. PPARγ1, PPARγ3, and PPARγ4 encode for the same protein product, while the PPARγ2 protein contains an additional 28 amino acids at its N-terminus. PPARγ ligands include the naturally occurring arachidonic acid metabolite, 15-deoxy-D12,14-prostaglandin J2 (15d-PGJ2), as well as the thiazolidinedione (TZD) group of drugs such as ciglitazone and certain novel non-TZD insulin-sensitizing agents [9, 10].
PPARγ expressed in murine T-cells plays a regulatory role in T-cell activation [11]. Previous experiments showed that murine helper-T-cell clones and freshly isolated splenocytes express PPARγ1 but not PPARγ2 mRNA and that 15d-PGJ2 and ciglitazone inhibited the proliferative responses and IL-2 production of these cells when stimulated with the specific antigen and anti-CD3 antibodies, respectively [11]. Similarly, it was reported that 15d-PGJ2 and troglitazone suppressed IL-2 production of PHA-stimulated peripheral blood T cells [12]. PPARγ has been shown to physically bind to the transcription factors AP-1 and NFAT [12, 13], which regulate the IL-2 promoter thus blocking their binding to the promoter and hence inhibiting the transcription of the IL-2 gene.
These studies indicate an important immunoregulatory role for PPARγ in T-cell function. It will, therefore, be interesting to investigate whether naïve and memory CD4+ T cells behave in the same manner with regard to the expression of PPARγ and whether their activation modulate the expression of the PPARγ receptor differently. It would also be important to explore the impact on cytokine expression in these T-cell subsets upon activation of PPARγ, in particular selected proinflammatory cytokines, which are important in autoreactivity such as autoimmune diabetes [14].
Most studies on the role of PPARγ have used semi-quantitative measurements to assess the mRNA level of the receptor. Since subtle changes in PPARγ levels may result in significant changes to various downstream events as postulated by other types of receptor-signaling molecules [15], an accurate quantification of PPARγ isoform levels following cellular activation would need to be carried out.
We propose to study the expression of PPARγ1 and PPARγ2 in unstimulated and stimulated naïve and memory CD4+ T-cell subsets using quantitative real-time PCR. To further dissect the role of PPARγ1 and PPARγ2 in immune activation, the PPARγ agonist, ciglitazone, was used to modulate the activation status of these cell types and assess the modulation of their expression levels as well as those of selected proinflammatory cytokines in these cells.
2. Materials and Methods
2.1. Isolation of Naïve and Memory CD4+ T Cells from Peripheral Blood
Peripheral blood collection has prior approval from the Universiti Sains Malaysia Ethics Committee and collected after informed consent was obtained. Human naïve and memory CD4+ T cells were isolated from the peripheral blood by immunomagnetic separation. Briefly, blood was obtained from normal donors, and the peripheral blood mononuclear cells (PBMCs) were isolated by the Ficoll gradient centrifugation and incubated with a panel of biotin-conjugated monoclonal antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγδ, and glycophorin A (Miltenyi Biotec, Germany). CD45RA and CD45RO microbeads were added reciprocally for the negative isolation of memory and naïve CD4+ T cells. The purity of the isolated naïve and memory CD4+ T cells were generally 90–95% as determined by flow cytometric analysis.
2.2. In Vitro Stimulation of Naïve and Memory CD4+ T Cells
Naïve and memory CD4+ T cells were suspended at 2 × 105 cells/mL in complete RPMI 1640 medium (10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin) containing CD3/CD28 beads at a 1 : 1 cell/bead ratio in 25 cm2 tissue culture flasks. Twenty μM of ciglitazone solution was added when required at day 0 of culture. This concentration of ciglitazone was determined based on the minimum concentration required to cause a reduction in cell proliferation as reported in the literature [11, 13, 16]. The flasks were incubated for 5 days in a humidified incubator at 37°C in 5% CO2.
2.3. Proliferation Assay
Naïve and memory CD4+ T cells were suspended in 200 μL of complete RPMI 1640 medium at a concentration of 1 × 103/well in triplicate wells of a 96-well flat-bottom plate and stimulated with CD3/CD28 beads for 5 days as previously described [17]. When required, ciglitazone (20 μM) was added at day 0 of culture. Ten μL of diluted [3H] thymidine (1 μCi) was added to each well at 0, 24, 48, 72, and 96 h after stimulation. After incubation for another 20–22 h, the cells were harvested to represent day 1, 2, 3, 4, and 5, respectively, using the Innotech cell harvester system (Innotech AG, Switzerland). The incorporation of [3H] thymidine into DNA was quantified using a liquid scintillation counter by Hidex data analysis software (Hidex, USA).
2.4. Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from unstimulated and stimulated naïve and memory CD4+ T cells with or without ciglitazone treatment using the RNeasy Mini kit (Qiagen, USA) and QIAshredder (Qiagen, USA) according to the manufacturer's instructions. Briefly, the cells were lysed in RLT buffer and the beads were depleted using Dynal MPC. The lysed cells were applied onto the QIAshredder column followed by the RNeasy Mini spin column after addition of 70% ethanol. The sample column was then centrifuged, and the flow-through discarded before 700 μL of RW1 buffer was added into the column. Following centrifugation, the mixture was washed twice in 500 μL RPE buffer before 50 μL of RNase free water was added into the column to dissolve the total RNA. The RNA was eluted by centrifugation, and its integrity was assessed by gel electrophoresis while RNA purity and concentration were measured by spectrophotometry (Biophotometer, Eppendorf, Germany).
Total RNA (between 0.5 to 5 μg) was reverse transcribed into cDNA using the RevertAid H Minus first strand cDNA synthesis kit (MBI Fermentas, USA) in the presence of 0.5 μg oligo(dT)18 primer in nuclease-free deionized water. The mixture was firstly incubated at 70°C for 5 minutes. The reaction mixture was then mixed with 4 μL of 5x reaction buffer, 20 unit ribonuclease inhibitor, and 2 μL of 10 mM dNTP mix, followed by incubation at 37°C for 5 minutes. The process of reverse transcription was performed at 42°C for 1 hour using 200 unit of RevertAid H Minus M-MuLV. Finally the process was terminated by heating at 70°C for 10 minutes. The success of cDNA synthesis was confirmed by running a PCR using human β-actin primer (Maxim Biotech, USA).
2.5. Competitive Real-Time PCR
The PPARγ1 gene was amplified and quantified using the following primers/probe: forward primer 5′-CTT TAT GGA GCC CAA GTT TGA GTT-3′; reverse primer 5′-GGC TTC ACA TTC AGC AAA CCT-3′ and TaqMan probe 5′-TGC CAA GTC GCT GTC ATC TAA TTC CAG TG-3′. The PPARγ2 gene was amplified and quantified using the following primers/probe: forward primer 5′-GGG TGA AAC TCT GGG AGA TTC TC-3′; reverse primer 5′-GAT GCC ATT CTG GCC CAC-3′ and TaqMan probe 5′-TGA CCC AGA AAG CGA TTC CTT CAC TGA-3′. A total volume of 22.5 μL master mix, which included the TaqMan Universal Master Mix (ABI, USA), TaqMan probe, forward and reverse primers, and sterile distilled water was added in each well of the PCR plate prior to the addition of 50 ng of target cDNA. The master mix contains a dye (ROX) for normalization. Five dilutions of internal standards (plasmids containing the PPARγ1 and PPARγ2 genes) were chosen from the range of 10−4 pmol to 10−8 pmol. For nontemplate control (NTC) wells, only water was added. The reaction plate was sealed with an optical adhesive cover, centrifuged briefly to avoid any bubbles, and placed in the real-time PCR apparatus to begin the reaction. All samples were run in triplicates.
The reaction was initiated at 50°C for 2 min. This step was required for optimal AmpErase uracil-N-glycosylase (UNG) enzyme activity to decontaminate any DNA carryover. The temperature was increased to 95°C for 10 min to activate the AmpliTaq Gold enzyme. This was followed by 45 cycles of denaturation at 95°C for 15 sec and primer annealing and extension stages at 60°C for 1 min each.
2.6. Multiplex PCR (MPCR)
The expression levels of TGFβ, IL-1β, IL-8, TNFα, GM-CSF, and IL-6 were measured in unstimulated and stimulated naïve and memory CD4+ T cells with or without treatment with ciglitazone using the MPCR kit for Human Inflammatory Cytokines Genes Set-1 (Maxim Biotech, USA). The expression of the house-keeping gene, GAPDH, was used for normalization. The MPCR was carried out according to the manufacturer's instructions. Briefly, 1x MPCR buffer, 1x MPCR primer mix, 2.5 units of Taq polymerase, and 0.1 μg cDNA template were mixed in a 50 μL reaction; the optimum annealing temperature for the MPCR analysis was 66°C and subjected to 35 cycles of PCR, with denaturing, annealing, and extension temperatures at 94, 58, and 70°C for 1 min each, respectively. Following MPCR, the products were fractionated electrophoretically in a 2% agarose gel containing 0.5 μg/mL ethidium bromide and analysed by the Image Master Total Lab v1.00 (Amersham Pharmacia, USA).
2.7. Statistical Analysis
The profiles of [3H] thymidine incorporation of naïve and memory CD4+ T cells after in vitro stimulation with or without ciglitazone treatment were compared and analysed using the Kruskal-Wallis test. The PPARγ1 and PPARγ2 expression and cytokine profiles of unstimulated and stimulated naïve and memory CD4+ T cells with or without ciglitazone treatment were compared and analysed using the Mann-Whitney U test by statistical program for social science (SPSS) version 11.0 computer program (SPSS Inc., USA).
3. Results
3.1. Proliferative Response of CD3/CD28-Stimulated Naïve and Memory CD4+ T Cells
The proliferative response of purified naïve and memory CD4+ T cells following in vitro stimulation with CD3/CD28 was assessed. Anti-CD3/CD28 enhanced proliferation in both naïve and memory CD4+ T cells as depicted by the incorporation of [3H] thymidine (Figure 1). From day 1 to 5 after stimulation, the cell proliferation rate increased by more than 20-fold. There was no significant difference in the proliferation rate between the naïve and memory CD4+ T cells. The addition of ciglitazone decreased the degree of proliferation in naïve and memory CD4+ T cells by about 10-fold. Ciglitazone significantly decreased the proliferation rate of activated naïve CD4+ T cells on days 3, 4, and 5 (P < 0.05) and that of activated memory CD4+ T cells on days 4 and 5 (P < 0.05).
Figure 1.
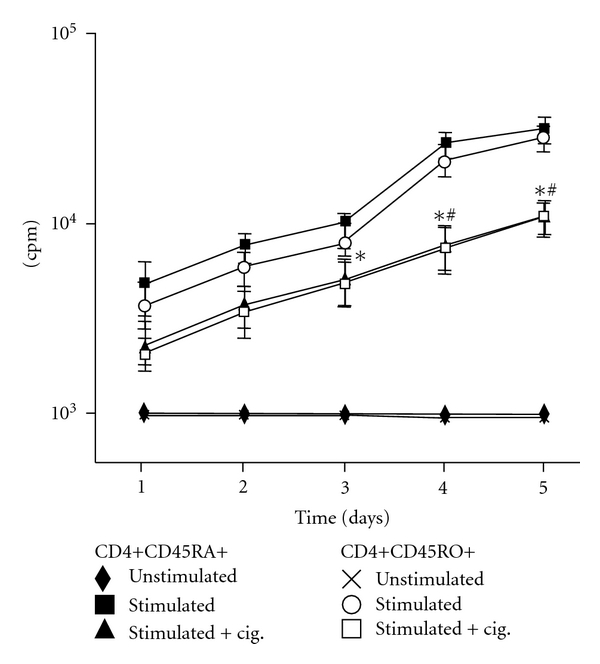
Proliferation assay. [3H] thymidine incorporation of naïve (CD45RA+) and memory (CD45RO+) CD4+ T cells following in vitro stimulation with CD3/CD28 beads, in the presence or absence of ciglitazone. Data are expressed as the mean cpm of triplicate cultures ± SEM. The experiments were repeated three times. Statistical analyses were performed using the Kruskal-Wallis test.*P < 0.05 (for naïve CD4+ T cells) or # P < 0.05 (for memory CD4+ T cells) of ciglitazone-treated, compared to untreated stimulated cells.
3.2. Quantification of PPARγ1 and PPARγ2
Unstimulated naïve and memory CD4+ T cells expressed low constitutive levels of PPARγ1 mRNA, whereas stimulated naïve and memory CD4+ T cells expressed significantly higher levels of the receptor in both cell types (average of 7 × 104 and 1.2 × 105 mRNA transcripts/μg of total RNA, for naïve and memory CD4+ T cells; resp., P > 0.05; Figure 2(a)). Stimulated memory CD4+ T cells displayed higher PPARγ1 expression than naïve CD4+ T cells (P < 0.05). Ciglitazone treatment significantly increased the expression of PPARγ1 by about 70-fold and 160-fold in naïve and memory CD4+ T cells (P < 0.01), respectively. PPARγ1 expression remained significantly higher in stimulated memory compared to stimulated naïve CD4+ T cells in the presence of ciglitazone (P < 0.01).
Figure 2.
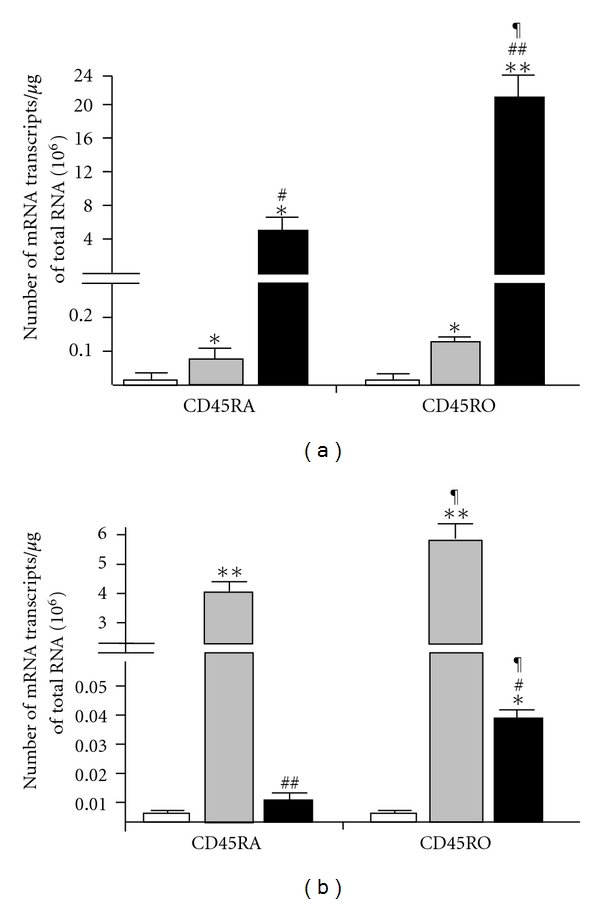
PPARγ1 and PPARγ2 mRNA expression. (a) PPARγ1 and (b) PPARγ2 gene expression levels in unstimulated (open bar, n = 13) and stimulated (grey bar, n = 13) naïve and memory CD4+ T cells or those treated with ciglitazone (solid bar, n = 8). The PPARγ1 and PPARγ2 gene expression levels were calculated as the number of mRNA transcripts per μg total RNA. The data plotted is the mean mRNA transcripts ± SEM. Statistical analyses were performed using the Mann-Whitney U test. *P < 0.05; **P < 0.01—significantly different from unstimulated cells. # P < 0.05; ## P < 0.01—significantly different from CD3/CD28–stimulated cells. ¶ P < 0.05—significantly different from correspondingly treated CD45RA+ cells.
Unstimulated naïve and memory CD4+ T cells expressed 10-fold lower constitutive levels of PPARγ2 mRNA compared to PPARγ1 (Figure 2(b)). Stimulated naïve and memory CD4+ T cells express very high levels of PPARγ2 mRNA in both cell types (average of 3.9 × 106 and 5.5 × 106 mRNA transcripts/μg of total RNA, in naïve and memory CD4+ T cells, resp.). PPARγ2 expression in stimulated memory CD4+ T cells expressed higher levels of the receptor compared to naïve CD4+ T cells. In contrast to PPARγ1, the addition of ciglitazone significantly decreased the expression of PPARγ2 by about 470-fold and 150-fold in naïve and memory CD4+ T cells, respectively (P < 0.01). However, after treatment with ciglitazone, PPARγ2 expression was significantly higher in stimulated memory compared to stimulated naïve CD4+ T cells (P < 0.01).
Figure 3 shows an example of a gel electrophoresis of the MPCR products of selected inflammatory cytokines in unstimulated and stimulated naïve and memory CD4+ T cells with or without ciglitazone treatment. The expression of various cytokines was compared by densitometric analyses and expressed as a ratio of GAPDH. The results were then plotted as histograms as depicted in Figure 4.
Figure 3.
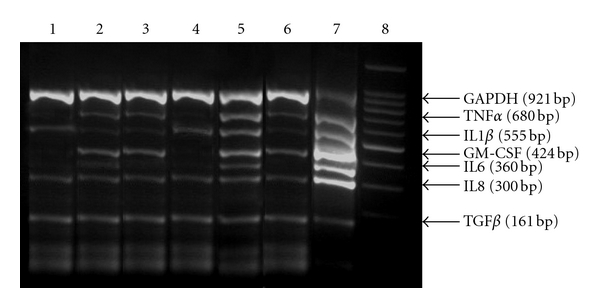
Example of multiplex PCR readout of inflammatory cytokine gene expression. Lane 1: unstimulated naïve CD4+ T cells. Lane 2: stimulated naïve CD4+ T cells. Lane 3: stimulated naïve CD4+ T cells + ciglitazone. Lane 4: unstimulated memory CD4+ T cells. Lane 5: stimulated memory CD4+ T cells. Lane 6: stimulated memory CD4+T cells + ciglitazone. Lane 7: positive control. Lane 8 : 100 bp marker.
Figure 4.
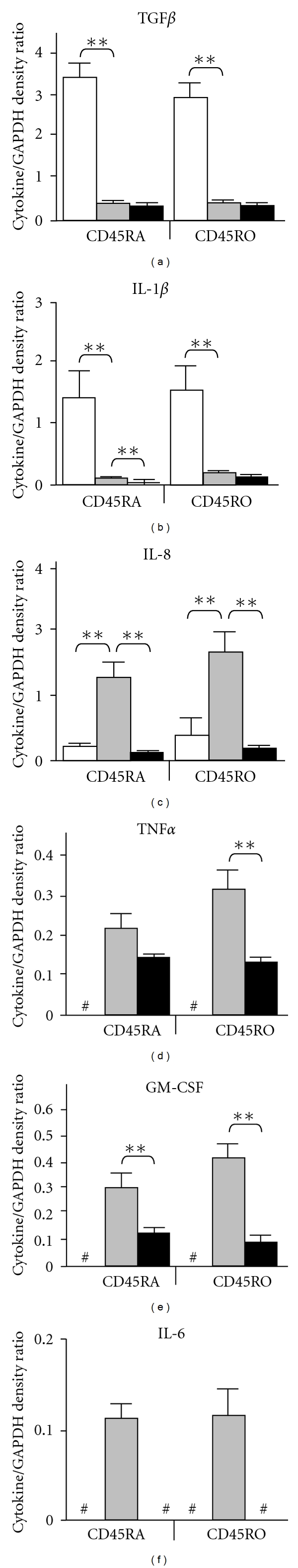
Inflammatory cytokine expression. Relative mRNA expression levels of selected cytokine genes in naïve and memory CD4+ T cells following CD3/CD28 stimulation in the presence or absence of ciglitazone (n = 5). Untreated cells (open bar), stimulated cells (grey bar), and ciglitazone-treated cells (solid bar) were assessed for their relative expression of (a) TGFβ, (b) IL-1β, (c) IL-8, (d) TNFα, (e) GM-CSF, and (f) IL-6, as a ratio of GAPDH. Statistical analyses were performed using the Mann-Whitney U test. *P < 0.05, **P < 0.01. #Significance levels cannot be analyzed because the gene expression was not detectable.
As shown in Figure 4(a), the expression levels of TGFβ gene were higher in unstimulated naïve and memory CD4+ T cells but decreased significantly in their stimulated state (P < 0.01). The addition of ciglitazone did not significantly alter the expression of TGFβ in both stimulated cells. IL-1β gene expression was also higher in unstimulated naïve and memory CD4+ T cells but decreased significantly in their stimulated state (P < 0.01). Ciglitazone further decreased the expression of IL-1β in stimulated naïve (P < 0.01) but not in stimulated memory CD4+ T cells (Figure 4(b)). IL-8 gene was expressed at low levels in unstimulated naïve and memory CD4+ T cells but significantly increased in both cell types upon activation (P < 0.01). IL-8 expression decreased in memory and naïve CD4+ T cells to its unstimulated states upon addition of ciglitazone (P < 0.01) (Figure 4(c)).
Figure 4(d) shows the de novo TNFα expression in stimulated naïve and memory CD4+ T cells. There was no significant difference in the expression of TNFα in both cell types after activation. Ciglitazone significantly decreased the expression of TNFα in stimulated memory (P < 0.01) but not in naïve CD4+ T cells. GM-CSF was also expressed in stimulated naïve and memory CD4+ T cells but not in their unstimulated state. There was no significant difference in the expression of GM-CSF in both cell types after activation. GM-CSF expression was significantly reduced in stimulated naïve and memory CD4+ T cells in the presence of ciglitazone (P < 0.01; Figure 4(e)). Figure 4(e) shows that only stimulated naïve and memory CD4+ T cells expressed IL-6. The addition of ciglitazone completely abolished the expression of IL-6 in both stimulated cells. The results clearly show de novo expression of TNF-α, GM-CSF, and IL-6 upon activation of naïve and memory CD4+ T cells.
4. Discussion
It is now established that PPARγ is involved in the regulation of T-cell function, as well as macrophage and dendritic cell activities [18–20]. In view of the fact that human naïve and memory CD4+ T cells differ in the requirements for activation and magnitude of their cellular responses [21] and autoreactivity [3, 4], we investigated the effect of the PPARγ agonist, ciglitazone, on the mRNA expression of PPARγ1 and PPARγ2 and on a number of inflammatory cytokines produced by these cells. No previous studies on the expression of PPARγ1 and PPARγ2 in human naïve and memory CD4+ T cells have been reported.
Consistent with previous reports [11, 13, 16, 20], ciglitazone treatment resulted in a tenfold reduction in the proliferative response of both CD3/CD28-stimulated naïve and memory CD4+ T-cell subsets. Inhibition of proliferation in activated naïve T cells by PPARγ agonists, such as ciglitazone, has been previously attributed to apoptosis [16], although whether this occurs via a PPARγ-dependent or independent pathway remains to be elucidated.
Using RT-PCR, PPARγ1 and PPARγ2 were found to be highly expressed in both naïve and memory CD4+ T cells upon activation through the TCR and costimulatory CD28 pathway. Consistent with previous findings [21], only low expression levels of both transcripts in unstimulated CD4+ T cells were recorded. Interestingly, previous studies reported that PPARγ is constitutively expressed in human peripheral blood mononuclear cells [6, 22]. However, this may be due to its expression by other cell subsets in the mononuclear cell population such as monocytes [18], B cells [23], and NK cells [24].
It is interesting to note the low level expression of PPARγ1 and PPARγ2 in resting human naïve and memory CD4+ T cells. This may suggest that their roles are primarily in the regulation of responding T cells. It is also noteworthy that higher levels of both transcripts are found in activated memory CD4+ T cells as opposed to their low level expression in activated naïve T cells, suggesting that regulation of memory CD4+ T cells may require higher-level expression of PPARγ compared to naïve CD4+ cells.
Treatment with ciglitazone enhanced the expression of PPARγ1 but greatly diminished that of PPARγ2 in both the naïve and memory CD4+ T cells. Previous studies have reported that PPARγ agonists such as troglitazone [12] and pioglitazone [22] attenuated the expression of the receptor. Here, we report that ciglitazone enhances the expression of PPARγ1 but greatly diminishes the expression of PPARγ2 in both naïve and memory CD4+ T cells. This apparent discrepancy can be attributed to the fact that the above studies did not distinguish between the two PPARγ isoforms. PPARγ1 can be regarded as a “subset” of PPARγ2 which contains additional 28 amino acids at its N-terminus. Thus, measuring PPARγ expression without distinguishing the two isoforms may not provide an accurate reflection of the receptor's role in immune regulation. The lack of specific antibodies against PPARγ1 has however impeded our attempt to differentiate the protein expression of these receptors in the current study. The decrease in PPARγ2 expression cannot be attributed to cell death via apoptosis [16] since the expression of PPARγ1 was enhanced and that the cell recovery after 5 days was above 90% (results not shown).
The different roles played by the two PPARγ isoforms in CD4+ T-cell regulation can be inferred from their expression levels displayed at pre- and posttreatment with ciglitazone. Thus, although the fold increase in PPARγ2 expression was higher than that observed for PPARγ1, it was almost completely abrogated upon addition of ciglitazone. A previous report [12] showed that troglitazone and 15d-PGJ2 inhibited IL-2 production in the PPARγ2-expressing but not in PPARγ2-nonexpressing transfected Jurkat T cells, suggesting that PPARγ2 is involved in regulating T cell function. The almost complete abrogation of PPARγ2 expression following treatment with ciglitazone is interesting and requires further investigations, such as inhibition studies. The present lack of specific chemical inhibitors for PPARγ2, however, would complicate such studies for the time being.
As mentioned above, activation of PPARγ by its ligands has been shown to induce apoptosis in T cells [16, 25]. Hence the question arises whether cells that express higher levels of PPARγ2 are more prone to apoptosis, resulting in the preferential “elimination” of PPARγ2-expressing cells. Single-cell analyses, including the measurement of PPARγ1 and PPARγ2 protein levels, should be carried out to address these questions. However, as anti-PPARγ1 antibodies are not available, such experiments may prove currently challenging. It will also be important to investigate the molecular regulation of PPARγ1 and PPARγ2 promoters in order to understand the possible differential control of their expression.
Since differential expression of PPAR has been shown to correlate with selected cytokine production [26, 27] and that naïve and memory CD4+ T cells may play a differential role in autoimmunity [3, 4], the level of various proinflammatory cytokines that were expressed in the resting and activated naïve and memory CD4+ T cells with or without treatment with ciglitazone was subsequently determined. While TGFβ, IL-8, and IL-1β expression in resting naïve and memory CD4+ T cells has previously been reported [28], their expression in activated naïve and memory CD4+ T cells has not been previously studied.
Activated naïve and memory CD4+ T cells displayed low expression levels of both TGF-β and IL-1β, further reduced upon stimulation with ciglitazone (in the case of IL-1β, further reduction was only observed in activated naïve CD4+ T cells). These findings are in agreement with those previously reported [13, 19]. Unstimulated naïve and memory CD4+ T cells displayed low levels of IL-8 which significantly increased upon activation. However, the addition of ciglitazone dramatically reduced IL-8 expression. This observation is in contrast to a previous finding that 15d-PGJ2, another PPARγ ligand, induced the expression of IL-8 in human T cells via a PPARγ-independent manner [29]. Thus there may be distinct response against different ligands with regard to the function of these receptors. Future studies will, therefore, need to include the use of several PPARγ ligands to determine the detailed mechanistic roles of the receptors in immune response.
Activation of both naïve and memory CD4+ T cells induced de novo expression of TNFα, GM-CSF, and IL-6, whereas treatment of these activated cells with ciglitazone diminished TNFα and GM-CSF expression, and totally abrogated IL-6 expression. Previous studies showed significant reduction in the release of LPS-stimulated TNFα upon activation of placental, amnion, and choriodecidual, tissues with both 15d-PGJ2 and troglitazone [30]. Ciglitazone, troglitazone, and 15d-PGJ2 also inhibited RSV-induced release of TNFα in A549 epithelial cells [31]. As previously reported [19], the expression of GM-CSF in activated naïve and memory CD4+ T cells may play a role in inducing the expression of PPARγ1 and PPARγ2 in both activated cells. Reduction of GM-CSF expression after ciglitazone treatment has also been reported in mast cells where a PPARγ agonist decreased the antigen-induced GM-CSF production [32].
The present observation that IL-6 is produced in similar levels by both naïve and memory CD4+ T cells has previously been reported [33]. IL-6 plays an essential role in activating naïve and memory CD4+ T cells through the CD2 molecule [34]. Unlike naïve T cells, CD4 memory T cells can undergo proliferation when stimulated with anti-CD2 in the absence of APCs since they are able to use self-produced IL-6 [35]. However, the current study shows that activation of naïve CD4+ T cells via the CD3 and CD28 pathways also induced the production of IL-6. This may have occurred through the engagement of the CD28 molecule which may act by amplifying the activation signals in an autocrine fashion.
A previous report [36] supports our observation that ciglitazone completely abolished the expression of IL-6 in activated naïve and memory CD4+ T cells. There is also evidence that chronic IL-6 treatment suppressed the expression of PPARγ [26], and the suppression of PPARγ functions resulted in excessive production of the cytokine [37]. The mechanism through which ciglitazone affects cytokine production remains to be elucidated. There is evidence [11, 12, 19] to suggest that this may occur through activation of transcription factors such as AP-1, STAT-1, and NF-κB. Since there are no reports to suggest that the cis-element of inflammatory cytokine genes contains PPARγ binding site, inhibition may occur indirectly via transrepression as described above [13]. It was also reported that 15d-PGJ2 treatment rendered IκB resistant to degradation upon cellular activation [38], hence, preventing NF-κB activation. However, since ciglitazone is structurally different from 15d-PGJ2, the mechanism of inhibition of NF-κB and AP-1 activity by ciglitazone may differ from its inhibition by 15d-PGJ2.
5. Conclusions
PPARγ1 and PPARγ2 have differential regulatory roles in responding naïve and memory CD4+ T cells. Overall, naïve CD4+ T cells seem to be more sensitive to PPARγ activation, although further studies need to be carried out to confirm this observation. The availability of specific antibodies and specific antagonists against these two isoforms is needed to enable a more precise elucidation of their purported differential functions in T-cell regulation. In addition, the precise mechanism of how PPARγ1 and PPARγ2 regulate the response of naïve and memory cells or the immune response in general will require further investigations utilizing single-cell analytical tools.
Acknowledgments
This study was funded by the Fundamental Research Grant Scheme, Ministry of Higher Education, Malaysia (203/PPSK/6170004), and the IRPA (Experimental & Applied) Grant, Ministry of Science, Technology and Innovation, Malaysia (305/PPSK/6112218).
References
- 1.Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annual Review of Immunology. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 3.Muraro PA, Pette M, Bielekova B, McFarland HF, Martin R. Human autoreactive CD4+ T cells from naive CD45RA+ and memory CD45RO+ subsets differ with respect to epitope specificity and functional antigen avidity. Journal of Immunology. 2000;164(10):5474–5481. doi: 10.4049/jimmunol.164.10.5474. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone DE, Golightly MG, Brannagan TH. High dose cyclophosphamide preferentially targets naïve T (CD45/CD4/RA+) cells in CIDP and MS patients. Journal of Neuroimmunology. 2007;190(1-2):121–126. doi: 10.1016/j.jneuroim.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 6.Greene ME, Blumberg B, McBride OW, et al. isolation of the human peroxisome proliferator activated receptor γ cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expression. 1995;4(4-5):281–299. [PMC free article] [PubMed] [Google Scholar]
- 7.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 8.Sundvold H, Lien S. Identification of a novel peroxisome proliferator-activated receptor (PPAR) γ promoter in man and transactivation by the nuclear receptor RORα1. Biochemical and Biophysical Research Communications. 2001;287(2):383–390. doi: 10.1006/bbrc.2001.5602. [DOI] [PubMed] [Google Scholar]
- 9.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 10.Willson TM, Cobb JE, Cowan DJ, et al. The structure activity relationship between peroxisome proliferator-activated receptor-γ agonist, and anti-hyperglycaemic activity of thiazolinediones. Journal of Medicinal Chemistry. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 11.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPARγ and immunoregulation: PPARγ mediates inhibition of helper T cell responses. Journal of Immunology. 2000;164(3):1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 12.Yang XY, Wang LH, Chen T, et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. Journal of Biological Chemistry. 2000;275(7):4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Anderson PO, Chen S, Paulsson KM, Sjögren HO, Li S. Inhibition of the transcription factors AP-1 and NF-κB in CD4 T cells by peroxisome proliferator-activated receptor γ ligands. International Immunopharmacology. 2001;1(4):803–812. doi: 10.1016/s1567-5769(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 14.Yaacob NS, Kaderi MA, Norazmi MN. The expression of cytokine genes in the peritoneal macrophages and splenic CD4- and CD8-positive lymphocytes of the nonobese diabetic mice. Journal of Clinical Immunology. 2004;24(2):177–184. doi: 10.1023/B:JOCI.0000019783.61674.1d. [DOI] [PubMed] [Google Scholar]
- 15.Germain RN. The art of the probable: system control in the adaptive immune system. Science. 2001;293(5528):240–245. doi: 10.1126/science.1062946. [DOI] [PubMed] [Google Scholar]
- 16.Harris SG, Phipps RP, Bernard I, et al. The nuclear receptor PPAR γ is expressed by mouse T lymphocytes and PPAR γ agonists induce apoptosis. European Journal of Immunology. 2001;31(4):1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Liu K, Li Y, Prabhu V, et al. Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. Journal of Immunology. 2001;166(12):7335–7344. doi: 10.4049/jimmunol.166.12.7335. [DOI] [PubMed] [Google Scholar]
- 18.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391(6662):82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 19.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L, Dani I, Edenhofer F, et al. Peroxisome proliferator-activated receptor γ control of dendritic cell function contributes to development of CD4+ T cell anergy. Journal of Immunology. 2007;178(4):2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper H, Brouwer M, De Boer M, Parren P, Van Lier RAW. Differences in responsiveness to CD3 stimulation between naive and memory CD4+ T cells cannot be overcome by CD28 costimulation. European Journal of Immunology. 1994;24(9):1956–1960. doi: 10.1002/eji.1830240903. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L, Schmidt M, Giese T, et al. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor γ levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. Journal of Immunology. 2005;175(8):4948–4955. doi: 10.4049/jimmunol.175.8.4948. [DOI] [PubMed] [Google Scholar]
- 23.Padilla J, Leung E, Phipps RP. Human B lymphocytes and B lymphomas express PPAR-γ and are killed by PPAR-γ agonists. Clinical Immunology. 2002;103(1):22–33. doi: 10.1006/clim.2001.5181. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Rodriguez-Galán MC, Subleski JJ, et al. Peroxisome proliferator-activated receptor-γ and its ligands attenuate biologic functions of human natural killer cells. Blood. 2004;104(10):3276–3284. doi: 10.1182/blood-2004-02-0664. [DOI] [PubMed] [Google Scholar]
- 25.Soller M, Tautenhahn A, Brüne B, et al. Peroxisome proliferator-activated receptor γ contributes to T lymphocyte apoptosis during sepsis. Journal of Leukocyte Biology. 2006;79(1):235–243. doi: 10.1189/jlb.0205058. [DOI] [PubMed] [Google Scholar]
- 26.Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochemical and Biophysical Research Communications. 2003;311(2):372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Trifilieff A, Bench A, Hanley M, Bayley D, Campbell E, Whittaker P. PPAR-α and -γ but not -δ agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-κB-independent effect. British Journal of Pharmacology. 2003;139(1):163–171. doi: 10.1038/sj.bjp.0705232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gossner AG, Bailey S, Hunter N, Hopkins J. Patterns of cytokine gene expression of naïve and memory T lymphocytes in vivo. Veterinary Immunology and Immunopathology. 2002;87(3-4):261–264. doi: 10.1016/s0165-2427(02)00050-8. [DOI] [PubMed] [Google Scholar]
- 29.Harris SG, Smith RS, Phipps RP. 15-Deoxy-Δ12,14-PGJ2 induces IL-8 production in human T cells by a mitogen-activated protein kinase pathway. Journal of Immunology. 2002;168(3):1372–1379. doi: 10.4049/jimmunol.168.3.1372. [DOI] [PubMed] [Google Scholar]
- 30.Lappas M, Permezel M, Georgiou HM, Rice GE. Regulation of proinflammatory cytokines in human gestational tissues by peroxisome proliferator-activated receptor-γ: effect of 15-deoxy-12,14-PGJ2 and troglitazone. Journal of Clinical Endocrinology and Metabolism. 2002;87(10):4667–4672. doi: 10.1210/jc.2002-020613. [DOI] [PubMed] [Google Scholar]
- 31.Arnold R, König W. Peroxisome-proliferator-activated receptor-γ agonists inhibit the release of proinflammatory cytokines from RSV-infected epithelial cells. Virology. 2006;346(2):427–439. doi: 10.1016/j.virol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: a possible role of these receptors in negative regulation of mast cell activation. European Journal of Immunology. 2000;30(12):3363–3370. doi: 10.1002/1521-4141(2000012)30:12<3363::AID-IMMU3363>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.van Kooten C, Rensink I, Pascual-Salcedo D, Van Oers R, Aarden LA. Monokine production by human T cells; IL-1α production restricted to memory T cells. Journal of Immunology. 1991;146(8):2654–2658. [PubMed] [Google Scholar]
- 34.Killeen N, Stuart SG, Littman DR. Development and function of T cells in mice with a disrupted CD2 gene. EMBO Journal. 1992;11(12):4329–4336. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasahara Y, Miyawaki T, Kato K, et al. Role of interleukin 6 for differential responsiveness of naive and memory CD4+ T cells in CD2-mediated activation. Journal of Experimental Medicine. 1990;172(5):1419–1424. doi: 10.1084/jem.172.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang THW, Razmovski-Naumovski V, Kota BP, Lin DSH, Roufogalis BD. The pathophysiological function of peroxisome proliferator-activated receptor-γ in lung-related diseases. Respiratory Research. 2005;6:p. 102. doi: 10.1186/1465-9921-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannisto K, Sutinen J, Korsheninnikova E, et al. Expression of adipogenic transcription factors, peroxisome proliferator-activated receptor γ co-activator 1, IL-6 and CD45 in subcutaneous adipose tissue in lipodystrophy associated with highly active antiretroviral therapy. AIDS. 2003;17(12):1753–1762. doi: 10.1097/00002030-200308150-00004. [DOI] [PubMed] [Google Scholar]
- 38.Su CG, Wen X, Bailey ST, et al. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. Journal of Clinical Investigation. 1999;104(4):383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]


