Abstract
The supporting role of urokinase-type plasminogen activator (uPA) and its inhibitor plasminogen activator inhibitor 1 (PAI-1) in migration and invasion is well known. In addition, both factors are key components in cancer cell-related signaling. However, little information is available for uPA and PAI-1-associated signaling pathways in primary cancers and corresponding lymph node metastases. The aim of this study was to compare the expression of uPA and PAI-1-associated signaling proteins in 52 primary breast cancers and corresponding metastases. Proteins were extracted from formalin-fixed paraffin-embedded tissue samples of the primary tumors and metastases. Protein lysates were subsequently analyzed by reverse phase protein array for the expression of members of the PI3K/AKT (FAK, GSK3-β, ILK, pGSK3-β, PI3K, and ROCK) and the MAPK pathways (pp38, pSTAT3, and p38). A solid correlation of uPA expression existed between primary tumors and metastases, whereas PAI-1 expression did not significantly correlate between them. The correlations of uPA and PAI-1 with signaling pathways found in primary tumors did not persist in metastases. Analysis of single molecules revealed that some correlated well between tumors and metastases (FAK, pGSK3-β, ILK, Met, PI3K, ROCK, uPA, p38, and pp38), whereas others did not (PAI-1 and GSK3-β). Whether the expression of a protein correlated between tumor and metastasis or not was independent of the pathway the protein is related to. These findings hint at a complete deregulation of uPA and PAI-1-related signaling in metastases, which might be the reason why uPA and PAI-1 reached clinical relevance only for lymph node-negative breast cancer tissues.
Introduction
The supporting role of urokinase-type plasminogen activator (uPA), its receptor uPAR, and the plasminogen activator inhibitor 1 (PAI-1) in cell migration and invasion is well described in both physiologic [1,2] and pathologic settings (for review, see McMahon and Kwaan [3]). Since the late 1980s, uPA and PAI-1 have been considered as clinically relevant predictive markers [4,5], and today, these findings are well confirmed (for review, see Schmitt et al. [6]). The levels of uPA and PAI-1 in the primary tumors of patients with node-negative breast cancer are used clinically to decide which patients require chemotherapy and which do not [7].
The soluble protein uPA is known to inhibit apoptosis [8,9] and actively induce proliferative signaling cascades, for example, the STAT3 pathway [10]. The main inhibitor of uPA, PAI-1, also has effects on cell adhesion and modulates intracellular signaling, primarily through up-regulation of apoptotic pathways [11,12]. The related signaling cascades are not well known. In a model of vascular smooth muscle cells, elevated levels of PAI-1 suppress apoptosis by directly interacting with and inhibiting the dominant death caspase 3 [13]. In different studies, PAI-1 was either upregulated or downregulated by the PI3K/AKT signaling pathway [14,15].
These results demonstrate that the functions of uPA and PAI-1 signaling are well described in cell culture systems. However, corresponding data for primary breast cancer tissues are very limited. We recently set up the assumption that PAI-1 might be associated mainly with PI3K/AKT signaling, whereas uPA may correlate with activation of ERK [16]. Herein, we used a reverse-phase protein array (RPPA) approach to analyze the proposed interaction of uPA and PAI-1 with ERK- and PI3K/AKT-related signaling cascades, respectively.
An additional matter discussed quite controversially in literature is the expression and/or activation of different signaling proteins in primary tumors and lymph node metastases [17–20]. However, no studies comparing uPA and PAI-1 expression and signaling in primary tumors versus lymph node metastases have been completed so far. Because the uPA/PAI-1 system not only is involved in early metastatic processes like cell detachment and migration but also plays important roles in inhibition of apoptosis, proliferation, and vascularization, we compared uPA- and PAI-1-associated signaling pathways in primary tumors and lymph node metastases.
Materials and Methods
Antibodies
The following 14 proteins were analyzed in this study: PAI-1, uPA, pER, focal adhesion kinase (FAK), glycogen synthase kinase 3-β (GSK3-β), phosphorylated GSK3-β, HSP27, integrin linked kinase (ILK), MET, phosphatidylinositol 3-kinase (PI3K), p38 MAP kinase (p38), phosphorylated p38 (pp38), Rho-associated kinase (ROCK), phosphorylated signal transducer and activator of transcription 3 (pSTAT3). Table W1 lists the antibodies against these proteins used in RPPAs. All antibodies have been validated by Western blot analysis using proteins extracted from formalin-fixed tissues or cells as described before [16]. Specificity of the antibodies is shown in Figure W1.
Tissue Samples
Formalin-fixed paraffin-embedded primary breast cancer tissues from 52 patients diagnosed between 2000 and 2008 were selected from the archive at the Institute of Pathology, Technische Universität München, Germany. In addition, the 52 corresponding lymph node metastases were also used in the study. Patients' characteristics are summarized in Table W2. Figure W2 provides an overview of the collectives and the workflow for their analysis.
The study was approved by the ethics committee of the Technische Universität München, and all patients gave informed consent. Reference hematoxylin/eosin-stained sections of the tissues were histologically verified by an experienced pathologist (A.W.). Only tissue sections containing more than 80% tumor cells were included in the study.
Protein Extraction
Protein extraction was performed as previously described [21], using buffer EXB Plus (Qiagen, Hilden, Germany) [22]. Briefly, tissue sections were deparaffinized, and proteins were extracted using EXB Plus. Tissue areas of approximately 0.25 cm2 from three 10-µm-thick sections were processed in 100 µl of extraction buffer. The Bradford protein assay (BioRad, Hercules, CA) was used according to the manufacturer's instructions to determine the protein concentrations. A Western blot probing for β-actin was performed from randomly selected lysates to demonstrate successful protein extraction and suitability for RPPA analysis. All protein lysates tested produced a clear β-actin band on the Western blot.
Reverse-Phase Protein Arrays
RPPAs were generated using the Aushon 2470 Arrayer (Aushon, Billerica, MA) or BioOdyssee Calligrapher (Biorad, Hercules, CA) as described before [23]. For every lysate and every dilution (undiluted, 1:2, 1:4, 1:8, 1:16, buffer), three replicates were applied onto a nitrocellulose-coated glass slide (Grace Bio-Labs, Bend, OR), which produced 18 data points per sample. Immunodetection was performed like for a Western blot and as previously described [24]. For the estimation of total protein amounts, parallel arrays were stained with Sypro Ruby Protein Blot Stain (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. For more detailed information and method validation, see Wolff et al. [16].
Quantitative Protein Analysis
The scanned films (1200 dpi) of the antibody-detected slides were saved as tiff images. These and the images of the Sypro Ruby-stained slides were analyzed with MicroVigene 3.5.0.0 (VigeneTech, Carlisle, MA) as described [25].
Statistical Analysis
The relationships between uPA or PAI-1 and the RPPA results, as well as with continuous patient and tumor characteristics, were investigated using the Spearman rank correlation test. The associations of uPA and PAI-1 levels with ordered clinical variables were tested using the nonparametric Wilcoxon rank sum test or the Kruskal-Wallis test. When appropriate, this was followed by a nonparametric test for trend. All tests were performed using PASW Statistics software version 18.0 (SPSS, Inc, Chicago, IL). To fit different linear regression lines to spread bivariate data, a mixture model was used [26,27]. Ninety-five percent of the confidence bands were provided for regression fit lines.
Holm-Bonferroni adjustment of P values was performed to correct for multiple testing. Adjusted two-sided P values ≤ .05 were considered to indicate statistical significance.
Results
Correlation of uPA and PAI-1 Expression with PI3K/AKT- and MAPK Signaling in Primary Breast Cancers
As we recently revealed first hints to possible interactions of PAI-1 and uPA with AKT and MAP kinase signaling, respectively, in vivo [16], we now examined the expression of uPA, PAI-1, and 12 further signaling molecules composed of members of the PI3K/AKT (FAK, GSK3-β, ILK, pGSK3-β, PI3K, and ROCK) and the MAPK pathways (pp38, pSTAT3, and p38). Furthermore, we included HSP27, MET, and pER to examine the relation of these tumor progression modulators with uPA and PAI-1. HSP27 was described to interact with AKT before [28]. Protein levels of PAI-1 and uPA were correlated with the expression levels of these key signaling proteins determined by RPPA. The Spearman correlation coefficient rs and P values are listed in Tables 1 and 2. All P values are adjusted for multiple testing as described in theMaterials and Methods section. Positive correlations were found for PAI-1 with FAK, ILK, ROCK, and PI3K (Table 1). In addition, PAI-1 expression correlated positively with the levels of HSP27 and pp38. GSK3-β, ILK, MET, pGSK3-β, pHSP27, and pSTAT3 did not correlate with PAI-1 levels. These results are in line with our suggestion that PAI-1 is correlated with the PI3K/AKT pathway.
Table 1.
Correlation of PAI-1 with Key Signaling Proteins Determined by RPPA.
| PAI-1 vs | rs | P |
| FAK | 0.495 | .029 |
| GSK3-β | 0.103 | >.999 |
| pGSK3-β | 0.297 | >.999 |
| HSP27 | 0.464 | .047 |
| ILK | 0.451 | .047 |
| MET | 0.286 | >.999 |
| p38 | 0.312 | .676 |
| pp38 | 0.482 | .029 |
| pER | 0.212 | >.999 |
| PI3K | 0.547 | .010 |
| pSTAT3 | 0.170 | >.999 |
| ROCK | 0.491 | .025 |
| uPA | 0.663 | .000 |
The Spearman correlation coefficient rs and P values are shown. All P values are adjusted for multiple testing as described in the Materials and Methods section. Statistically significant correlations are in bold face emphases.
Table 2.
Correlation of uPA with Key Signaling Proteins Determined by RPPA.
| uPA vs | rs | P |
| FAK | 0.598 | .002 |
| GSK3-β | 0.336 | .543 |
| pGSK3-β | 0.468 | .055 |
| HSP27 | 0.579 | .002 |
| ILK | 0.514 | .012 |
| MET | 0.410 | .151 |
| p38 | 0.420 | .102 |
| pp38 | 0.584 | .002 |
| PAI-1 | 0.663 | .000 |
| pER | 0.329 | .610 |
| PI3K | 0.471 | .072 |
| pSTAT3 | -0.013 | .932 |
| ROCK | 0.574 | .002 |
The Spearman correlation coefficient rs and P values are shown. All P values are adjusted for multiple testing as described in the Materials and Methods section. Statistically significant correlations are in bold face emphases.
For uPA, we found positive correlations with FAK, HSP27, ILK, pp38, and ROCK (Table 2). This fits with our expectation that uPA might interact with the MAP kinase pathway but also hints at some connection with cytoskeleton regulation through ILK, FAK, and ROCK. Also, uPA and PAI-1 correlated with each other in this study set.
Analysis of Pathway Activation in Single Tumors
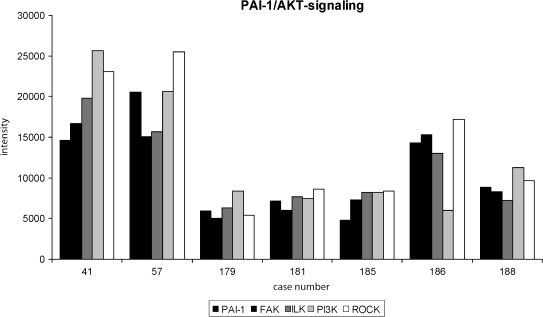
Because we found FAK, ILK, PI3K, and ROCK to be correlated with PAI-1, we wanted to know next if this connection of PI3K/AKT signaling and PAI-1 can also be seen on a single-patient level. Expression levels of PAI-1, FAK, ILK, PI3K, and ROCK detected in seven patients are shown in Figure 1. The examples are representative and show coexpression of the molecules at the patient level. From the 52 cases analyzed, only 4 showed a differential expression of a single molecule.
Figure 1.
Parallel analysis of FAK, ILK, PI3K, and ROCK expression in correlation to PAI-1 expression. A selection of seven representative tumors is shown in the figure. Normalized signal intensity of the different proteins obtained by RPPA analysis is depicted. All four proteins of the PI3K/AKT signaling pathway are expressed at similar levels compared with PAI-1 expression in six of seven patients. For one patient (no. 186), a single protein (PI3K) is expressed in a different manner than the rest of the pathway. In only 4 of the analyzed 52 patients, a single protein was regulated in a different manner than the rest of the pathway.
Correlation of Protein Expression in Primary Breast Cancers versus Their Corresponding Lymph Node Metastases
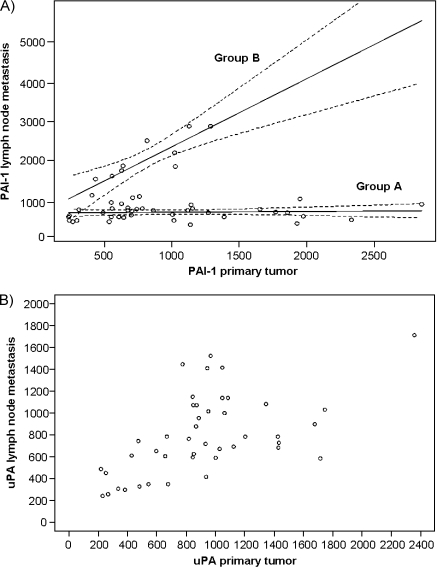
As it is known that both uPA and PAI-1 play a role in the metastatic cascade and are potentially involved in metastasis establishment by regulation of vascularization, we analyzed uPA and PAI-1 expression in the corresponding 52 lymph node metastases. The analysis revealed that PAI-1 did not correlate between primary tumors and lymph node metastases (rs = +0.137, P = .336). However, we found that two distinguishable groups exist within the patients regarding their PAI-1 expression profiles. These groups can be functionally described by linear regression equations. For one group (A), the expression of PAI-1 in the lymph node is always low and almost independent of PAI-1 expression of the primary tumor. For the other patient group (B), the expression of PAI-1 in primary tumors and lymph node metastases correlated with a correlation coefficient of rs = +0.799 and P < .001 (Figure 2A). This raised the question whether these two groups could be distinguished in respect of the clinical classification of the patients. However, we could not find any difference between the groups regarding their correlation with nodal status, tumor size, grading, steroid hormone receptor status, and occurrence of distant metastasis (data not shown).
Figure 2.
Correlations of uPA and PAI-1 expression in primary tumors versus lymph node metastases. (A) PAI-1 expression did not correlate in the two entities, rs = 0.094, P = .516, when analyzing all patients. Two groups (groups A and B) of PAI-1 expression could be discriminated by a statistical mixture model and 95% confidence intervals. In group A, PAI-1 expression can be described by the quotation: PAI-1 expression in lymph node metastases = 622.6 (relative expression units) + 0.02 x PAI-1 expression in primary tumors. In group B, PAI-1 expression can be described by the quotation PAI-1 expression in lymph node metastases = 587.7 (relative expression units) + 1.79 x PAI-1 expression in primary tumors. (B) A significant correlation of uPA expression in primary tumors versus lymph node metastases, rs = 0.566, P ≤ .001, was found.
In contrast, the expression of uPA correlated significantly between primary tumors and corresponding lymph node metastases with a correlation coefficient of rs = +0.566 and P < .001 (Figure 2B).
Because we found differential expression of uPA and PAI-1 in primary tumors and lymph node metastases, we also analyzed uPA- and PAI-1-associated pathways in lymph node metastases. As expected, the correlations found in primary tumors were not seen in lymph node metastasis. Whereas we could not find any correlation between PAI-1 and the signaling molecules analyzed herein (Table 3), uPA expression correlated with the expression of pp38 and pGSK3-β in lymph node metastases (Table 4). Tables 3 and 4 list the Spearman correlation coefficients rs and P values. All P values are adjusted for multiple testing as described in the Materials and Methods section. Because we could not find the correlations for uPA and PAI-1 found in primary tumors to persist in lymph node metastases, we next analyzed the correlation of the expression levels of single molecules between primary tumors and lymph node metastases. Whereas the expression of several molecules (FAK, ILK,MET, pGSK3-β, PI3K, pp38, p38, ROCK, and uPA) correlated significantly between primary tumors and lymph node metastases, the expression of GSK3-β and PAI-1 did not (Table W3).
Table 3.
Correlation of PAI-1 with Key Signaling Proteins Determined by RPPA in Lymph Node Metastases.
| PAI-1 vs | rs | P (two-sided) |
| FAK | -0.041 | >.999 |
| GSK3-β | -0.035 | >.999 |
| pGSK3-β | 0.136 | >.999 |
| HSP27 | -0.046 | >.999 |
| ILK | 0.035 | >.999 |
| MET | -0.035 | >.999 |
| p38 | 0.104 | >.999 |
| pp38 | -0.023 | >.999 |
| pER | 0.105 | >.999 |
| PI3K | -0.019 | >.999 |
| pSTAT3 | 0.215 | >.999 |
| ROCK | -0.029 | >.999 |
| uPA | 0.264 | >.999 |
The Spearman correlation coefficient rs and P values are shown. All P values are adjusted for multiple testing as described in the Materials and Methods section. Statistically significant correlations are in bold face emphases.
Table 4.
Correlation of uPA with Key Signaling Proteins Determined by RPPA in Lymph Node Metastases.
| uPA vs | rs | P (two-sided) |
| FAK | 0.418 | .073 |
| GSK3-β | 0.243 | >.999 |
| pGSK3-β | 0.461 | .030 |
| HSP27 | 0.274 | .106 |
| ILK | 0.217 | >.999 |
| MET | 0.389 | .144 |
| p38 | 0.343 | .374 |
| pp38 | 0.456 | .032 |
| PAI | 0.264 | >.999 |
| pER | 0.367 | .248 |
| PI3K | 0.199 | >.999 |
| pSTAT3 | 0.419 | .074 |
| ROCK | 0.359 | .293 |
The Spearman correlation coefficient rs and P values are shown. All P values are adjusted for multiple testing as described in the Materials and Methods section. Statistically significant correlations are in bold face emphases.
Discussion
Signaling Pathways in Primary Breast Cancers
During the last few years, cell-based models have provided insight into the binding and signaling of the members of the plasminogen activator system. Lately, we were able to confirm some of the cell culture studies in mammary carcinoma, and we could integrate them into a broader in vivo picture [16]. In the current study, we analyzed uPA, PAI-1, and 12 proteins potentially involved in uPA and PAI-1 signaling. Related to the AKT pathway, we found PAI-1 to be positively correlated with FAK, PI3K, ROCK, and ILK (Figure W3). In an earlier study of our institute, we could demonstrate PAI-1 correlation with the integrin subunit αV and pAKT. Together, these findings substantiate a positive correlation of PAI-1 with various members of the PI3K/AKT pathway in breast cancer. These correlation results were strengthened by the finding that there is clear coexpression of the proteins of the PI3K/AKT pathway (FAK, PI3K, ROCK, and ILK) with PAI-1 expression in individual patients. We found one of the five proteins differentially expressed compared to the others in only 4 of 52 patients analyzed here. In the literature, it is controversially discussed whether PAI-1 has an inhibiting or activating effect on the PI3K/AKT pathway and thus on cell proliferation and survival [13–15]. However, these studies were all realized in different cell culturemodels. Because of our findings, we can now state that, in breast cancer tissue, in which PAI-1 plays an important role in diagnosis, there is an activating connection between the protein and thementioned pathway. Furthermore, the results strengthen the assumption that the PI3K/AKT pathway is a majormediator on PAI-1-induced antiapoptotic activity. Because there are PI3K inhibitors already tested in clinical trials, our findings may be of clinical relevance. However, with our data, it is not possible to predicate who is influencing who, especially because there is also evidence that FAK, PI3K, ROCK, and ILK play a crucial role in the induction of PAI-1 expression [11,12]. Because GSK3-β seems not to be the preferential target of the PAI-1-induced signaling, additional studies will be needed to further characterize the outcome of this PI3K/AKT activation.
In addition to these core proteins of the AKT pathway, we also found PAI-1 to correlate with pp38 and HSP27. Functional studies demonstrated that an activation of p38 induces up-regulation of PAI-1 expression [29]. Accordingly, an inhibition of p38 phosphorylation leads to a decrease of PAI-1 levels [30,31]. Furthermore, it is a known fact that, through MAPKAPK activation, pp38 leads to an activation of HSP27 [32,33], which, in turn, stimulates activation of ROCK [33]. Unfortunately, we did not analyze the activated forms of HSP27 and ROCK, so that these findings could not be verified for breast cancer tissue samples. Nevertheless, there is evidence that, beside activation, p38 also increases the expression of HSP27 [34]. Such an overexpression of HSP27 then in turn leads to an up-regulation of PAI-1 [35]. Combined with the known facts in the literature, the found correlations support the interactions of PAI-1, p38, and HSP27.
For the correlation of uPA with cell signaling pathways, the results we obtained are less clear than for PAI-1. We found uPA to correlate with pERK but not pAKT in our previous study [16]. Here, we found interactions of uPA with pp38 but also with ILK, FAK, ROCK, and HSP27. Thus, we could not identify a single pathway that seems to be preferentially involved in uPA-mediated signaling so far.
Expression Studies in Primary Tumors versus Lymph Node Metastases
Analysis of the expression of uPA and PAI-1 in primary tumors versus lymph node metastases revealed that, whereas uPA expression correlated well between primary tumors and lymph nodes, PAI-1 seems to be differentially expressed in these entities. This finding might explain why the prognostic value of uPA and PAI-1 expression found in node-negative patients is lost in breast cancer patients bearing lymph node metastases. While the primary tumor might still react to chemotherapy as predicted by the uPA/PAI-1 status, the correlating lymph node metastases might be differentially regulated by a different expression of PAI-1, thus changing the outcome for the patient. The separation of the patients into two groups (compare Figure 2A) is of special interest in this regard. Although we could not correlate the two groups with any known clinical parameters (grading, staging, tumor size, hormone receptor status, and occurrence of distant metastases), it is plausible to argue that the finding might give a hint why analysis of uPA and PAI-1 in primary tumors does not predict therapy outcome in patients with node-positive breast cancer. The differences of PAI-1 expression in lymph node metastases may at least in part account for the loss of predictive value of uPA and PAI-1 seen in patients with node-positive cancers so far. Thus, our data show that a reevaluation of the predictive value of uPA and PAI-1 in node-positive patients taking the uPA and PAI-1 status of lymph node metastases into account might be auxiliary to further optimize patient stratification for chemotherapy beyond the node-negative status.
In addition to the deregulation of PAI-1 in lymph node metastases, we also found that uPA and PAI-1 correlated signaling was changed in metastases compared with primary tumors. None of the correlations found for PAI-1 in primary tumors is still present in metastases, hinting at differential signaling regulation between primary tumor and metastasis. On the basis of this assumption, we looked at the correlation the proteins analyzed in this study (n = 14) between primary tumor and metastasis individually. Here we found that, while most of the proteins analyzed in this study (uPA, FAK, HSP27, ILK, MET, p38, pER, pGSK3-β, PI3K, pp38, and pSTAT3) correlate between primary tumors and lymph node metastases, PAI-1 and GSK3-β did not (Table W3). This fits quite well with literature where differences of expression between primary tumors and their corresponding lymph node metastases are discussed quite controversially. There are studies demonstrating similar expressions of defined protein patterns in primary tumors and the correlating lymph node metastases [18,20] as well as such describing proteins to be differentially expressed in primary tumors and lymph node metastases [17,19]. Interestingly, a study by Hao et al. [36] concluded that both findings may be true depending on the individual protein analyzed. In our study, the similar versus differential expression of single proteins cannot be associated to distinct pathways, which indicates that the overall regulation of protein expression may well differ between primary tumors and the correlating lymph node metastases, thus leading to alternate signaling activity and thereby influencing further metastatic potential as well as therapy response.
In summary, we could pin down the correlation of PAI-1 expression with the PI3K/AKT pathway, whereas the results for uPA were not as clear. We found uPA to correlate with proteins of the MAPK pathway as well as with the PI3K/AKT pathway. The association with the MAPK pathway is supported by our previous study [16]. To get insight into the correlation of uPA with the PI3K/AKT pathway, further studies are planned. Furthermore, with this study, we provided insight into the differential expression of proteins and pathways between primary tumors and metastases demonstrating that there is no uniform change of expression between these entities. Regarding the potential of uPA and PAI-1 as predictive markers to stratify patients for chemotherapy, our results provide an interesting starting point to reevaluate the value of the factors in node-positive patients with consideration of the uPA and PAI-1 expression in lymph node metastasis.
Supplementary Material
Acknowledgments
The authors want to thank Kai Tran, Christina Schott, and Kerstin Schragner for excellent technical support.
Footnotes
This study was supported by the Nationale Genomforschungsnetz Project of Bundesministerium für Bildung und Forschung (grant no. 01GR0805 to K.F.B.). A.W. gratefully acknowledges the financial support of the DFG (WA 1565/3-1). No conflict of interests is declared by the authors.
This article refers to supplementary materials, which are designated by Tables W1 to W3 and Figures W1 to W3 and are available online at www.transonc.com.
References
- 1.Kroon ME, Koolwijk P, van der Vecht B, van Hinsbergh VW. Urokinase receptor expression on human microvascular endothelial cells is increased by hypoxia: implications for capillary-like tube formation in a fibrin matrix. Blood. 2000;96:2775–2783. [PubMed] [Google Scholar]
- 2.Schafer BM, Maier K, Eickhoff U, Todd RF, Kramer MD. Plasminogen activation in healing human wounds. J Pathol. 1994;44:1269–1280. [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiol Haemost Thromb. 2008;6:184–194. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- 4.Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996:613–618. [PubMed] [Google Scholar]
- 5.Janicke F, Schmitt M, Graeff H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost. 1991;17:303–312. doi: 10.1055/s-2007-1002624. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt M, Mengele K, Gkazepis A, Napieralski R, Magdolen V, Reuning U, Harbeck N. Assessment of urokinase-type plasminogen activator and its inhibitor PAI-1 in breast cancer tissue: historical aspects and future prospects. Breast Care. 2008;3:3–10. doi: 10.1159/000151737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Look MP, van Putten WL, Duffy MJ, Harbeck N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Ferno M, Eppenberger-Castori S, et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst. 2002;94:116–128. doi: 10.1093/jnci/94.2.116. [DOI] [PubMed] [Google Scholar]
- 8.Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Downregulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
- 9.Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood. 2009;113:1383–1390. doi: 10.1182/blood-2008-06-164210. [DOI] [PubMed] [Google Scholar]
- 10.Shetty S, Rao GN, Cines DB, Bdeir K. Urokinase induces activation of STAT3 in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L772–L780. doi: 10.1152/ajplung.00476.2005. [DOI] [PubMed] [Google Scholar]
- 11.Soeda S, Oda M, Ochiai T, Shimeno H. Deficient release of plasminogen activator inhibitor-1 from astrocytes triggers apoptosis in neuronal cells. Brain Res Mol Brain Res. 2001;91:96–103. doi: 10.1016/s0169-328x(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 12.Soeda S, Shinomiya K, Ochiai T, Koyanagi S, Toda A, Eyanagi R, Shimeno H. Plasminogen activator inhibitor-1 aids nerve growth factor-induced differentiation and survival of pheochromocytoma cells by activating both the extracellular signal-regulated kinase and c-Jun pathways. Neuroscience. 2006;141:101–108. doi: 10.1016/j.neuroscience.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Kelm RJ, Jr, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 14.Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG. Akt kinase targets association of CBP with SMAD 3 to regulate TGFβ-induced expression of plasminogen activator inhibitor-1. J Cell Physiol. 2008;214:513–527. doi: 10.1002/jcp.21236. [DOI] [PubMed] [Google Scholar]
- 15.Mukai Y, Wang CY, Rikitake Y, Liao JK. Phosphatidylinositol 3-kinase/protein kinase Akt negatively regulates plasminogen activator inhibitor type 1 expression in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H1937–H1942. doi: 10.1152/ajpheart.00868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff C, Malinowsky K, Berg D, Schragner K, Schuster T, Walch A, Bronger H, Hofler H, Becker KF. Signalling networks associated with urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1 in breast cancer tissues: new insights from protein microarray analysis. J Pathol. 2011;223:54–63. doi: 10.1002/path.2791. [DOI] [PubMed] [Google Scholar]
- 17.Gschwantler-Kaulich D, Fink-Retter A, Czerwenka K, Hudelist G, Kaulich A, Kubista E, Singer CF. Differential expression pattern of estrogen receptors, aromatase, and sulfotransferase in breast cancer tissue and corresponding lymph node metastases. Tumour Biol. 2011;32:501–508. doi: 10.1007/s13277-010-0144-3. [DOI] [PubMed] [Google Scholar]
- 18.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 19.Vecchi M, Confalonieri S, Nuciforo P, Vigano MA, Capra M, Bianchi M, Nicosia D, Bianchi F, Galimberti V, Viale G, et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene. 2008;27:2148–2158. doi: 10.1038/sj.onc.1210858. [DOI] [PubMed] [Google Scholar]
- 20.Weigelt B, Wessels LF, Bosma AJ, Glas AM, Nuyten DS, He YD, Dai H, Peterse JL, van't Veer LJ. No common denominator for breast cancer lymph node metastasis. Br J Cancer. 2005;93:924–932. doi: 10.1038/sj.bjc.6602794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker KF, Schott C, Hipp S, Metzger V, Porschewski P, Beck R, Nahrig J, Becker I, Hofler H. Quantitative protein analysis from formalin-fixed tissues: implications for translational clinical research and nanoscale molecular diagnosis. J Pathol. 2007;211:370–378. doi: 10.1002/path.2107. [DOI] [PubMed] [Google Scholar]
- 22.Wolff C, Schott C, Porschewski P, Reischauer B, Becker KF. Successful protein extraction from over-fixed and long-term stored formalin-fixed tissues. PLoS One. 2011;6:e16353. doi: 10.1371/journal.pone.0016353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brase J, Mannsperger H, Frohlich H, Gade S, Schmidt C, Wiemann S, Beissbarth T, Schlomm T, Sultmann H, Korf U. Increasing the sensitivity of reverse phase protein arrays by antibody-mediated signal amplification. Proteome Sci. 2010;8:36. doi: 10.1186/1477-5956-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handschuh G, Candidus S, Luber B, Reich U, Schott C, Oswald S, Becke H, Hutzler P, Birchmeier W, Hofler H, et al. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene. 1999;18:4301–4312. doi: 10.1038/sj.onc.1202790. [DOI] [PubMed] [Google Scholar]
- 25.Neeley ES, Kornblau SM, Coombes KR, Baggerly KA. Variable slope normalization of reverse phase protein arrays. Bioinformatics. 2009;25:1384–1389. doi: 10.1093/bioinformatics/btp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner TR. Estimating the propagation rate of a viral infection of potato plants via mixtures of regressions. J R Stat Soc C Appl Stat. 2000;49:371–384. [Google Scholar]
- 27.Turner TR. mixreg: Functions to Fit Mixtures of Regressions. 2009. R package version 0.0-3. Available at: http://CRAN.R-project.org/package=mixreg.
- 28.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 29.Jaulmes A, Sansilvestri-Morel P, Rolland-Valognes G, Bernhardt F, Gaertner R, Lockhart BP, Cordi A, Wierzbicki M, Rupin A, Verbeuren TJ. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb Res. 2009;124:439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Vayalil PK, Iles KE, Choi J, Yi AK, Postlethwait EM, Liu RM. Glutathione suppresses TGF-β-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1281–L1292. doi: 10.1152/ajplung.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nerurkar SS, Olzinski AR, Frazier KS, Mirabile RC, O'Brien SP, Jing J, Rajagopalan D, Yue TL, Willette RN. P38 MAPK inhibitors suppress biomarkers of hypertension end-organ damage, osteopontin and plasminogen activator inhibitor-1. Biomarkers. 2007;12:87–112. doi: 10.1080/13547500600944930. [DOI] [PubMed] [Google Scholar]
- 32.Hong Z, Zhang QY, Liu J, Wang ZQ, Zhang Y, Xiao Q, Lu J, Zhou HY, Chen SD. Phosphoproteome study reveals Hsp27 as a novel signaling molecule involved in GDNF-induced neurite outgrowth. J Proteome Res. 2009;8:2768–2787. doi: 10.1021/pr801052v. [DOI] [PubMed] [Google Scholar]
- 33.Pramanik R, Qi X, Borowicz S, Choubey D, Schultz RM, Han J, Chen G. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J Biol Chem. 2003;278:4831–4839. doi: 10.1074/jbc.M207732200. [DOI] [PubMed] [Google Scholar]
- 34.Hirade K, Kozawa O, Tanabe K, Niwa M, Matsuno H, Oiso Y, Akamatsu S, Ito H, Kato K, Katagiri Y, et al. Thrombin stimulates dissociation and induction of HSP27 via p38 MAPK in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;283:H941–H948. doi: 10.1152/ajpheart.00060.2001. [DOI] [PubMed] [Google Scholar]
- 35.Aldrian S, Kindas-Mugge I, Trautinger F, Frohlich I, Gsur A, Herbacek I, Berger W, Micksche M. Overexpression of Hsp27 in a human melanoma cell line: regulation of E-cadherin, MUC18/MCAM, and plasminogen activator (PA) system. Cell Stress Chaperones. 2003;8:249–257. doi: 10.1379/1466-1268(2003)008<0249:oohiah>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao X, Sun B, Hu L, Lahdesmaki H, Dunmire V, Feng Y, Zhang SW, Wang H, Wu C, Fuller GN, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100:1110–1122. doi: 10.1002/cncr.20095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.