Abstract
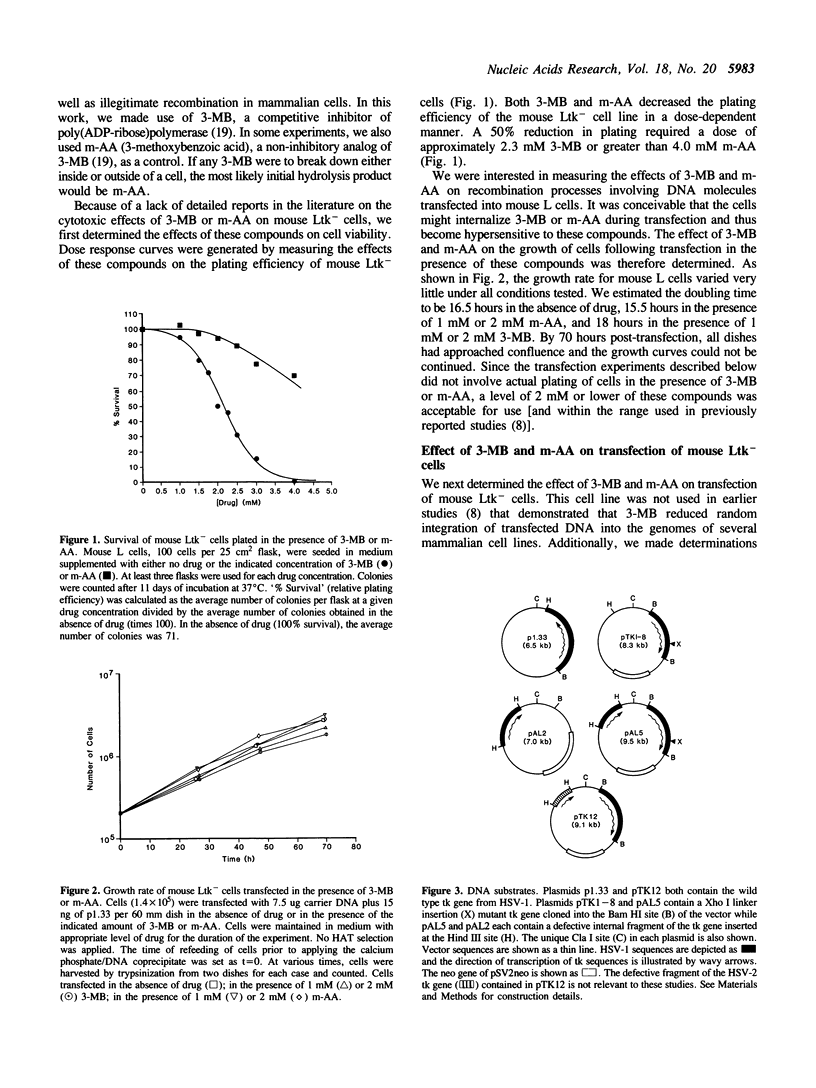
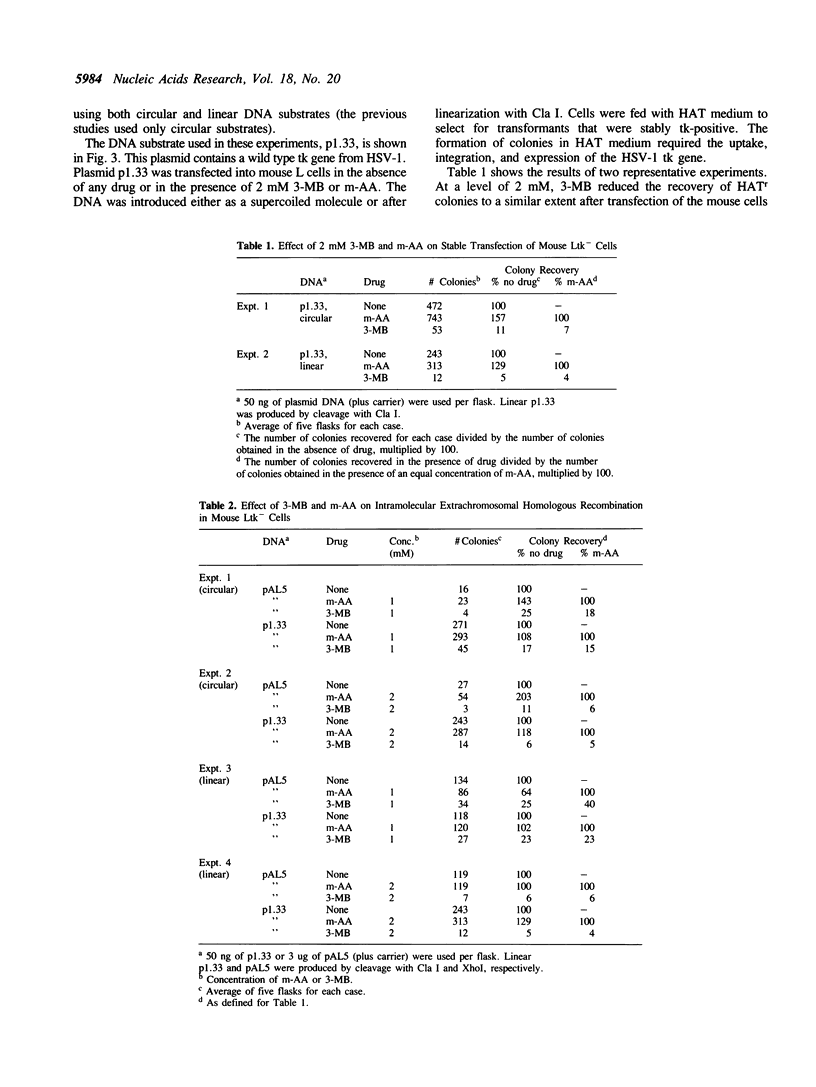
We determined the effect of 3-methoxybenzamide (3-MB), a competitive inhibitor of poly(ADP-ribose)polymerase (E.C. 2.4.2.30), on illegitimate and extrachromosomal homologous recombination in mouse Ltk- cells. Cells were transfected with a wild type Herpes thymidine kinase (tk) gene or with two defective tk gene sequences followed by selection for tk-positive colonies. Using a wild type tk gene, colony formation required uptake, integration, and expression of the tk gene. Using defective tk genes, colony formation had the additional requirement for homologous recombination to reconstruct a functional tk gene. The presence of non-cytotoxic levels of 3-MB during and after transfection reduced the number of colonies recovered with a wild type tk gene in a dose-dependent manner, with 2 mM 3-MB causing a 10 to 20-fold reduction. 3-MB reduced the number of colonies recovered with defective tk genes only to the same extent as in transfections with a wild type gene. Treatment with 3-methoxybenzoic acid, a non-inhibitory analog of 3-MB, did not reduce the recovery of colonies in any experiment. Similar results were obtained using linear or supercoiled molecules and when defective tk genes were transfected into cells on one or two different DNA molecules. By assaying for transient expression of the tk gene, we found that 3-MB did not inhibit uptake or expression of the tk gene. We conclude that poly(ADP-ribosylation) plays a role in random integration (illegitimate recombination) of DNA but does not play an important role in extrachromosomal homologous recombination, demonstrating that these two recombination pathways in cultured mouse fibroblasts are biochemically distinct.
Full text
PDF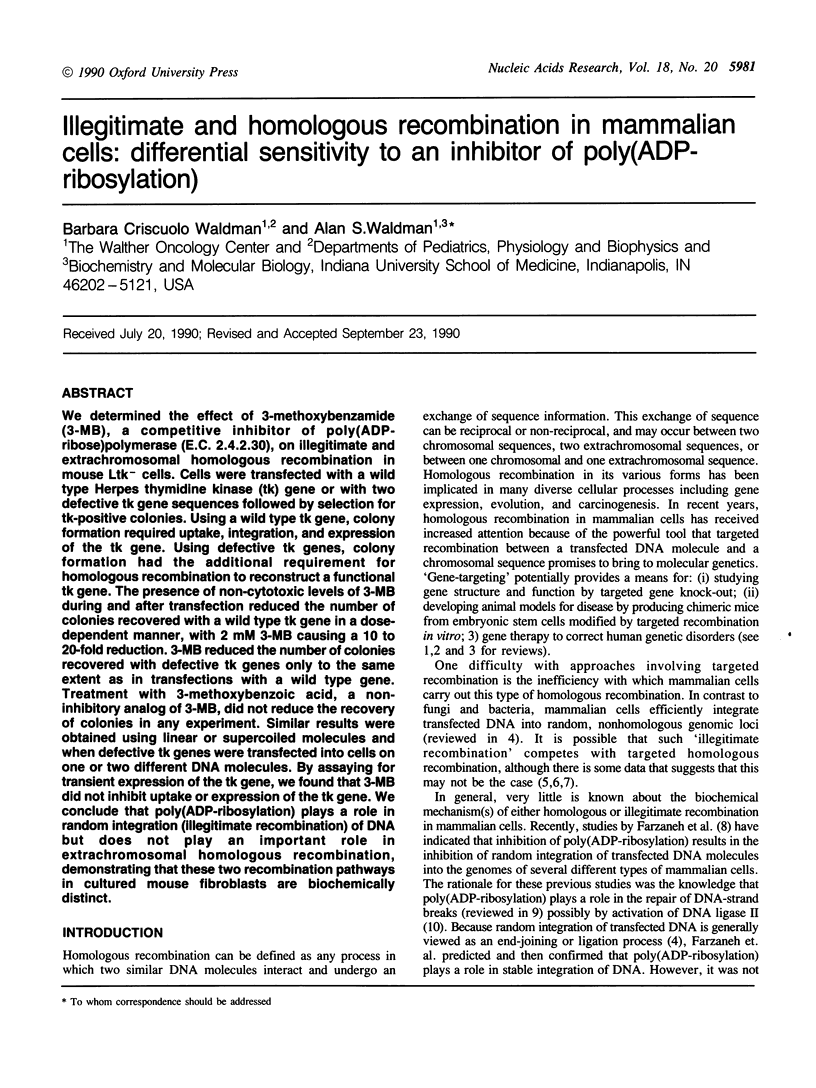



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Panayotou G. N., Bowler L. D., Hardas B. D., Broom T., Walther C., Shall S. ADP-ribosylation is involved in the integration of foreign DNA into the mammalian cell genome. Nucleic Acids Res. 1988 Dec 9;16(23):11319–11326. doi: 10.1093/nar/16.23.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A. M., McElwain M. C., Olivera B. M. Poly(ADP-ribosylation) of DNA topoisomerase I: a nuclear response to DNA-strand interruptions. Cold Spring Harb Symp Quant Biol. 1984;49:683–690. doi: 10.1101/sqb.1984.049.01.077. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri C. P., West M. H., Ramirez M. L., Smulson M. Nuclear protein modification and chromatin substructure. 2. Internucleosomal localization of poly(adenosine diphosphate-ribose) polymerase. Biochemistry. 1978 Aug 22;17(17):3501–3504. doi: 10.1021/bi00610a012. [DOI] [PubMed] [Google Scholar]
- Kit S., Kit M., Qavi H., Trkula D., Otsuka H. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim Biophys Acta. 1983 Nov 17;741(2):158–170. doi: 10.1016/0167-4781(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Malik N., Miwa M., Sugimura T., Thraves P., Smulson M. Immunoaffinity fractionation of the poly(ADP-ribosyl)ated domains of chromatin. Proc Natl Acad Sci U S A. 1983 May;80(9):2554–2558. doi: 10.1073/pnas.80.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommerskirch W., Graeber I., Grässmann M., Grässmann A. Homologous recombination of SV40 DNA in COS7 cells occurs with high frequency in a gene dose independent fashion. Nucleic Acids Res. 1988 Feb 11;16(3):941–952. doi: 10.1093/nar/16.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Differential effects of base-pair mismatch on intrachromosomal versus extrachromosomal recombination in mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5340–5344. doi: 10.1073/pnas.84.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A. S., Milman G. Transfer of herpes simplex virus thymidine kinase synthesized in bacteria by a high-expression plasmid to tissue culture cells by protoplast fusion. Mol Cell Biol. 1984 Aug;4(8):1644–1646. doi: 10.1128/mcb.4.8.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. A., Capecchi M. R. Homologous recombination between coinjected DNA sequences peaks in early to mid-S phase. Mol Cell Biol. 1987 Jun;7(6):2294–2295. doi: 10.1128/mcb.7.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Wilson J. H. Gene targeting in normal and amplified cell lines. Nature. 1990 Mar 8;344(6262):170–173. doi: 10.1038/344170a0. [DOI] [PubMed] [Google Scholar]
- Zipser D., Lipsich L., Kwoh J. Mapping functional domains in the promoter region of the herpes thymidine kinase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6276–6280. doi: 10.1073/pnas.78.10.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]