Abstract
Objective: High temperature adversely affects quality and yield of tomato fruit. Polyamine can alleviate heat injury in plants. This study is aimed to investigate the effects of polyamine and high temperature on transcriptional profiles in ripening tomato fruit. Methods: An Affymetrix tomato microarray was used to evaluate changes in gene expression in response to exogenous spermidine (Spd, 1 mmol/L) and high temperature (33/27 °C) treatments in tomato fruits at mature green stage. Results: Of the 10 101 tomato probe sets represented on the array, 127 loci were differentially expressed in high temperature-treated fruits, compared with those under normal conditions, functionally characterized by their involvement in signal transduction, defense responses, oxidation reduction, and hormone responses. However, only 34 genes were up-regulated in Spd-treated fruits as compared with non-treated fruits, which were involved in primary metabolism, signal transduction, hormone responses, transcription factors, and stress responses. Meanwhile, 55 genes involved in energy metabolism, cell wall metabolism, and photosynthesis were down-regulated in Spd-treated fruits. Conclusions: Our results demonstrated that Spd might play an important role in regulation of tomato fruit response to high temperature during ripening stage.
Keywords: Solanum lycopersicum L., Spermidine, High temperature, Microarray, Gene expression
1. Introduction
High temperature (HT) is one of the main environmental constraints to agricultural productivity worldwide. Many efforts have been made to explain the mechanisms of HT tolerance in plants through molecular and genomic approaches, and a number of genes involved in HT stress response at the transcriptional level have been reported (Wahid et al., 2007). Immediately after exposure to HT, changes at the molecular level alter the expression of many genes involved in various pathways, thereby leading to the synthesis of stress-related proteins as a stress-tolerance strategy (Iba, 2002). Some of these genes might play important roles in protecting plants from heat stress through stress perception, signal transduction, and transcriptional regulatory networks in cellular responses.
Tomato (Solanum lycopersicum L.) is an important commercial crop and has been proved to be a highly useful model system for fruit development and ripening. HT is a major factor limiting the productivity in warm seasons and adversely influences the vegetative and reproductive phases of tomato, which ultimately reduces in yield and quality (Sato et al., 2001; Pressman et al., 2002). Exposure to HT adversely affects tomato ripening and nutrient and flavor quality, inducing poor fruit coloring, vacuous fruit, low sugar/acid ratio, and decreasing lycopene content (Yakir et al., 1984; Paull and Chen, 2000), and finally causing tissue damage and physiological disorder (Inaba and Crandall, 1988). Furthermore, heat treatment leads to an inhibition of expression of tomato-ripening genes and thus has a negative impact on fruit quality (Picton and Grierson, 1988). The mRNA levels of 1-aminocyclopropane-1-carboxylic acid oxidase, phytoene synthase, and polygalacturonase decreased dramatically during the heat treatment but recovered afterwards, whereas the mRNA of heat shock protein (HSP) 17 increased during the HT treatment and then decreased when fruits were removed from heat stress (Lurie et al., 1996). So the inhibition of fruit ripening by HT might occur at the level of gene expression. However, the entire molecular events occurring in the fruit under HT stress were not investigated until now.
Aliphatic polyamines [putrescine (Put), spermidine (Spd), and spermine (Spm)] are ubiquitous compounds involved in various physiological processes, including plant growth and development, flowering, fruit growth and development, and stress response, senescence, and fruit ripening (Mariani et al., 1989). In recent years, a protective role against stress has been attributed to polyamines, both in the free and soluble conjugated forms, during mineral nutrient deficiency, and osmotic, salt, drought, heat, chilling, and oxidation stresses (Liu et al., 2000; Perez-Amador et al., 2002; Nayyar and Chander, 2004; Renaut et al., 2005; Todorova et al., 2007). Manipulation of polyamine biosynthesis may lead to improvement of plant tolerance against multiple environmental stresses. Transgenic rice plants expressing an oat ADC cDNA exhibited an increased polyamine accumulation, which enhanced plant biomass under salinity (Roy and Wu, 2001). Transgenic tomato seedlings overexpressing yeast S-adenosyl-l-methionine decarboxylase gene would improve the tolerance to HT stress as compared to wild plants (Cheng et al., 2009). Furthermore, Mattoo et al. (2002) proved that higher polyamines enhanced the accumulation of lycopene in the tomato. Since polyamines have been described as anti-senescence agents and a higher endogenous level of polyamines is associated with delayed fruit ripening (Valero et al., 2002), many attempts have recently been made to explain the role of exogenous polyamines on fruit ripening. Pre-harvest and post-harvest applications of polyamines have been demonstrated to delay the fruit ripening and extend shelf life in mango, peach, plum, apple, and tomato (Law et al., 1991; Pérez-Vicente et al., 2002; Torrigiani et al., 2004). However, controversial results on the effect of exogenous polyamines on fruit ripening have also been obtained (Wang C.Y. et al., 1993; Escribano and Merodio, 1994). Therefore, much deeper insight is still required to understand the role of polyamines in fruit development and ripening.
In recent decades, there is increasing evidence demonstrating that polyamines act as antioxidants under environmental adverse conditions (Groppa et al., 2001; Kakkar and Sawhney, 2002). Exogenous application of polyamines reduced the H2O2 level and malondialdehyde content and increased the level of antioxidants in chickpea plants subjected to water deficiency and cold stress (Nayyar and Chander, 2004). Exogenous Put improved Indian mustard seedling growth by preventing lipid peroxidation and denaturation of macromolecules through the induction of antioxidative enzymes and the increase of glutathione and carotenoid under NaCl stress (Verma and Mishra, 2005). Conversely, polyamines may act as a mediator or secondary messenger to activate a vast genetic network with a potential to provide defense against biotic and abiotic stresses (Paschalidis and Roubelakis-Angelakis, 2005; Cona et al., 2006). It has been proved that a wide array of genes regulating transcription, translation, signal transduction, stress protein biosynthesis, ethylene biosynthesis and action, isoprenoid and flavonoid biosyntheses was activated by polyamines. However, there are still many doubts concerning the role of polyamines in stress tolerance (Groppa and Benavides, 2008).
More recently, high-throughput screening techniques such as microarray analysis have been used to monitor the expression of genes that respond to biotic and abiotic stresses (Zhang H. et al., 2010; Lang et al., 2011). Investigating how polyamines alleviate the HT injury could facilitate a better understanding of the genetic bases of heat tolerance. Thus, in the present study, to investigate the effects of HT stress and Spd application on the gene expression in tomato fruit during ripening, mature green tomatoes were harvested and treated with Spd under different temperature regimes. The transcription profiles were compared using Affymetrix microarray analysis, and the differentially expressed genes involved in primary metabolism, stress response, and hormone biosynthesis were identified after HT and Spd treatments.
2. Materials and methods
2.1. Plant materials and growth conditions
Tomatoes [Solanum lycopersicum L. (formerly Lycopersicon esculentum Miller)] cv. Zhongshu No. 6 obtained from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China were grown in a commercial greenhouse (21–29 °C day, >12 °C night) at the experimental farm in Zhejiang University, China. Seeds were sown in pots (30 cm×20 cm) containing potting media (mixture of peat and vermiculite) at the end of Jan. 2008, and seedlings with five growing leaves were transplanted to a field in a Venlo-type greenhouse. The plants were arranged at a density of four plants per m2. Plant nutrition, pest and disease controls were in accordance with commercial practices.
2.2. High temperature and exogenous spermidine treatments
Tomato fruits were harvested at the mature green stage approximately four months after sowing in June 2008. Only fruits at a firm, mature green color classification with uniform shape and size and free from fungal infection were selected. After harvest, fruits were washed in tap water, air-dried at 25 °C, and individually labeled.
Mature green fruits were randomly divided into two batches. One batch was immersed in 1.0 mmol/L Spd solutions with 0.1% Tween-20 surfactant (5 L, 20 °C) for 30 min, and the other in distilled water containing 0.1% Tween-20 surfactant for 30 min. After air-drying for 10 min, the fruits of Spd-treatment and control were respectively subdivided into two sub-batches with 60 fruits each, and furthermore, each sub-batch was kept under normal (26/20 °C) or heat temperature (33/27 °C) condition. Finally, there were four different treatments, including normal temperature (C26) and HT (C33) without Spd treatment, Spd pre-treatment under normal (Spd26) and HT (Spd33) conditions. Detached mature green fruits were incubated in controlled environment chambers with 16/8 h (light/dark) period and 85% relative humidity (RH). The fruits were sampled at 1, 6, and 12 h, respectively. One gram of fresh peel and flesh were taken at the fruit shoulder position, immediately frozen in liquid nitrogen, and stored at −80 °C for further use.
2.3. Microarray analysis
Total RNA was extracted from the fruits of C26, C33, Spd26, and Spd33 at 1, 6, and 12 h, respectively, using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s recommendations. Each pooled RNA sample was generated by mixing equal amounts of RNA from three intervals and then the mRNA was purified from 80‒90 μg mixed total RNA using the RNeasy Plant mini kit (QIAGEN, Germany) according to the instructions. Then the double stranded cDNA was synthesized using the GeneChip® two-cycle cDNA synthesis kit. Tomato genome array (Affymetrix, CA, USA), designed specifically to monitor gene expression in tomato, was used. All procedures for probe preparation, hybridization, washing, staining, and scanning of the GeneChip® tomato arrays, as well as data collection, were performed at Affymetrix custom service (CapitalBio, Beijing, China) by following the standard protocol (http://www.affymetrix.com/support/technical/manual/expression_manual.affx). Normalization was performed according to the standard Affymetrix protocol to allow the comparison of the samples from each set of experiments. Fold changes and P-values of probe sets were calculated using limma nested F-test, and the P-values for multiple testing were corrected using the false discovery rate (Benjamini and Hochberg, 1995). The Excel add-in for significance analysis of microarrays was used to identify differentially expressed genes between different treatments.
2.4. Databases for tomato functional genomics analysis
The data described here have been deposited in the Tomato Expression Database (http://ted.bti.cornell.edu/) and are available for public access (Fei et al., 2006). Nucleic acid sequence and annotation data pertaining to the TOM1 microarray are available via the sol genomics network (SGN) database (Mueller et al., 2005) (http://sgn.cornell.edu/).
2.5. Quantitative real-time polymerase chain reaction (PCR)
To validate the expression patterns revealed by microarray results, 15 genes identified through microarray analysis, which represent up-regulated, down-regulated, and unchanged genes, respectively, were analyzed using quantitative real-time PCR. The RNA samples for microarray analysis were also used for quantitative real-time PCR. Equal amounts of total RNA for each sample from three sampling time points (1, 6, and 12 h) were mixed and then the first-strand cDNA was synthesized from the mixed total RNA using ImProm-II™ reverse transcription system (Promega, Madison, USA) according to the manufacture’s instructions. Specific primers (Table 1) for selected genes were designed for 80‒120 bp amplicon with melting temperature at 50‒52 °C by DNAMAN software. The cDNA obtained (1 μg) was subjected to real-time PCR in a final volume of 20 μl containing 12.5 μl SYBR Green Master Mix Reagent (TaKaRa, Japan) and specific primers (3 pmol). Two biological and three technical replicates for each sample were performed in an iCycler iQ™ real-time PCR system (Bio-Rad, USA) programmed to heat for 4 min at 95 °C, followed by cycling conditions (melting step for 30 s at 95 °C, annealing for 30 s at 54 °C, and extension for 30 s at 72 °C) repeated for 40 cycles. To normalize the total amount of cDNA present in each reaction, Ubi3 gene was co-amplified as an endogenous control for calibration of relative expression. Melting curves were performed using Dissociation Curves software (Applied Biosystems, Foster City, CA) to ensure only a single product was amplified. The PCR efficiency was estimated by the data obtained from the exponential phase of each individual amplification plot and the equation (1+Eff)=10slope (Ramakers et al., 2003). The comparative cycle threshold (ΔΔC t) method of relative gene quantification recommended by Applied Biosystems was used to calculate the expression level of different treatments.
Table 1.
Primers for real-time PCR analysis
| Accession No. | Primer sequence (5′→3′) | |
| BG629220 | F | CTTCAAACCTTGGCTCTT |
| R | TCATCTATCCAGCACCAG | |
| BM535342 | F | CTCACCTCACTTCGCTTC |
| R | GAGATGGTCAGGTGAGAGAG | |
| U66300 | F | AAGCGGAACTGAAGAATG |
| R | GGACATCAATCACCTTTTTCTC | |
| BF112635 | F | GGGGATTCTTTGAGGTGA |
| R | GCCTTTACTTGCCACCAT | |
| BE459581 | F | ACAGGAGTTGCTGGTTCATTT |
| R | TTGAGAAAGCCAATGAAGAC | |
| BG628248 | F | GTTTATCCTTACGGTTTCTCC |
| R | GCGGCTGATAAAGGTGAG | |
| AY128100 | F | CAAGAATTGCTTTGCCTG |
| R | AACACTTTGGGATTGCTACT | |
| AI781668 | F | TGCTGTATCAATGCCTGTG |
| R | TGAACAGACATGAACCCT | |
| BE432137 | F | CAGAGGATGTTGGAGGCA |
| R | GACACGCAACATTTACCC | |
| BG129203 | F | GGCAAAGTTCACTGGGATA |
| R | TTTCCCGTGGTTGGTT | |
| X95296 | F | GGCACATACAAACAAAGGAG |
| R | CCATCGGAGACGACAAC | |
| BT014421 | F | CATTCGTGAAGGGTGATC |
| R | TGGATAAAGTTCCGAGGC | |
F: forward; R: reverse
3. Results
3.1. Differentially expressed genes in response to high temperature stress
Using a microarray platform consisting of approximately 10 101 tomato probe sets, we found that a total of 249 genes exhibited significant up-regulation (>2.0-fold), while 112 genes exhibited down-regulated expression in HT-treated fruits (C33) compared with those under normal condition (C26). The Affymetrix identification (ID) number, National Center for Biotechnology Information (NCBI) accession number, GenBank homologue, and Arabidopsis genome initiative number of the best hit from translated BLAST (TBLASTX) search are listed in Tables 2‒5 and Tables S1‒S2 using a significance threshold of 10−4.
Table 2.
Defense responses and oxidation reduction genes with significant (P<0.01) differential expression (≥2.0-fold)
| Contig ID | Accession No. | Annotation | Fold difference |
||||
| C33 vs. C26 | Spd26 vs. C26 | Spd33 vs. C26 | Spd33 vs. C33 | Spd33 vs. Spd26 | |||
| Affx.11839.1.S1_at | BI208864 | NADH dehydrogenase subunit 3 | 2.08 | 1.25 | −1.56 | −3.24 | −1.12 |
| Affx.44224.1.A1_at | AI487590 | NADH-ubiquinone oxidoreductase | 4.29 | 1.44 | 1.68 | −2.56 | 1.33 |
| Affx.44474.1.S1_at | AJ831993 | NADH dehydrogenase subunit 4 | 2.30 | −1.09 | 1.30 | −1.76 | 1.07 |
| Affx.70450.1.S1_at | AW033442 | NADH dehydrogenase subunit 6 | 3.85 | −1.03 | 2.56 | −1.50 | 1.23 |
| Les.3311.3.S1_at | AI782246 | NADP-isocitrate dehydrogenase | −2.52 | −1.10 | −1.56 | 1.61 | −1.69 |
| Les.3677.1.S1_at | AY128100.1 | Small heat shock protein | 3.50 | −1.10 | 3.30 | −1.06 | 5.24 |
| Affx.10807.1.S1_at | BM408547 | Heat shock protein 70 | 2.55 | 1.10 | 2.12 | −1.20 | 1.82 |
| Les.3739.1.S1_at | AB026983.1 | Small heat shock protein | 6.29 | 1.05 | 6.54 | 1.04 | 13.71 |
| Affx.69957.1.S1_at | BM410870 | 26.5 kDa class I small heat shock protein-like | 2.23 | −1.23 | 2.66 | 1.20 | 3.82 |
| Affx.63687.1.S1_at | CN384460 | 26.5 kDa class P-related heat shock protein | 3.20 | 1.02 | 2.42 | −1.32 | 2.82 |
| Les.3550.1.A1_s_at | AF096251.1 | Ethylene-responsive heat shock protein cognate | 2.51 | 1.06 | 2.25 | −1.12 | 1.49 |
| Les.269.1.S1_at | U66300.1 | Heat shock protein | 2.94 | 1.35 | 3.29 | 1.12 | 5.48 |
| Affx.9815.1.S1_at | CK715796 | DNAJ-like protein | 2.90 | 1.05 | 3.43 | 1.63 | 1.89 |
| Affx.56637.1.S1_at | BI208173 | DNAJ heat shock N-terminal domain-containing protein | 3.40 | 1.12 | 2.31 | −1.47 | 2.56 |
| Affx.45577.1.A1_at | CN384902 | DNAJ heat shock N-terminal domain-containing protein | −2.40 | 1.09 | 1.27 | 3.04 | 1.16 |
| Affx.23109.1.S1_at | AI782520 | Cold shock domain protein 1 | −2.63 | −1.03 | 1.01 | 2.66 | −1.02 |
| Les.124.1.S1_at | AY034473.1 | CRT/DRE-binding protein 1 | 1.20 | 1.06 | 3.70 | 3.10 | 3.76 |
| Les.4880.1.S1_at | BT012820.1 | Cytochrome P450 | 2.83 | 1.01 | 2.47 | −1.14 | 2.12 |
| Les.3534.1.S1_at | AJ270961.1 | Putative cytochrome P450 | −1.04 | −1.18 | −5.29 | −1.10 | 1.10 |
| Affx.24042.1.S1_at | CN385197 | Cytochrome P450 76A1 | −2.28 | 1.02 | 1.61 | 3.67 | 1.61 |
| Les.4528.1.A1_at | AJ635324.1 | Polyphenol oxidase A | −6.22 | 1.06 | −10.94 | −1.76 | −15.78 |
Table 5.
Metabolism-related genes with significant (P<0.01) differential expression (≥2.0-fold)
| Contig ID | Accession No. | Annotation | Fold difference |
||||
| C33 vs. C26 | Spd26 vs. C26 | Spd33 vs. C26 | Spd33 vs. C33 | Spd33 vs. Spd26 | |||
| Les.5934.1.S1_at | AI895164 | Omega-6 fatty acid desaturase | −3.70 | −1.05 | −2.54 | 1.46 | −3.32 |
| Les.5240.1.S1_at | BT013554.1 | Serine protease | −3.15 | 1.13 | −2.94 | 1.07 | −3.56 |
| Les.5402.1.S1_at | BT013846.1 | Serine carboxypeptidase | −2.53 | 1.04 | −3.27 | −1.29 | −2.34 |
| Les.3610.1.S1_at | BG629712 | Glycine rich protein | −3.13 | 1.08 | −3.06 | 1.02 | −4.67 |
| Les.3035.1.A1_at | BI423372 | Cathepsin D inhibitor protein | −3.10 | −1.22 | −7.01 | −2.26 | −1.35 |
| Les.2809.1.S1_at | BT014540.1 | Putative glucosyltransferase | −2.66 | −1.23 | −3.35 | −1.26 | −2.77 |
| Les.5832.1.S1_at | BT014414.1 | UDP-glucoronosyl family protein | −2.31 | −1.08 | −1.08 | 2.13 | 2.60 |
| Affx.15898.2.S1_at | BE434722 | UDP-xylose phenolic glycosyltransferase | 2.00 | −1.01 | 2.39 | 1.20 | 2.03 |
| Les.3696.1.S1_at | AF311943.1 | UDP-galactose:myo-inositol galactosyltransferase | 5.49 | 1.35 | −1.48 | −8.13 | −3.55 |
| Affx.51348.1.S1_at | AJ785026 | Aspartyl protease family protein | −2.26 | 1.07 | −2.19 | −1.09 | −1.78 |
| Les.4317.1.S1_at | AW625684 | Asparagine synthetase | −2.20 | 1.01 | −2.83 | −1.28 | −1.21 |
| Affx.58104.1.S1_at | AW036288 | Globulin precursor | −2.16 | 1.14 | −5.52 | −4.62 | −6.36 |
| Affx.58041.1.A1_at | CN385714 | Glycosyl hydrolase family 1 protein | −2.16 | −1.01 | −1.41 | 1.53 | −1.32 |
| Affx.19924.1.S1_at | BE459775 | Glycosyl hydrolase family 17 protein | −2.10 | −1.11 | −1.94 | 1.08 | −2.05 |
| Les.2817.2.S1_at | BI933507 | ATP-citrate lyase A-1 | −2.05 | 1.34 | −1.77 | 1.16 | −1.72 |
| Les.4868.1.S1_at | BT012795.1 | ADP/ATP translocator | 2.63 | −1.22 | 2.44 | −1.08 | 3.08 |
| Affx.51975.2.S1_at | BI930488 | Cytochrome c oxidase subunit | 2.00 | 1.24 | 1.60 | −1.25 | 1.21 |
| Affx.51226.1.S1_at | AI779132 | Cytochrome f | 2.88 | 1.15 | 1.08 | −3.11 | −1.04 |
| Affx.30946.1.A1_at | AJ785184 | Cytochrome b6 | 3.02 | −1.16 | 2.13 | −1.42 | 1.73 |
| Affx.9007.1.S1_at | CN385923 | Lactoylglutathione lyase family protein | 3.06 | −1.01 | 3.53 | 1.15 | 2.68 |
| Affx.51975.1.A1_at | AF362735.1 | Succinate dehydrogenase subunit 4 | 3.35 | −1.08 | 1.59 | −2.11 | 1.16 |
| Les.5956.1.S1_at | CN385508 | Proline dehydrogenase | 3.74 | −1.21 | −1.07 | −4.01 | −1.05 |
| Affx.71476.1.S1_at | AW036283 | 11S globulin seed storage protein 2 precursor | −1.41 | 1.04 | −8.32 | −5.90 | −9.86 |
| Les.3980.1.S1_at | U37839.1 | Lipoxygenase | −1.82 | 1.04 | −3.55 | −1.95 | −2.83 |
| Les.3273.1.S1_at | BG627786 | Proline rich protein | −1.44 | 1.66 | −4.44 | −3.08 | −1.09 |
| Affx.58104.1.A1_at | AJ785426 | Globulin precursor | −1.20 | 1.14 | −5.52 | −4.62 | −6.36 |
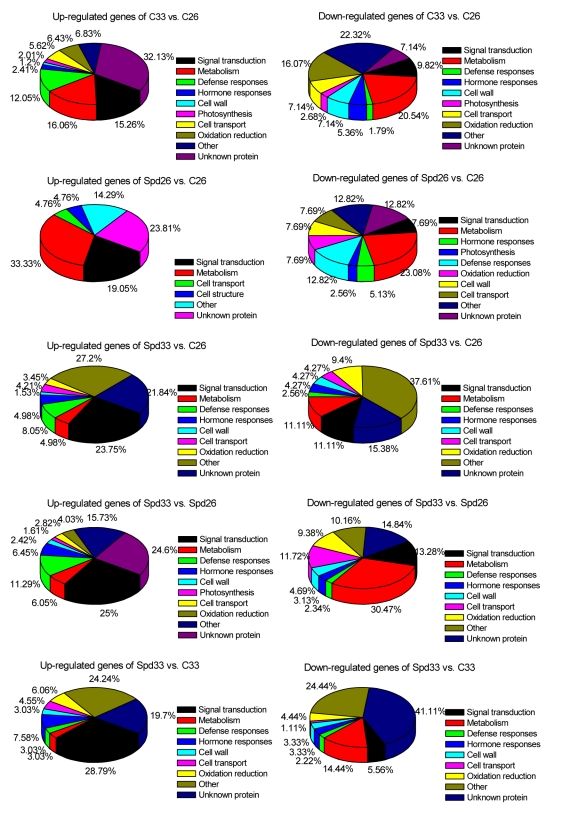
The differentially expressed genes identified were functionally annotated and subsequently classified into ten functional categories according to their putative functions (Fig. 1). Many loci encoding translational machinery, transcription factors, signal transduction components, in addition to genes associated with primary metabolism, photosynthesis, cell wall metabolism, and hormone responses were involved in tomato fruit responses to HT stress. The genes in the categories such as “defense responses”, “signal transduction” and “metabolism” were mostly up-regulated.
Fig. 1.
Functional categorization of differentially expressed genes in response to different treatments
Among 361 HT-regulated genes, 32 genes were previously shown to be involved in stress response genes in other plant systems (Sun and Callis, 1997; Bohnert et al., 2006) (Table 2). Different kinds of HSP genes were accumulated, while thaumatin-like protein (PR-5x) gene, cold shock domain protein (CSDP) transcript, was down-regulated. Previous reports indicated that the tolerance conferred by HSPs resulted in improved physiological phenomena such as photosynthesis, assimilate partitioning, and membrane stability (Momcilovic and Ristic, 2007). In addition to specific members of the classical HT-responsive genes, a major proportion of the genes presented in Table 2 are those that were only recently implicated in heat shock response (HSR) and/or HT stress signaling in other plant systems (Kotak et al., 2007). Functional classification of the 361 heat stress regulated genes indicates that 49 loci (13.57%) share homology with signal transduction including the genes of calcium binding protein, PP2C, protein kinases or transcription factors including C2H2 zinc finger, MYB, heat stress transcription factor (HSF), NAC, AP2, bHLH, and WRKY (Table 3). The majority of those genes involved in transcription regulation and carbohydrate metabolism were also stress- or defense-related genes. Another group of HT-regulated genes seems to be involved in hormone metabolism or hormonal response (Table 4). Microarray data indicate that the genes of AUX/IAA and jasmonate-zim-domain protein (JAZ) were up-regulated, while the genes of salicylic acid methyltransferase, ACC oxidase (ACO), auxin-responsive family protein, ornithine decarboxylase (ODC), and β-carotene hydroxylase were down-regulated.
Table 3.
Signal transduction genes with significant (P<0.01) differential expression (≥2.0-fold)
| Contig ID | Accession No. | Annotation | Fold difference |
||||
| C33 vs. C26 | Spd26 vs. C26 | Spd33 vs. C26 | Spd33 vs. C33 | Spd33 vs. Spd26 | |||
| Affx.57054.2.A1_at | AI487567 | Calcium-binding EF hand family protein | 2.10 | −1.05 | 3.10 | 1.48 | 2.37 |
| Affx.16164.1.S1_at | CN385704 | Calcium-binding EF hand family protein | 2.73 | −1.02 | 2.73 | 1.00 | 3.81 |
| Affx.57454.1.S1_at | BI923132 | Calmodulin | 2.17 | −1.29 | 2.94 | 1.35 | 2.57 |
| Affx.70732.1.S1_at | AI897832 | Calmodulin-related protein | 11.77 | −1.29 | 3.21 | −3.67 | 1.18 |
| Affx.9367.1.S1_at | CN385420 | Ca2+-binding protein 1 | 4.65 | 1.27 | 1.98 | −2.35 | 2.16 |
| Affx.66814.1.S1_at | AW621230 | Calmodulin binding | 2.02 | −1.14 | 2.64 | 1.31 | 1.94 |
| Affx.66436.1.S1_at | AW040280 | Calcium binding protein | 3.24 | −1.11 | 3.30 | 1.02 | 4.47 |
| Affx.50750.1.A1_at | AI771837 | MYB1 | 2.25 | 1.16 | 3.49 | 1.55 | 2.35 |
| Affx.53591.1.S1_at | AI899018 | MYB domain protein 107 | −4.78 | −1.22 | −3.01 | 1.59 | −2.36 |
| Les.5017.1.S1_at | BT013110.1 | MYB domain protein 111 | 1.40 | 1.15 | 2.19 | 1.07 | 1.77 |
| Les.2084.1.S1_at | BF097539 | NAC domain protein | 1.54 | 1.08 | 2.20 | 1.44 | 1.91 |
| Les.4483.1.S1_at | AY498713.1 | NAC domain protein | 2.16 | −1.04 | 1.63 | −1.33 | 1.36 |
| Affx.37343.1.A1 at | AI488497 | NAC domain protein | 2.51 | 1.06 | 3.02 | 1.20 | 1.83 |
| Les.2667.3.S1_at | BE433811 | WRKY-type transcription factor 2 | 2.62 | −1.12 | 2.89 | 1.11 | 2.11 |
| Les.3964.1.S1_at | AY157060.1 | WRKY transcription factor IId-2 | −3.73 | 1.10 | −2.45 | 1.52 | −1.78 |
| Affx.64823.1.S1_at | AI778204 | Zinc finger like protein | 2.23 | 1.49 | 4.30 | 1.93 | 4.50 |
| Affx.68645.1.S1_at | BM411685 | Zinc finger protein | 1.23 | 1.05 | 2.10 | 1.71 | 2.01 |
| Affx.30683.1.S1_at | AI486655 | Zinc finger protein | 3.02 | −1.16 | 3.25 | 1.08 | 2.82 |
| Affx.70855.1.S1_at | AW220130 | Zinc finger protein | 1.01 | −1.03 | 2.03 | 2.71 | 1.93 |
| Affx.35255.1.S1_at | AW623792 | Zinc finger protein 1 | 2.21 | −1.29 | 2.14 | −1.03 | 1.67 |
| Les.5126.1.S1_at | BT013336.1 | Zinc finger protein | −1.16 | −1.07 | 2.17 | 2.52 | 2.30 |
| Affx.71311.1.S1_at | CN384672 | Zinc finger (C2H2 type) family protein | 2.53 | 1.02 | 3.78 | 1.49 | 2.76 |
| Les.5244.1.S1_at | BT013559.1 | Zinc finger (DHHC type) family protein | 2.74 | −1.01 | −1.05 | −2.89 | −1.28 |
| Les.1287.2.S1_at | AW621773 | Zinc finger (DHHC type) family protein | 2.99 | 1.17 | 3.00 | 1.01 | 3.77 |
| Les.1287.1.A1_at | BG630588 | Zinc finger (DHHC type) family protein | 2.64 | 1.05 | 2.37 | −1.11 | 2.43 |
| Affx.30683.2.S1_at | AI897122 | Zinc finger (C3HC4 type) family protein | 3.12 | −1.12 | 3.13 | 1.00 | 2.62 |
| Affx.53941.1.S1_at | BF176552 | Zinc finger (C3HC4 type) family protein | 4.10 | −1.01 | 3.48 | −1.18 | 2.12 |
| Affx.3163.2.S1_at | BM412856 | Zinc finger (AN1-like) family protein | 1.68 | −1.02 | 2.22 | 1.32 | 2.49 |
| Les.3549.1.S1_at | AF096252.1 | Ethylene-responsive catalase | 1.71 | −1.28 | 2.58 | −1.22 | 1.29 |
| Les.4102.1.S1_at | AY192368.1 | Ethylene response factor 2 | 1.68 | 1.24 | 2.19 | 1.30 | 2.21 |
| Les.4139.1.S1_at | AY192370.1 | Ethylene response factor 4 | 1.57 | 1.13 | 2.05 | 1.31 | 1.00 |
| Les.271.1.S1_at | CN384665 | Ethylene-responsive methionine synthase | −1.76 | 1.23 | −2.00 | −1.13 | −2.43 |
| Les.3465.1.S1_at | AY079426.1 | Ethylene receptor-like protein | −2.66 | −1.37 | −2.43 | 1.09 | −2.20 |
| Les.3766.1.S1_at | U77719.1 | Ethylene-responsive late embryogenesis-like protein | −7.48 | −1.06 | −4.21 | 1.78 | −1.44 |
| Affx.16424.1.A1_at | AI487449 | Mitogen-activated protein kinase 3 | −1.04 | 1.10 | 2.11 | 2.17 | 2.13 |
| Affx.26003.1.S1_at | CK715553 | Serine/threonine-protein kinase | 2.22 | 1.09 | −1.20 | −1.13 | −1.12 |
Table 4.
Hormone-related genes with significant (P<0.01) differential expression (≥2.0-fold)
| Contig ID | Accession No. | Annotation | Fold difference |
||||
| C33 vs. C26 | Spd26 vs. C26 | Spd33 vs. C26 | Spd33 vs. C33 | Spd33 vs. Spd26 | |||
| Affx.23969.1.S1_at | AA824862 | Brassinosteroid-responsive ring-h2 | 2.00 | 1.07 | 1.16 | −1.20 | 1.02 |
| Affx.1251.1.S1_at | BG125851 | Auxin-responsive family protein | 2.17 | −1.04 | 2.08 | −1.04 | 1.43 |
| Les.5138.1.S1_at | BT013365.1 | Auxin-responsive family protein | −2.22 | 1.04 | −2.00 | 1.11 | −1.70 |
| Affx.63209.1.S1_at | AW092854 | Auxin-induced SAUR-like protein | 1.55 | 1.01 | 2.87 | 1.85 | 2.54 |
| Affx.71035.1.S1_at | BI207404 | Auxin-induced SAUR-like protein | 2.91 | −1.08 | 1.81 | −1.61 | 1.04 |
| Les.3707.1.S1_at | AF022013.1 | IAA2 protein | 2.03 | 1.09 | 10.86 | 3.34 | 6.40 |
| Les.3706.1.A1_at | AF022014.1 | IAA3 protein | −1.20 | 1.18 | 2.73 | 3.28 | 2.97 |
| Les.256.1.A1_at | AF022017.1 | IAA6 protein | 1.65 | 1.11 | 2.15 | 1.30 | 1.72 |
| Les.4097.1.A1_at | AF022018.1 | IAA7 protein | 2.40 | 1.06 | 2.53 | 1.05 | 2.77 |
| Les.5442.1.S1_at | BT013931.1 | IAA19 protein | −3.35 | −1.18 | −2.51 | 1.56 | −1.80 |
| Les.5917.1.S1_at | AJ715790.1 | 1-Aminocyclopropane-1-carboxylate oxidase | 1.83 | 1.19 | 2.36 | 1.29 | 2.99 |
| Les.3225.3.S1_at | BF112635 | 1-Aminocyclopropane-1-carboxylate oxidase | −3.20 | 1.01 | −2.05 | 1.56 | −2.97 |
| Les.3769.1.S1_at | AB013100.1 | 1-Aminocyclopropane-1-carboxylate synthase | 2.48 | −1.60 | 2.85 | 1.15 | 2.28 |
| Les.3358.1.S1_at | AI771286 | Arginine decarboxylase | 1.64 | −1.15 | 2.25 | 1.37 | 1.82 |
| Les.3525.1.S1_at | AF029349.2 | Ornithine decarboxylase (ODC) | −2.17 | −6.06 | −2.31 | −1.06 | −1.37 |
| Les.3728.1.A1_at | AJ278743.1 | Gibberellin 2 (GA2) protein | −1.82 | −1.02 | −2.19 | −1.20 | 1.19 |
| Affx.30832.1.S1_at | CN384615 | Jasmonate-zim-domain protein 7 | 4.05 | 1.08 | 1.78 | −2.28 | 2.04 |
| Les.612.1.S1_at | BE459581 | Salicylic acid methyltransferase | −3.95 | 1.21 | −3.40 | 1.16 | −2.37 |
3.2. Transcriptome profiling of ripening fruit in response to exogenous spermidine treatment
Under normal temperature, a total of 21 genes were up-regulated, whilst 40 genes were down-regulated in Spd-treated fruits (Spd26), compared with those in non-treated fruits (C26). The genes of ethylene-responsive catalase, ethylene response factor 4, calmodulin-related protein were up-regulated, whilst those of MADS-box protein, S-adenosyl-L-methionine, ethylene-responsive late embryogenesis-like protein, and gibberellin 2 (GA2) protein were down-regulated in Spd26 fruits.
Furthermore, the expression profiles in Spd-treated fruits under HT condition (Spd33) were compared with those in untreated fruits (C33). A total of 66 genes were up-regulated in Spd-treated fruits, whilst 90 genes were down-regulated. Among these Spd-regulated genes, 24 candidate regulatory factors were identified, including the genes of ethylene-responsive transcription factor, zinc finger, mitogen-activated protein kinase (MAPK), WRKY, auxin response factors (ARF), and calmodulin-like protein (Table 3). Twelve genes were involved in defense and oxidation reduction, including the genes of LeCBF1 protein, HSP, salt responsive protein, cytochrome P450, NADH dehydrogenase, and NAD(P)H-quinone oxidoreductase (Table 2). Eight genes were involved in hormone pathways, such as the genes of IAA, Spd synthase, and brassinosteroid sulfotransferase (Table 4).
3.3. Expression changes under both high temperature and spermidine treatment
A total of 261 genes were up-regulated in Spd-treated fruits under HT condition (Spd33), while 117 genes were down-regulated compared with those in control tomatoes (C26) (Fig. 2).
Fig. 2.
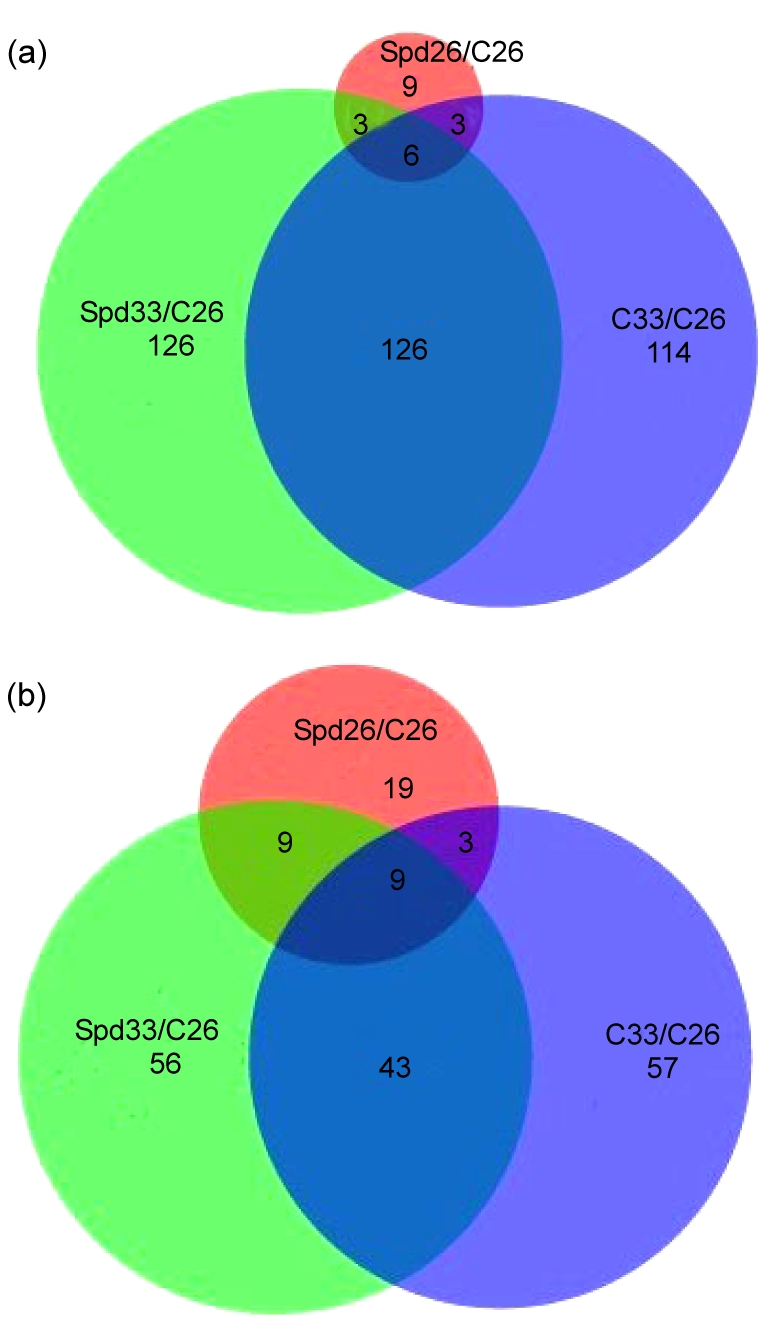
Venn diagram showing the numbers of up- and down-regulated genes under different temperature conditions
The tomato Affymetrix GeneChip® contains 10 101 probe sets. (a) Numbers of significantly up-regulated genes in Spd treatment under normal temperature (Spd26), high temperature (C33), and Spd treatment under high temperature (Spd33), compared with control treatment (C26), respectively. The number of up-regulated genes common between these treatments is shown within the overlapping circular. (b) Numbers of down-regulated genes in Spd26, C33, and Spd33, compared with C26, respectively. The number of down-regulated genes common between these treatments is shown within the overlapping circular
Functional classification indicates that the largest group consists of 75 candidate regulatory factors, many of which are likely to be regulators of specific genes or gene sets, including the genes of ethylene cascade protein, MAPK, NAC, MYB, WRKY, and auxin-responsive family protein (Tables 3 and 4). Members of the ethylene response element binding protein (EREBP) family may play important roles in the cross-talk of different kinds of abiotic stress and signaling pathways. The EREBP family genes are highly regulated by various kinds of abiotic factors and plant hormones, such as low temperature, drought, high salinity, ethylene, abscisic acid, and jasmonate (Zhang H.W. et al., 2004).
Several HSPs were identified as the products of HT- and polyamine-regulated genes (Table 2). The HSPs, acting as molecular chaperons, play a crucial role in protecting plants against stress by re-establishing normal protein conformations and maintaining cellular homeostasis (Wang W.X. et al., 2004; Ouyang et al., 2007). In the suspension culture of tobacco and alfalfa, polyamines can affect the membrane system further to influence the HSP synthesis in response to HT stress (Königshofer and Lechner, 2002).
Many genes involved in hormone pathways changed in response to HT and Spd treatments (Table 4). The genes of IAA protein, early auxin-responsive (SAUR), ACC synthase (ACS), ACO, and ADC accumulated after treatment with Spd under HT condition. In particular IAA2 exhibited a 10.86-fold increase in expression. The ACO and ACS genes, which are crucial in ethylene biosynthesis, were also highly regulated by HT and Spd treatments, which indicated that Spd might play a role in ethylene signal pathway under HT stress.
3.4. Verification of the expression patterns with quantitative real-time PCR
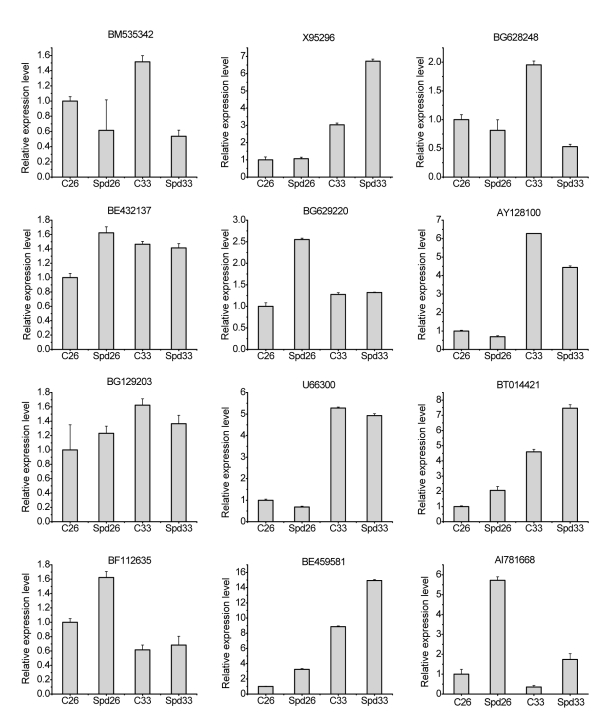
To validate the expression differences detected by the microarray analysis, we analyzed expression patterns of 15 randomly selected genes using quantitative real-time reverse transcription (RT)-PCR (Fig. 3). These genes represent different groups with diverse change patterns, including up-regulation, down-regulation, and no significant changes. Real-time RT-PCR analyses confirmed the change of mRNA level. Out of 15 genes detected, the expressions of 12 (80%) genes agree with the microarray analyses, indicating that the expression patterns detected by microarray analyses are in good agreement with those detected by quantitative real-time PCR. HSP genes (AY128100.1, U66300.1 and BG129203) were significantly up-regulated by HT stress, but relieved by exogenous Spd treatment. The expression levels of 1-aminocyclopropane-1-carboxylate oxidases (BF112635 and BG629220) were significantly increased by Spd treatment. The mRNA levels of salicylic acid methyltransferase (BE459581), cytosolic ascorbate peroxidase (BE432137), and phenylcoumaran benzylic ether reductase (BT014421.1) were up-regulated by HT or Spd treatment. The mRNA level of the cell wall protein (BG628248) was also up-regulated in response to Spd and HT treatments.
Fig. 3.
Verification of microarray results by quantitative real-time PCR
Total RNA was isolated from tomato fruits of C26, Spd26, C33, and Spd33 for cDNA synthesis, respectively, then for quantitative real-time PCR. The presented data are the averages of two independent experiments [±standard error (SE)]. AY128100.1, U66300.1, and BG129203: heat shock protein genes; BF112635 and BG629220: ACO gene; BE459581: salicylic acid methyltransferase gene; BE432137: cytosolic ascorbate peroxidase gene; BT014421.1: phenylcoumaran benzylic ether reductase gene; AI781668: proteinase inhibitor 1 PPI3A4 gene; X95296: THM27 protein gene; BM535342: I2C protein gene; BG628248: calcium-binding EF-hand protein gene
4. Discussion
The ripening process of tomato fruit involves a complex and coordinated series of changes in pigmentation, flavor, texture, and aroma resulting from physiological and biochemical activities, which were caused by alterations in gene expression (Lurie et al., 1996). HT has been found to inhibit ethylene synthesis and proteoglycan accumulation, to interfere with lycopene synthesis, and finally to inhibit fruit ripening. In this work, microarray approach was used to examine the gene expression profile of tomato mature green fruit in response to HT and exogenous Spd treatments. The transcript abundance changes were monitored and the HT- or/and Spd-regulated genes were identified in the early ripening stages. There were a total of 361 genes regulated in response to HT stress (33 °C), and only 61 genes exhibited regulation following the exogenous Spd-treatment. Meanwhile, 378 genes were simultaneously regulated by the HT and Spd treatments. A total of 185 genes changed together in both C33 and Spd33 (each normalized to C26). However, there were 114 and 126 specific genes up-regulated only in C33 and Spd33, respectively, while 57 and 56 genes down-regulated in C33 and Spd33, respectively (Fig. 2). All the differentially expressed genes were also functionally annotated and subsequently classified into ten functional categories according to their putative functions (Fig. 1). Most of these genes representing four groups of notably differentially regulated genes appear to be involved in the HSR of mature tomato fruits: genes involved in primary metabolism, defense responses, signal transduction, and hormone metabolism or hormonal response. The microarray analysis indicated that 49 differentially expressed genes were involved in signal transduction and reported as transcription factors in mature fruits in response to HT stress (C33) (Table 3), including the genes of EREBP family, MAPK, WRKY, MYB, NAC, LOB domain (LBD), zinc finger family protein, calmodulin-related protein, and serine/threonine-protein kinase. Some EREBP family genes also significantly accumulated in Spd-treated fruits under HT condition, including ethylene response factors, ethylene-responsive HSP cognate 70, ethylene-responsive late embryogenesis-like protein, ethylene receptor-like protein, and ethylene-responsive methionine synthase, which were not changed in response to HT. Interestingly, the expressions of MAPK family genes were up-regulated after the Spd treatment. MAPK cascades play an important role in signal transduction pathways in plants and function ubiquitously in many responses to external signals (Kaur and Gupta, 2005). In order to cope with heat stress, plants implement various mechanisms, such as MAPK cascades (Wahid et al., 2007). Many genes of MAPK cascades changed in Spd33 other than in C33 indicated Spd might play important roles in alleviating HT injury by signal pathways. In a previous study, WRKY25 was involved in heat stress tolerance in Arabidopsis thaliana (Li et al., 2009). MYB4 was found to be involved in drought, salt, UV, ozone, viruses, bacteria, and fungi stresses and represents a crucial knot in the cross-talk of stress signaling cascades through the activation of multiple components in rice (Vannini et al., 2006). The NAC domain protein could interact with leaf curl virus in tomato (Selth et al., 2005).
The microarray analysis indicated HT induction of many metabolism-related genes in mature fruits, including energy metabolism genes, amino acid and protein metabolism genes, nucleic acid metabolism genes, and secondary metabolism genes. Under HT treatment, the metabolism of development was deeply disordered, many genes involved were changed to adapt to HT stress. However, when the fruits were pretreated with exogenous Spd, the damage to metabolism was alleviated, and the number of those genes in response to stress was significantly decreased (Table 5).
Some genes involved in defense responses and oxidation reduction categories, were also decreased under HT and Spd treatments. There were 76 genes changed by HT stress, whilst 44 genes changed under HT and Spd treatments (Table 2). It indicated that Spd significantly promoted the plant response to HT at the molecular level to relieve the injury by heat stress.
The hormone metabolism was also involved in responses to HT stress (Table 4). Under HT and Spd treatments, ACS, ACC and IAA genes were up-regulated. Previous studies showed that polyamines were indispensable for IAA occurrence (Couée et al., 2004). The action of auxin and polyamine may be closely related to each other (Rastogi and Davies, 1991; Nag et al., 2001). Polyamines are able to replace auxin effects, suggesting that they could mimic hormonal responses (Pal Bais and Ravishankar, 2002). Numerous reports have shown that different pathways are interconnected and together regulate the plant response to biotic and abiotic stresses (Ludwig et al., 2005; Ma et al., 2006). Abscisic acid and ethylene play an important role in the complicated story of abiotic stress and, consequently, cross-talk between these two kinds of plant hormone has been reported (Yamamoto et al., 2005). The relationship between polyamine and ethylene was complex. Because of their common pre-requisite S-adenosylmethionine (SAM), there exists a competitive mechanism, which may be affected by species, environment, or limited SAM storage. Polyamines affect the levels of ACS and ACO gene transcriptions, thereby affecting the synthesis and conversion of ACC. Meanwhile, polyamines influence the nature of the ACO on the membrane system, so inhibit the conversion of ACC to ethylene. Polyamines, as an effective scavenger of free radicals, improve the protective enzyme activity and inhibit ethylene production (Apelbaum et al., 1981; Walden et al., 1997). Conversely, ethylene affected the activities of ADC, SAM decarboxylase (SAMDC) and other key enzymes in polyamine biosynthesis (Thu-Hang et al., 2002). Polyamines may have a dual function in plant stress tolerance, as a protectant in reactive oxygen species (ROS)-scavenging and a membrane-protecting compound and as a signaling regulator in stress signaling pathways that lead to the build-up of stress-tolerant mechanism (Kasukabe et al., 2004). However, there are contradictory research data providing evidence for the lack of antioxidant activity and even prooxidant action of polyamines (Todorova et al., 2007).
A large number of early response genes regulated by HT or/and Spd in this study encode unknown proteins, indicating that there is still a great deal of uncertainty with regard to the mechanism of the HT tolerance and in how polyamines affect the fruit ripening-related gene expression in tomato. This study has practical importance for subtropical and tropical tomatoes that are unable to ripen if grown under HT conditions.
5. Conclusions
The data presented here provide genome-wide expression profiles of mature green tomato fruit following their exposure to a short-term HT treatment and exogenous Spd application. An Affymetrix tomato genome array was successfully used to identify HT- or/and Spd-regulated genes representing the classical HSR and thermotolerance mechanisms. The results indicate HT regulates HSP and heat shock factor family members, carbohydrate metabolism genes, stress- or defense-related signal transduction genes, and hormone metabolism genes or hormonal response elements. Under normal temperature, the Spd-regulated genes were quite different with stress-related genes in response to HT. However, under HT conditions, when pre-treated with exogenous Spd, the number of genes involved in signal transduction was significantly increased. Many regulatory factors, ethylene-related genes, polyamine biosynthesis genes, hormone pathways genes, and oxidation reduction genes exhibited the regulation in response to Spd treatment. So our results indicated that Spd might alleviate the heat stress injury during tomato fruit ripening. However, more complete understanding of the molecular mechanisms that contribute to fruit thermotolerance requires additional data, including the functional analyses of a large part of the above differentially expressed genes, under both short-term HT and longer durations of moderate HT conditions.
List of electronic supplementary materials
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2009CB119000), the National Natural Science Foundation of China (Nos. 31071804 and 30771470), and the Zhejiang Provincial Natural Science Foundation (Nos. R3110209 and 2009C32025), China
Electronic supplementary materials: The online version of this article (doi:10.1631/jzus.B1100060) contains supplementary materials, which are available to authorized users
References
- 1.Apelbaum A, Burgoon AC, Anderson JD, Lieberman M. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 1981;68(2):453–456. doi: 10.1104/pp.68.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289–300. [Google Scholar]
- 3.Bohnert HJ, Gong QQ, Li PH, Ma SS. Unraveling abiotic stress tolerance mechanisms—getting genomics going. Curr Opin Plant Biol. 2006;9(2):180–188. doi: 10.1016/j.pbi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Zou YJ, Ding SL, Zhang JJ, Yu XL, Cao JS, Lu G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol. 2009;51(5):489–499. doi: 10.1111/j.1744-7909.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- 5.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11(2):80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Couée I, Hummel I, Sulmon C, Gouesbet G, El Amrani A. Involvement of polyamines in root development. Plant Cell Tiss Org Cult. 2004;76(1):1–10. doi: 10.1023/A:1025895731017. [DOI] [Google Scholar]
- 7.Escribano MI, Merodio C. The relevance of polyamine levels in cherimoya (Annona cherimola Mill.) fruit ripening. J Plant Physiol. 1994;143(2):207–212. doi: 10.1016/S0176-1617(11)81688-3. [DOI] [Google Scholar]
- 8.Fei ZJ, Tang XM, Alba R, Giovannoni J. Tomato Expression Database (TED): a suite of data presentation and analysis tools. Nucl Acids Res. 2006;34(90001):D766–D770. doi: 10.1093/nar/gkj110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34(1):35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- 10.Groppa MD, Tomaro ML, Benavides MP. Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci. 2001;161(3):481–488. doi: 10.1016/S0168-9452(01)00432-0. [DOI] [Google Scholar]
- 11.Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53(1):225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 12.Inaba M, Crandall PG. Electrolyte leakage as an indicator of high-temperature injury to harvested mature green tomatoes. J Am Soc Hort Sci. 1988;113(1):96–99. [Google Scholar]
- 13.Kakkar RK, Sawhney VK. Polyamine research in plants—a changing perspective. Physiol Plant. 2002;116(3):281–292. doi: 10.1034/j.1399-3054.2002.1160302.x. [DOI] [Google Scholar]
- 14.Kasukabe Y, He LX, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress regulated genes in transgenic Arabidopsis thaliana . Plant Cell Physiol. 2004;45(6):712–722. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- 15.Kaur N, Gupta AK. Signal transduction pathways under abiotic stresses in plants. Curr Sci India. 2005;88(11):1771–1780. [Google Scholar]
- 16.Königshofer H, Lechner S. Are polyamines involved in the synthesis of heat-shock proteins in cell suspension cultures of tobacco and alfalfa in response to high-temperature stress? Plant Physiol Biochem. 2002;40(1):51–59. doi: 10.1016/S0981-9428(01)01347-X. [DOI] [Google Scholar]
- 17.Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10(3):310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Lang QL, Zhou XC, Zhang XL, Drabek R, Zou ZX, Ren YL, Li TB, Chen JS, Gao XL. Microarray-based identification of tomato microRNAs and time course analysis of their response to Cucumber mosaic virus infection. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(2):116–125. doi: 10.1631/jzus.B1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law DM, Davies PJ, Mutschler MA. Polyamine-induced prolongation of storage in tomato fruits. Plant Growth Regul. 1991;10(4):283–290. doi: 10.1007/BF00024588. [DOI] [Google Scholar]
- 20.Li SJ, Fu QT, Huang WD, Yu DQ. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep. 2009;28(4):683–693. doi: 10.1007/s00299-008-0666-y. [DOI] [PubMed] [Google Scholar]
- 21.Liu K, Fu HH, Bei QX, Luan S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000;124(3):1315–1325. doi: 10.1104/pp.124.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, Boller T, Jones JDG, Romeis T. Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. PNAS. 2005;102(30):10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lurie S, Handros A, Fallik E, Shapira R. Reversible inhibition of tomato fruit gene expression at high temperature—effects on tomato fruit ripening. Plant Physiol. 1996;110(4):1207–1214. doi: 10.1104/pp.110.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma SS, Gong QQ, Bohnert HJ. Dissecting salt stress pathways. J Exp Bot. 2006;57(5):1097–1107. doi: 10.1093/jxb/erj098. [DOI] [PubMed] [Google Scholar]
- 25.Mariani P, Dorazi D, Bagni N. Polyamines in primary walls of carrot cells: endogenous content and interactions. J Plant Physiol. 1989;135(4):508–510. doi: 10.1016/S0176-1617(89)80113-0. [DOI] [Google Scholar]
- 26.Mattoo A, Cassol T, Mehta R, Handa A, Ali N, Abdul-Baki A. Genetic engineering of tomato fruit for sustained accumulation of polyamines during ripening to study their physiological role(s) Acta Hort (ISHS) 2002;575(1-2):157–161. [Google Scholar]
- 27.Momcilovic I, Ristic Z. Expression of chloroplast protein synthesis elongation factor, EF-Tu, in two lines of maize with contrasting tolerance to heat stress during early stages of plant development. J Plant Physiol. 2007;164(1):90–99. doi: 10.1016/j.jplph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Mueller LA, Solow TH, Taylor N, Skwarecki B, Buels R, Binns J, Lin CW, Wright MH, Ahrens R, Wang Y. The SOL Genomics Network. A comparative resource for Solanaceae biology and beyond. Plant Physiol. 2005;138(3):1310–1317. doi: 10.1104/pp.105.060707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nag S, Saha K, Choudhuri MA. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul. 2001;20(2):182–194. doi: 10.1007/s003440010016. [DOI] [Google Scholar]
- 30.Nayyar H, Chander S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci. 2004;190(5):355–365. doi: 10.1111/j.1439-037X.2004.00106.x. [DOI] [Google Scholar]
- 31.Ouyang B, Yang T, Li HX, Zhang L, Zhang YY, Zhang JH, Fei ZJ, Ye ZB. Identification of early salt stress response genes in tomato root by suppression subtractive hybridization and microarray analysis. J Exp Bot. 2007;58(3):507–520. doi: 10.1093/jxb/erl258. [DOI] [PubMed] [Google Scholar]
- 32.Pal Bais H, Ravishankar GA. Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tiss Org Cult. 2002;69(1):1–34. doi: 10.1023/A:1015064227278. [DOI] [Google Scholar]
- 33.Paschalidis KA, Roubelakis-Angelakis KA. Sites and regulation of polyamine catabolism in the tobacco plant. Correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol. 2005;138(4):2174–2184. doi: 10.1104/pp.105.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paull RE, Chen NJ. Heat treatment and fruit ripening. Postharvest Biol Technol. 2000;21(1):21–37. doi: 10.1016/S0925-5214(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Amador MA, Leon J, Green PJ, Carbonell J. Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in arabidopsis. Plant Physiol. 2002;130(3):1454–1463. doi: 10.1104/pp.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Vicente A, Martínez-Romero D, Carbonell Á, Serrano M, Riquelme F, Guillén F, Valero D. Role of polyamines in extending shelf life and the reduction of mechanical damage during plum (Prunus salicina Lindl.) storage. Postharvest Biol Technol. 2002;25(1):25–32. doi: 10.1016/S0925-5214(01)00146-6. [DOI] [Google Scholar]
- 37.Picton S, Grierson D. Inhibition of expression of tomato-ripening genes at high-temperature. Plant Cell Environ. 1988;11(4):265–272. doi: 10.1111/j.1365-3040.1988.tb01145.x. [DOI] [Google Scholar]
- 38.Pressman E, Peet MM, Pharr DM. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann Bot. 2002;90(5):631–636. doi: 10.1093/aob/mcf240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysisi of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339(1):62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 40.Rastogi R, Davies PJ. Polyamine metabolism in ripening tomato fruit: 2. polyamine metabolism and synthesis in relation to enhanced putrescine content and storage life of alc tomato fruit. Plant Physiol. 1991;95(1):41–45. doi: 10.1104/pp.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renaut J, Hoffmann L, Hausman JF. Biochemical and physiological mechanisms related to cold acclimation and enhanced freezing tolerance in poplar plantlets. Physiol Plant. 2005;125(1):82–94. doi: 10.1111/j.1399-3054.2005.00554.x. [DOI] [Google Scholar]
- 42.Roy M, Wu R. Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 2001;160(5):869–875. doi: 10.1016/S0168-9452(01)00337-5. [DOI] [PubMed] [Google Scholar]
- 43.Sato S, Peet MM, Gardner RG. Formation of parthenocarpic fruit, undeveloped flowers and aborted flowers in tomato under moderately elevated temperatures. Sci Hort. 2001;90(3-4):243–254. doi: 10.1016/S0304-4238(00)00262-4. [DOI] [Google Scholar]
- 44.Selth LA, Dogra SC, Rasheed MS, Healy H, Randles JW, Rezaian MA. A NAC domain protein interacts with tomato leaf cuil virus replication accessory protein and enhances viral replication. Plant Cell. 2005;17(1):311–325. doi: 10.1105/tpc.104.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun CW, Callis J. Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs of and in response to environmental changes. Plant J. 1997;11(5):1017–1027. doi: 10.1046/j.1365-313X.1997.11051017.x. [DOI] [PubMed] [Google Scholar]
- 46.Thu-Hang P, Bassie L, Safwat G, Trung-Nghia P, Christou P, Capell T. Expression of a heterologous S-adenosylmethionine decarboxylase cDNA in plants demonstrates that changes in S-adenosyl-L-methionine decarboxylase activity determine levels of the higher polyamines spermidine and spermine. Plant Physiol. 2002;129(4):1744–1754. doi: 10.1104/pp.010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todorova D, Sergiev I, Alexieva V, Karanov E, Smith A, Hall M. Polyamine content in Arabidopsis thaliana (L.) Heynh during recovery after low and high temperature treatments. Plant Growth Regul. 2007;51(3):185–191. doi: 10.1007/s10725-006-9143-1. [DOI] [Google Scholar]
- 48.Torrigiani P, Bregoli AM, Ziosi V, Scaramagli S, Ciriaci T, Rasori A, Biondi S, Costa G. Pre-harvest polyamine and aminoethoxyvinylglycine (AVG) applications modulate fruit ripening in Stark Red Gold nectarines (Prunus persica L. Batsch) Postharvest Biol Technol. 2004;33(3):293–308. doi: 10.1016/j.postharvbio.2004.03.008. [DOI] [Google Scholar]
- 49.Valero D, Perez-Vicente A, Martinez-Romero D, Castillo S, Guillen G, Serrano M. Plum storability improved after calcium and heat postharvest treatments: role of polyamines. J Food Sci. 2002;67(7):2571–2575. doi: 10.1111/j.1365-2621.2002.tb08778.x. [DOI] [Google Scholar]
- 50.Vannini C, Iriti M, Bracale M, Locatelli F, Faoro F, Croce P, Pirona R, Di Maro A, Coraggio I, Genga A. The ectopic expression of the rice Osmyb4 gene in Arabidopsis increases tolerance to abiotic, environmental and biotic stresses. Physiol Mol Plant Pathol. 2006;69(1-3):26–42. doi: 10.1016/j.pmpp.2006.12.005. [DOI] [Google Scholar]
- 51.Verma S, Mishra SN. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol. 2005;162(6):669–677. doi: 10.1016/j.jplph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61(3):199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 53.Walden R, Cordeiro A, Tiburcio AF. Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol. 1997;113(4):1009–1013. doi: 10.1104/pp.113.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang CY, Conway WS, Abbott JA, Kramer GF, Sams CE. Postharvest infiltration of polyamines and calcium influences ethylene production and texture changes in ‘Golden Delicious’ apples. J Am Soc Hort Sci. 1993;118(6):801–806. [Google Scholar]
- 55.Wang WX, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9(5):244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Yakir D, Sadovski A, Rabinowitch HD, Rudich J. Effect of high-temperature on quality of processing-tomatoes of various genotypes ripened off the vine. Sci Hort. 1984;23(4):323–330. doi: 10.1016/0304-4238(84)90028-1. [DOI] [Google Scholar]
- 57.Yamamoto A, Bhuiyan NH, Waditee R, Tanaka Y, Esaka M, Oba K, Jagendorf AT, Takabe T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J Exp Bot. 2005;56(417):1785–1796. doi: 10.1093/jxb/eri167. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Ma XY, Qian YJ, Zhou XY. Molecular characterization and infectivity of Papaya leaf curl China virus infecting tomato in China. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(2):109–114. doi: 10.1631/jzus.B0900176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang HW, Huang ZJ, Xie BY, Chen Q, Tian X, Zhang XL, Zhang HB, Lu XY, Huang DF, Huang RF. The ethylene-, jasmonate-, abscisic acid- and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box-containing genes and salt tolerance in tobacco. Planta. 2004;220(2):262–270. doi: 10.1007/s00425-004-1347-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.