Abstract
As a component of diesel exhaust particles, 3-methyl-4-nitrophenol (4-nitro-m-cresol, PNMC) is also a metabolite of the insecticide fenitrothion and imposes hazardous effects on human health. In the present study, the alleviative effect of a common antioxidant flavonoid quercetin on mouse germ cells intoxicated by PNMC was investigated. Results showed that a single intraperitoneal injection of PNMC at 100 mg/kg induced severe testicular damage after one week. PNMC-treated mice showed a significant loss of germ cells (approximate 40% loss of round germ cells). PNMC caused an increase of hydroxyl radical and hydrogen peroxide production and lipid peroxidation, as well as a decrease in glutathione level, superoxide dismutase and glutathione peroxidase activities. Furthermore, treatment of PNMC increased expression of the pro-apoptotic protein Bax and decreased expression of the anti-apoptotic protein Bcl-XL in germ cells. In addition, testicular caspase-3 activity was significantly up-regulated and germ cell apoptosis was significantly increased in the PNMC-treated mice. In contrast, combined administration of quercetin at 75 mg/kg significantly attenuated PNMC-induced testicular toxicity. These results indicate that the antioxidant quercetin displays a remarkable protective effect on PNMC-induced oxidative damage in mouse testes and may represent an efficient supplement to attenuate reproductive toxicity by environmental toxicants to ensure healthy sperm production.
Keywords: Quercetin, 3-Methyl-4-nitrophenol, Oxidative damage, Apoptosis, Germ cell, Testis
1. Introduction
There is a growing international concern about hazardous effects of air pollution, which has become an important public health problem. Vast amounts of diesel exhaust particles (DEPs) are released into the atmosphere in many countries. In Japan, 58 902 t of DEPs were emitted each year, ranking third, behind only the European Union (240 000 t) and the United States (111 530 t). Noya et al. (2008) revealed that 1 kg of DEPs contain an average of 79.1 mg 3-methyl-4-nitrophenol (4-nitro-m-cresol, PNMC) and PNMC was present in a significantly higher concentration than those reported previously (Miyabara et al., 1998). As a consequence of the use of diesel engines, DEP imposes hazardous effects on human health that include lung cancer, allergic rhinitis, and bronchial asthma-like diseases by polluting the atmosphere. Different studies have also reported that DEP pollutants exert toxic effects on the male reproductive system in vivo (Watanabe and Oonuki, 1999; Yoshida et al., 1999).
As a component of DEP, PNMC is also a major breakdown product of the insecticide fenitrothion, which is a broad-spectrum insecticide used extensively throughout the world (Wright et al., 1982). Once used, fenitrothion is rapidly metabolized by microorganisms, plants, and animals, with PNMC as a major metabolite. Several research studies have been performed to elucidate the diverse toxic effects induced by PNMC on vertebrates. Mori et al. (2003) isolated PNMC from DEPs and showed that it has a vasodilatation activity, while Huang et al. (2010) reported that PNMC induced an acute cardiac dysfunction after short-term DEPs exposure of rats. Similarly, exposure of DEPs in vitro impaired early embryonic development and quality in mice (Januario et al., 2010). PNMC has been reported to exhibit estrogenic (Furuta et al., 2004; 2005) and antiandrogenic activity (Li et al., 2006) in rats both in vitro and in vivo, and induced impairment of testicular function in adult male Japanese quail (Coturnix japonica) as well as in male rats by reduced daily sperm production. PNMC has also been reported to inhibit the activity of ribonucleotide reductase (Wright et al., 1982).
DEP has been reported to be able to generate reactive oxygen species (Ross and Kasum, 2002) and induce oxidative stress and inflammation in the lung and respiratory tract (Park et al., 2006). Han et al. (2001) reported that DEP generated hydroxyl radical (·OH) in the lung of mice. Chemicals in DEPs have also been reported to generate reactive oxygen radicals and induce apoptosis in macrophages (Hiura et al., 1999). Our previous study in vitro showed that PNMC induced oxidative stress on the spermatogonial cells in chicken embryo (Mi et al., 2009). Though PNMC-induced reproductive toxicity has been reported, its detailed mechanism is not well known.
Quercetin, as an effective antioxidant flavonoid, is ubiquitously distributed in vegetables and fruits. Quercetin can prevent oxidative injury and cell death by chelating metal ions, scavenging oxygen radicals, and protecting against lipid peroxidation. It has been reported that quercetin has alleviative effects against male reproductive toxicity induced by DEP (Izawa et al., 2008). Previous results also indicate that quercetin showed protective effects on male reproductive toxicity caused by PNMC in embryonic chicken and cadmium in mice (Mi et al., 2009; Bu et al., 2011). However, the effect of quercetin on PNMC-induced testicular toxicity in vivo is not well known.
Therefore, the present study was performed to reveal the relationship between oxidative stress and PNMC-induced testicular toxicity and to explore the protective effect of quercetin on PNMC-induced reproductive toxicity in male mice.
2. Materials and methods
2.1. Animals
Adult male Institute of Cancer Research (ICR) mice were purchased from the Laboratory Animal Center of Zhejiang University (Hangzhou, China). Animals were housed at conditions of (24±2) °C, (45±5)% humidity, and 12-h light/12-h dark cycle, with free access to food and water. The animals were acclimatized for one week prior to beginning the experiments. The experimental procedures were carried out in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhejiang University.
2.2. Treatments
In the first experiment, mice received a single intraperitoneal (i.p.) injection of PNMC (Alfa Aesar Co., Massachusetts, USA) at 1, 10, or 100 mg/kg body weight (BW). The control received the vehicle (phosphate buffered saline containing 0.5% (v/v) dimethyl sulfoxide and 0.05% (v/v) Tween 80). There were six animals in each group. The mice were sacrificed by cervical dislocation one week after injection. The toxic effect was estimated by conventional histological hematoxylin and eosin (HE) staining of the testis sections. Since the dose of 100 mg/kg induced significant germ cell damage, this dosage was selected for the subsequent studies.
In the second study, mice were randomly divided into four groups with six animals per group. The control received vehicle only. The animals in Group II received PNMC (100 mg/kg, i.p.). The animals in Group III received quercetin (75 mg/kg, i.p.) for 3 d. The animals in Group IV received PNMC (100 mg/kg) plus quercetin (75 mg/kg, i.p.) for 3 d. After one week the right testes were immediately collected and fixed in 4% (v/v) paraformaldehyde for histological studies, and the left testes were stored at −80 °C for analysis of biochemical parameters including ·OH, hydrogen peroxide (H2O2), malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidation (GSH-Px), and caspase-3.
2.3. Histological studies
The fixed testes were dehydrated and embedded in paraffin, sectioned at 5 µm for HE staining and immunohistochemical staining. The morphological changes were examined under a microscope (Eclipse 80i, Nikon, Kanagawa, Japan) and images were captured with a digital camera (DS-Fi1, Nikon, Kanagawa, Japan).
2.4. Determination of biochemical parameters
2.4.1. Preparation of testicular homogenate
Frozen testicular tissue was homogenized with ice-cold 9 g/L NaCl solution. The homogenate was centrifuged at 1 700×g for 15 min at 4 °C and the supernatant was used for further measurements.
2.4.2. Biochemical analysis of antioxidant status
·OH and H2O2 were measured by detecting the absorbance at 405 nm according to the commericial kit’s instruction. MDA as an endpoint of lipid peroxidation was calculated by detecting the absorbance of thiobarbituric acid reactive substances (TBARSs) at 532 nm. Reduced GSH was determined by measuring the absorbance at 412 nm. SOD activity was determined by measuring the absorbance at 560 nm. GSH-Px activity was assayed by the method based on the reaction between GSH remaining after the action of GSH-Px and 5,5′-dithiobis-2-nitrobenzoic acid to form a complex with the maximal absorbance at 412 nm (Rotruck et al., 1973). One unit of GSH-Px activity was defined as the decrease of 1 µmol/L of GSH per min per mg protein deducting the effect of nonenzyme-catalyzed reaction. The biochemical parameters (·OH, H2O2, MDA, GSH, SOD, and GSH-Px) were determined using commercial kits, which were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5. Immunohistochemistry of Bax and Bcl-XL
The pro-apoptotic protein Bax and anti-apoptotic protein Bcl-XL were evaluated by immunohistochemical staining using commercial kits (Boster Bio-engineering Co., Ltd., Wuhan, China). Histological sections were treated with 3% (v/v) H2O2 to block endogenous peroxides and antigen retrieval was carried out in nitrate buffer at 95 °C for 20 min. Fetal bovine serum (FBS, 10% (v/v)) was used to block nonspecific staining. Then, the sections were respectively incubated with a rabbit anti-Bax or Bcl-XL polyclonal antibody (Boster Bioengineering Co., Ltd., Wuhan, China) at a dilution of 1:200 (v/v) overnight at 4 °C. The immunoreaction was achieved with the goat anti-rabbit antibody (Boster Bioengineering Co., Ltd., Wuhan, China) at a dilution of 1:200 (v/v) and developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained with hematoxylin. The images of the seminiferous epithelium were captured with a digital camera. Germ cells with a brown cytoplasm were considered positive, and the number of positive cells was counted in each image using the Imaging Software (NIS-Elements BR 2.30, Nikon, Kanagawa, Japan).
2.6. Determination of caspase-3 activity
The testicular activity of caspase-3 was determined using caspase-3 assay kit (Beyotime Institute of Biotechnology, Jiangsu, China). The activity was expressed as the activity relative to the control group.
2.7. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay
Apoptotic cells were detected with a Promega apoptosis detection TUNEL kit in the seminiferous epithelium. Cells with a brown nucleus were considered positive. Germ cells with a brown cytoplasm were considered positive, and the number of positive cells was counted in each image using the Imaging Software (NIS-Elements BR 2.30, Nikon, Kanagawa, Japan).
2.8. Statistical analysis
All data are expressed as mean±standard error mean (SEM) and analyzed by one-way analysis of variance (ANOVA). The statistical analysis was performed using the generalized linear model (GLM) procedure of Statistical Analysis System (SAS) software (Version 6). Significance was declared at P<0.05.
3. Results
3.1. Morphological changes of testes
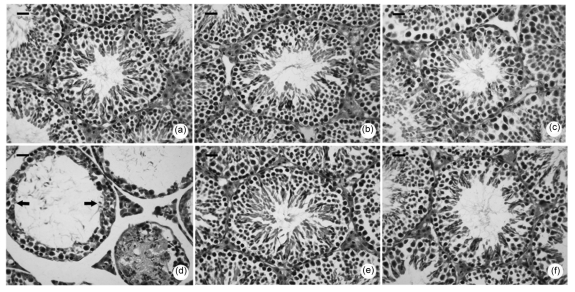
After one week of treatment, control mice showed normal morphology of seminiferous tubules (Fig. 1a). Treatments with PNMC at doses of 1 or 10 mg/kg induced no significant changes of the seminiferous tubules compared with the control (Figs. 1b and 1c). However, PNMC at the dose of 100 mg/kg resulted in remarkable damage to the seminiferous tubules. As shown in Fig. 1d, PNMC-treated mice showed approximately 40% loss of round germ cells. Almost no elongated spermatozoa could be found and the diameters of seminiferous tubules were markedly increased (approximately half that of control). Moreover, atrophy of testes was also detected.
Fig. 1.
Morphological changes of the seminiferous tubules one week after treatments of PNMC and quercetin
(a) Control; (b−d) PNMC treatments (1, 10, and 100 mg/kg, respectively); (e) Quercetin (75 mg/kg); (f) PNMC (100 mg/kg)+quercetin. Scale bar: 20 µm
Histological results showed that in the quercetin (75 mg/kg)-treated group, the mice had normal seminiferous epithelia (Fig. 1e). After combined treatment with quercetin, the PNMC-induced damage was attenuated significantly. There was no germ cell decline in the seminiferous tubules of the co-treated mice as shown in Fig. 1f.
3.2. Antioxidant status
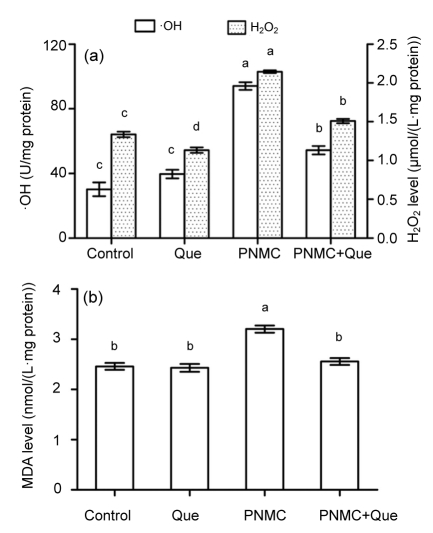
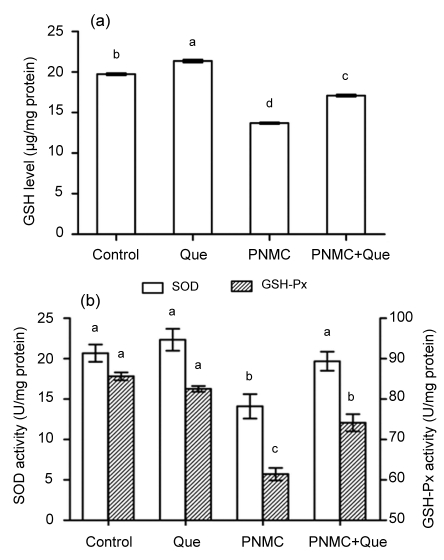
Treatment with PNMC (100 mg/kg) markedly destroyed the antioxidant system of the testes. PNMC significantly induced increases of ·OH and H2O2 levels (Fig. 2a; P<0.05). MDA level was also significantly increased (Fig. 2b; P<0.05). Administration of quercetin to PNMC-treated mice significantly reduced ·OH and H2O2 levels, as well as MDA level. PNMC significantly depleted testis GSH level (Fig. 3; P<0.05), while treatment with quercetin caused a significant increase in GSH level (P<0.05). The activities of SOD and GSH-Px were significantly reduced in mice treated with PNMC (P<0.05); however, there was a tendency for treatment with quercetin to enhance the activities of both enzymes (P>0.05).
Fig. 2.
Changes of ·OH, and H2O2 (a) and MDA (b) production in testes after treatments of PNMC and quercetin (Que)
Columns represent mean±SEM (n=6). Bars with different letters were statistically different (P<0.05)
Fig. 3.
Changes of GSH level (a) and SOD and GSH-Px activities (b) of testes after treatments of PNMC and quercetin (Que)
Columns represent mean±SEM (n=6). Bars with different letters were statistically different (P<0.05)
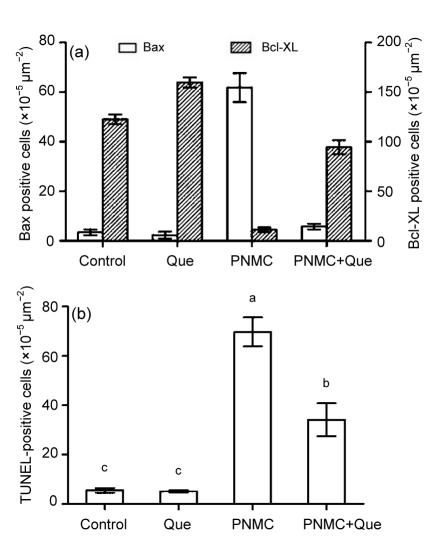
3.3. Bax and Bcl-XL expression
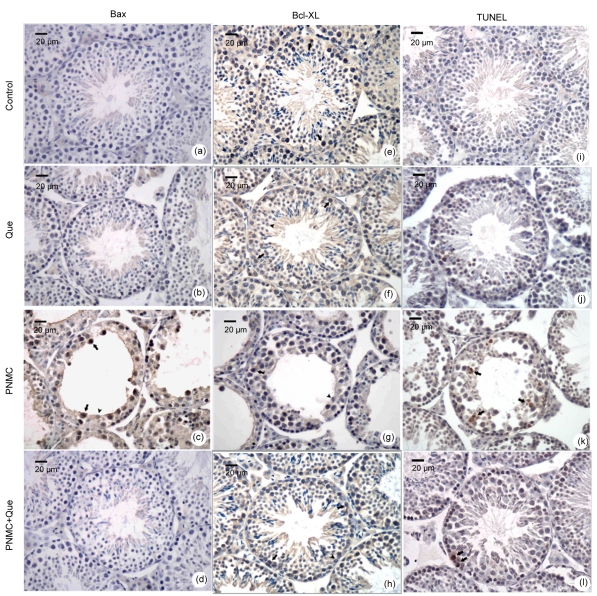
As shown in Fig. 4, PNMC significantly up-regulated Bax expression and down-regulated Bcl-XL expression in the germ cells, compared to the control, while quercetin up-regulated Bcl-XL expression in germ cells compared with the control. Moreover, in combination with PNMC, quercetin significantly down-regulated PNMC-induced increase of Bax expression, and further up-regulated the expression of Bcl-XL (Fig. 5a; P<0.05).
Fig. 4.
Changes of Bax (a–d) and Bcl-XL (e–h) expression and TUNEL staining (i–l) of germ cells in seminiferous epithelia
(a, e, i) Control; (b, f, j) Quercetin (75 mg/kg); (c, g, k) PNMC (100 mg/kg); (d, h, l) PNMC+quercetin. Arrows: positive cells; Arrowheads: negative cells
Fig. 5.
Changes of Bax and Bcl-XL expression (a) and TUNEL-positive germ cells (b) after treatments of PNMC and quercetin (Que)
Columns represent mean±SEM (n=6). Bars with different letters were statistically different (P<0.05)
3.4. TUNEL-positive cell number
The immunohistochemical results showed that, in the control and quercetin-treated group, mice showed almost no apoptotic cells (Figs. 4i–4l). However, TUNEL-positive cell number increased significantly in the PNMC-treated mice testes compared to the control (Fig. 5b; P<0.05). The apoptotic cells mainly included spermatocyte and round spermatid. Moreover, in combination with PNMC, quercetin markedly reduced the PNMC-induced increase of TUNEL-positive cell number (P<0.05).
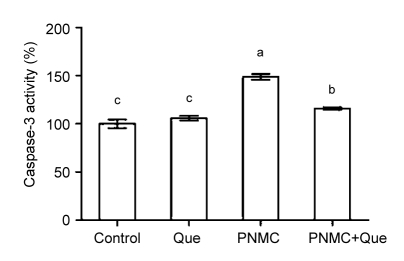
3.5. Caspase-3 activity
The activation of caspase-3 plays a pivotal role in the execution of apoptosis. Our results showed that PNMC induced a significant increase of caspase-3 activity compared to the control (Fig. 6; P<0.05). There was no significant change in caspase-3 activity of quercetin-treated mice relative to the control. However, in combination with PNMC, quercetin was demonstrated to effectively suppress caspase-3 activity.
Fig. 6.
Changes of caspase-3 activity in testicular homogenate after treatments of PNMC and quercetin (Que)
Columns represent percents of control (mean±SEM, n=6). Bars with different letters were statistically different (P<0.05)
4. Discussion
Our previous in vitro studies have reported that PNMC induced oxidative damage to chicken embryonic spermatogonial cells (Mi et al., 2009). Our present study found that PNMC treatment at 1 or 10 mg/kg (one week after a single i.p. injection) showed no significant changes of seminiferous tubules in mature male mice; however, at the dose of 100 mg/kg, it induced focal severe damage to the seminiferous epithelium. Moreover, germ cell loss was observed in PNMC-treated mice and almost no elongated spermatozoa were found.
It is known that reactive oxygen species play a key role in the pathophysiological processes (Rodrigo and Bosco, 2006). A previous study in vitro has reported that oxidative stress plays a critical role in PNMC-induced cytotoxicity (Mi et al., 2009). Our biochemical results showed that PNMC treatment induced oxidative stress on testes. With significant increases of ·OH and H2O2 levels and lipid peroxidation, the antioxidant system of testis was significantly disturbed, and at the same time the antioxidant GSH, enzymatic antioxidants SOD and GSH-Px were also significantly inhibited. The antioxidant GSH functions as a direct reactive free-radical scavenger (Romão et al., 2006). SOD and GSH-Px are two main components of antioxidant enzymes. As an important superoxide radical scavenger, SOD converts superoxide anions to hydrogen peroxide, which then can be detoxified by GSH/GSH-Px to yield reduced GSH.
There is a close relation between oxidative stress and apoptosis. The Bcl-2 family consists of pro-apoptotic (e.g., Bax) and anti-apoptotic (e.g., Bcl-XL) members, which play an important role in the mitochondrial pathway of apoptosis. The altered expression of Bax and Bcl-XL may occur before the activation of apoptosis (Adams and Cory, 1998; Hengartner, 2000; Gustafsson and Gottlieb, 2007). There are many factors which can alter the expression of Bcl-2 proteins, such as hypoxia, oxidative stress, and DNA damage (Gustafsson and Gottlieb, 2007). DEP induce down-regulation of Bcl-XL (Landvik et al., 2007) and the change of Bcl-2 family proteins may trigger caspase cascade activation. Activation of caspase-3 is known to be an important step in the progress of apoptosis. Our results showed that the activity of caspase-3 in PNMC-treated mice was significantly up-regulated.
It is known that reactive oxygen species are able to induce DNA damage. The most potent reactive oxygen species to react with DNA is ·OH, which generates damage to both DNA bases and deoxyribose residues. It has been reported that exposure to DEP extracts induced apoptosis in different cell types (Baulig et al., 2003). As shown in our results, one week of PNMC exposure (100 mg/kg) induced significant DNA damage of germ cells in the mice testis. TUNEL-positive cells in PNMC-treated mice were significantly increased.
Quercetin, as a natural antioxidant, is ubiquitously distributed in fruits and vegetables. Our previous study found that flavonoid quercetin and daizein have protective effects on cadmium or polychlorinated biphenyls-induced oxidative damage in mice testes (Zhang et al., 2008; Bu et al., 2011). In the present study, we found that quercetin supplement attenuated PNMC-induced increase of ·OH and H2O2, and further decreased lipid peroxidation, suggesting that superoxides ·OH and H2O2 are the main damage factors in PNMC-induced oxidative stress in mice testis. Furthermore, quercetin supplementation significantly restored the depletion level of antioxidant GSH and the activities of enzymatic antioxidants SOD and GSH-Px. These results are in agreement with our studies using chicken testicular germ cells (Mi et al., 2009).
Structure-activity relationships of quercetin were investigated by Cao et al. (1997) and the antioxidant activities of quercetin may depend upon its hydroxyl groups. It is supposed that flavonoids prevent the progression of the radical chain reaction by traping free radicals at the interface of the membranes (Ross and Kasum, 2002). In addition to its free-radical scavenging properties, quercetin can also chelate those transition metal ions responsible for the generation of reactive oxygen species (Rice-Evans et al., 1996). Our present study also revealed that quercetin exerts antioxidant abilities by enhancing the activities of endogenous antioxidant and enzymes.
It is reported that quercetin also has antiapoptotic function (Chao et al., 2009; Kim et al., 2009). Our previous studies have shown that quercetin has effectively inhibited pro-apoptotic member Bax and up-regulated anti-apoptotic member Bcl-XL (Bu et al., 2011; Jia et al., 2011). In the present study, quercetin treatment effectively down-regulated the expression of pro-apoptotic protein Bax and up-regulated the expression of Bcl-XL induced by PNMC. The change of the ratio of Bax/Bcl-XL would prevent apoptosis by suppressing caspase-3 activity. Furthermore, suppression of apoptosis was demonstrated by the decreased number of TUNEL-positive cells in quercetin-treated mice.
In conclusion, the present study suggests that PNMC has reproductive toxic effects on mouse germ cells involving mitochondrial oxidative damages via the inhibition of Bcl-XL expression, activation of Bax expression and caspase-3 activity. However, as an effective antioxidant, quercetin manifested a protective effect against PNMC-induced toxicity through attenuating lipid peroxidation, renewing the activities of antioxidant enzymes, and alleviating apoptosis by modulating Bax and Bcl-XL expression and inhibiting caspase-3 activity. Therefore, these observations suggest that the dietary antioxidant quercetin potentially reduces environmental PNMC-induced toxicity and improves reproductive health.
Acknowledgments
We thank Wei-dong ZENG (College of Animal Sciences, Zhejiang University) for help in the experiments and Dr. Imdad Hussain LEGHARI (College of Animal Sciences, Zhejiang University) for English improvement of the manuscript.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31001041), and the Chinese Universities Scientific Fund and Project of the Bureau of Education of Zhejiang Province (No. Y201018833), China
References
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, Baeza-Squiban A. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L671–L679. doi: 10.1152/ajplung.00419.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bu T, Mi Y, Zeng W, Zhang C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat Rec. 2011;294(3):520–526. doi: 10.1002/ar.21317. [DOI] [PubMed] [Google Scholar]
- 4.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22(5):749–760. doi: 10.1016/S0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 5.Chao CL, Hou YC, Chao PDL, Weng CS, Ho FM. The antioxidant effects of quercetin metabolites on the prevention of high glucose-induced apoptosis of human umbilical vein endothelial cells. Brit J Nutr. 2009;101(8):1165–1170. doi: 10.1017/S0007114508073637. [DOI] [PubMed] [Google Scholar]
- 6.Furuta C, Suzuki AK, Taneda S, Kamata K, Hayashi H, Mori Y, Li C, Watanabe G, Taya K. Estrogenic activities of nitrophenols in diesel exhaust particles. Biol Reprod. 2004;70(5):1527–1533. doi: 10.1095/biolreprod.103.024810. [DOI] [PubMed] [Google Scholar]
- 7.Furuta C, Li C, Taneda S, Suzuki AK, Kamata K, Watanabe G, Taya K. Immunohistological study for estrogenic activities of nitrophenols in diesel exhaust particles. Endocrine. 2005;27(1):33–36. doi: 10.1385/ENDO:27:1:033. [DOI] [PubMed] [Google Scholar]
- 8.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292(1):C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 9.Han JY, Takeshita K, Utsumi H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med. 2001;30(5):516–525. doi: 10.1016/S0891-5849(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 10.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999;163(10):5582–5591. [PubMed] [Google Scholar]
- 12.Huang CH, Lin LY, Tsai MS, Hsu CY, Chen HW, Wang TD, Chang WT, Cheng TJ, Chen WJ. Acute cardiac dysfunction after short-term diesel exhaust particles exposure. Toxicol Lett. 2010;192(3):349–355. doi: 10.1016/j.toxlet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Izawa H, Kohara M, Aizawa K, Suganuma H, Inakuma T, Watanabe G, Taya K, Sagai M. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci Biotechnol Biochem. 2008;72(5):1235–1241. doi: 10.1271/bbb.70705. [DOI] [PubMed] [Google Scholar]
- 14.Januario DA, Perin PM, Maluf M, Lichtenfels AJ, Nascimento Saldiva PH. Biological effects and dose-response assessment of diesel exhaust particles on in vitro early embryo development in mice. Toxicol Sci. 2010;117(1):200–208. doi: 10.1093/toxsci/kfq165. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y, Lin J, Mi Y, Zhang C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod Toxicol. 2011;31(4):477–485. doi: 10.1016/j.reprotox.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Kim BM, Choi YJ, Han Y, Yun YS, Hong SH. N,N-dimethyl phytosphingosine induces caspase-8-dependent cytochrome c release and apoptosis through ROS generation in human leukemia cells. Toxicol Appl Pharm. 2009;239(1):87–97. doi: 10.1016/j.taap.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Landvik NE, Gorria M, Arlt VM, Asare N, Solhaug A, Lagadic-Gossmann D, Holme JA. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: possible implications for their mutagenic and carcinogenic effects. Toxicology. 2007;231(2-3):159–174. doi: 10.1016/j.tox.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Anti-androgenic activity of 3-methyl-4-nitrophenol in diesel exhaust particles. Eur J Pharmacol. 2006;543(1-3):194–199. doi: 10.1016/j.ejphar.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Mi Y, Zhang C, Li C, Taneda S, Watanabe G, Suzuki AK, Taya K. Quercetin protects embryonic chicken spermatogonial cells from oxidative damage intoxicated with 3-methyl-4-nitrophenol in primary culture. Toxicol Lett. 2009;190(1):61–65. doi: 10.1016/j.toxlet.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Miyabara Y, Ichinose T, Takano H, Lim HB, Sagai M. Effects of diesel exhaust on allergic airway inflammation in mice. J Allergy Clin Immunol. 1998;102:805–812. doi: 10.1016/S0091-6749(98)70021-1. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Kamata K, Toda N, Hayashi H, Seki K, Taneda S, Yoshino S, Sakushima A, Sakata M, Suzuki AK. Isolation of nitrophenols from diesel exhaust particles (DEP) as vasodilatation compounds. Biol Pharm Bull. 2003;26(3):394–395. doi: 10.1248/bpb.26.394. [DOI] [PubMed] [Google Scholar]
- 22.Noya Y, Mikami Y, Taneda S, Mori Y, Suzuki AK, Ohkura K, Yamaki K, Yoshino S, Seki K. Improvement of an efficient separation method for chemicals in diesel exhaust particles: analysis for nitrophenols. Environ Sci Pollut Res Int. 2008;15(4):318–321. doi: 10.1007/s11356-008-0006-3. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Nam H, Chung N, Park JD, Lim Y. The role of iron in reactive oxygen species generation from diesel exhaust particles. Toxicol in Vitro. 2006;20(6):851–857. doi: 10.1016/j.tiv.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo R, Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and reodent kidney. A review. Comp Biochem Phys C. 2006;142(3-4):317–327. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Romão PR, Tovar J, Fonseca SG, Moraes RH, Cruz AK, Hothersall JS, Noronha-Dutra AA, Ferreira SH, Cunha FQ. Glutathione and the redox control system trypanothione/trypanothione reductase are involved in the protection of Leishmania spp. against nitrosothiol-induced cytotoxicity. Braz J Med Biol Res. 2006;39(3):355–363. doi: 10.1590/S0100-879X2006000300006. [DOI] [PubMed] [Google Scholar]
- 27.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 28.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium-biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, Oonuki Y. Inhalation of diesel engine exhaust affects spermatogenesis in growing male rats. Environ Health Perspect. 1999;107(7):539–544. doi: 10.2307/3434395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JA, Hermonat MW, Hards RG. A degradation product of fenitrothion, 3-methyl-4-nitrophenol, is an inhibitor of mammalian ribonucleotide reductase. Bull Environ Contam Toxicol. 1982;28(4):480–483. doi: 10.1007/BF01607715. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida S, Sagai M, Oshio S, Umeda T, Ihara T, Sugamata M, Sugawara I, Takeda K. Exposure to diesel exhaust affects the male reproductive system of mice. Int J Androl. 1999;22(5):307–315. doi: 10.1046/j.1365-2605.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DL, Mi YL, Wang KM, Zeng WD, Zhang CQ. Attenuating effect of daidzein on polychlorinated biphenyls-induced oxidative toxicity in mouse testicular cells. J Zhejiang Univ Sci B. 2008;9(7):567–571. doi: 10.1631/jzus.B0820048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Recommended reading
- 33.Lin JX, Jia YD, Zhang CQ. Effect of epidermal growth factor on follicle-stimulating hormone-induced proliferation of granulosa cells from chicken prehierarchical follicles. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2011;12(11):875–883. doi: 10.1631/jzus.B1100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu HY, Zeng WD, Cao AL, Zhang CQ. Follicle-stimulating hormone promotes proliferation of cultured chicken ovarian germ cells through protein kinases A and C activation. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2010;11(12):952–957. doi: 10.1631/jzus.B1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang DL, Mi YL, Wang KM, Zeng WD, Zhang CQ. Attenuating effect of daidzein on polychlorinated biphenyls-induced oxidative toxicity in mouse testicular cells. J Zhejiang Univ-Sci B. 2008;9(7):567–571. doi: 10.1631/jzus.B0820048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang DL, Wang KM, Zhang CQ. Ginsenosides stimulated the proliferation of mouse spermatogonia involving activation of protein kinase C. J Zhejiang Univ-Sci B. 2009;10(2):87–92. doi: 10.1631/jzus.B0820133. [DOI] [PMC free article] [PubMed] [Google Scholar]