Abstract
Evolution often results in morphologically similar solutions in different organisms, a phenomenon known as convergence. However, there is little knowledge of the processes that lead to convergence at the genetic level. The genes of the Hox cluster control morphology in animals. They may also be central to the convergence of morphological traits, but whether morphological similarities also require similar changes in Hox gene function is disputed. In arthropods, body subdivision into a region with locomotory appendages (“thorax”) and a region with reduced appendages (“abdomen”) has evolved convergently in several groups, e.g., spiders and insects. In insects, legs develop in the expression domain of the Hox gene Antennapedia (Antp), whereas the Hox genes Ultrabithorax (Ubx) and abdominal-A mediate leg repression in the abdomen. Here, we show that, unlike Antp in insects, the Antp gene in the spider Achaearanea tepidariorum represses legs in the first segment of the abdomen (opisthosoma), and that Antp and Ubx are redundant in the following segment. The down-regulation of Antp in A. tepidariorum leads to a striking 10-legged phenotype. We present evidence from ectopic expression of the spider Antp gene in Drosophila embryos and imaginal tissue that this unique function of Antp is not due to changes in the Antp protein, but likely due to divergent evolution of cofactors, Hox collaborators or target genes in spiders and flies. Our results illustrate an interesting example of convergent evolution of abdominal leg repression in arthropods by altering the role of distinct Hox genes at different levels of their action.
Keywords: Chelicerata, homeosis, tagmosis, homeotic genes, animal bauplan
The evolution of similar morphologies in unrelated species, so-called convergent evolution, is among the most fascinating outcomes of mutation and natural selection. Textbook examples for convergent evolution include the patagium (wing skin) of bats and pterosaurs, or the streamlined body with fins instead of legs in water-living animals like whales, ichthyosaurs, penguins, and fishes. Superficially speaking, it appears that convergent evolution means that evolution is repeating itself and provides similar solutions under similar selectional forces. However, how is this convergence achieved at the level of the genetic programs that control the development of these morphologies? Are these genetic programs modified at the same point again and again to reproduce similar morphologies? Or can similar morphologies also result from changes at different points in the genetic programs?
Arthropods form an interesting group to study such questions because their segmented body shows a remarkable morphological diversity and, thus, provide many opportunities for convergent evolution. For example, in the posterior body part, which often bears the reproductive and digestive organs, appendages are frequently reduced or lacking. This abdomen has evolved convergently in the body plan of several arthropod groups, e.g., in insects and spiders. The first genes that were found to be able to alter the arthropod body plan were the Hox genes (1–3). This discovery led to the proposal that changes in Hox gene regulation and function fueled the evolution of arthropod body plan diversity (4, 5). Hox genes encode transcription factors and are expressed in partially overlapping expression domains along the anterior-posterior axis, subdivide the body into discrete portions, and determine the morphology of these segments (6). For instance, in insects, the Hox genes determine the presence or absence of appendages in different parts of the body. Locomotory legs develop on the thorax in the expression domain of the Antennapedia (Antp) gene (7–10), whereas the Ultrabithorax (Ubx) gene represses legs on the abdomen (11). Hox proteins can control their target genes directly by binding to their regulatory sequences, but for binding specificity, they require cofactors that interact directly with the Hox protein or collaborators that act together with the Hox protein, but without physically binding it (12). The interplay of the Hox proteins and the upstream and downstream factors that influence their action has been termed the Hox gene pathway (13). Changes at multiple levels of this pathway are linked to morphological diversity: Changes in the expression of Ubx have been implicated in mouthpart diversity in crustaceans (14–16), evolution of femur shape in Drosophila (17, 18), or the evolution of novel appendage morphologies in grasshoppers (19) and waterstriders (20). Changes can also take place in the targets of a Hox gene, as has been suggested for Ubx as the cause for the differences in insect hindwing morphology (21, 22), or for Sex combs reduced (Scr) as the cause for the evolution of the dorsal helmet in treehoppers (23). Finally, there are instances where changes in the Hox protein itself caused changes in the body plan, for example the evolution of a novel domain in insect Ubx proteins that is required for abdominal limb repression (24, 25). These examples show that Hox genes are crucial for the evolution of body plan diversity and might therefore also be responsible for convergences in body plan morphology.
The spider body plan is (similar to insects) composed of a part that bears locomotory legs (prosoma or “cephalothorax”) and a part that has only reduced appendages (opisthosoma or abdomen). Clearly, these features have evolved convergently in insects and spiders, but the underlying genetic factors and the role of Hox genes in body plan specification in spiders are not well known. Previous work on Hox gene expression in spiders has shown that the Antp gene is not expressed in the walking leg-bearing segments. Thus, unlike its insect homolog, spider Antp cannot be the factor that determines the presence of locomotory legs. This difference points to evolutionary changes of the role of Antp in the specification of the spider body plan. We have therefore studied the role of Antp in the spider Achaearanea tepidariorum (At-Antp). We show that At-Antp has a leg-repressive role in the spider opisthosoma reminiscent of the role of Ubx in the insect abdomen. This observation suggests that the evolution of limb-repression in the abdomen in flies and spiders is at least in part driven by divergent roles of different Hox genes. However, unlike insect Ubx (24, 25) the Antp gene of A. tepidariorum does not repress the expression of the limb-inducing gene Distal-less (Dll) in Drosophila embryos. Thus, convergent evolution of limb repression has been more complicated than just exchanging one Hox gene for another and involves changes at different levels of the Hox gene pathway as well. These findings provide unique insight into the genetic basis of convergently evolved morphologies and form a basis for further research into the diversification of Hox gene function and its influence on divergent or convergent evolution of morphological characters.
Results
Expression of At-Antp in the Opisthosoma.
Expression of Antp has been studied in the spider Cupiennius salei (26). We have studied the expression of Antp in A. tepidariorum by whole-mount in situ hybridization to compare it to Antp expression in C. salei. As in C. salei, At-Antp is not expressed in the walking leg segments L1 to L4, but is strongly expressed in all opisthosomal (O) segments (Fig. 1A). Interestingly, At-Antp expression levels are highest in the limbless O1 segment (Fig. 1B). In older embryonic stages, after germ band inversion, the expression levels of At-Antp in the opisthosoma are decreasing, but in the O1 segment At-Antp expression levels remain high (Fig. 1C).
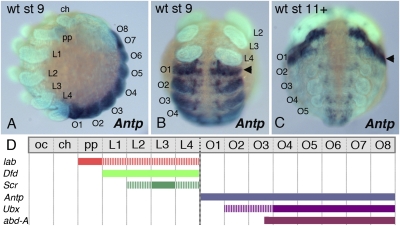
Fig. 1.
Embryonic expression of the A. tepidariorum Antp gene. (A and B) At developmental stage 9, eight abdominal segments have formed (A, lateral view; B, ventral view). At-Antp is expressed in the opisthosoma with strongest expression in O1 (arrowhead in B). (C) Older embryo at late stage 11 in ventral view. The strong O1 expression domain of At-Antp becomes more prominent as expression levels in the remaining opisthosoma decrease. (D) Summary of the segmental expression domains of the Hox genes At-lab, At-Dfd, At-Scr, At-Antp, At-Ubx, and At-abd-A at stage 9. Note that the O1 segment only expresses At-Antp. ch, cheliceral segment; L1–L4, walking leg segments; O1–O8, opisthosomal segments; oc, ocular segment; pp, pedipalpal segment, wt, wild type.
To evaluate the conservation of expression of At-Antp in relation to other Hox genes in A. tepidariorum, we have also examined the expression of several other Hox genes (27): labial (At-lab) is significantly expressed only in the pedipalp segment; expression of At-lab in the leg segments is very low (Fig. S1A). Deformed (At-Dfd) is expressed in all four leg segments (Fig. S1B), and At-Scr is expressed in L2 to L4, with strongest expression in L3 (Fig. S1C; see also below). At-Ubx and abdominal-A (At-abd-A) are expressed in the opisthosoma from segment O2 and posterior O3, respectively. A summary of the Hox gene expression patterns is given in Fig. 1D. The expression patterns in A. tepidariorum are very similar to those reported previously for other spider species (26, 28, 29). Our results show that, similar to previously studied spiders, the O1 segment in A. tepidariorum expresses only a single Hox gene, At-Antp, and support the notion that At-Antp has a role in producing the limbless state of the O1 segment.
At-Antp RNAi Results in 10-Legged Spiders.
To test whether At-Antp is required for the limbless state of the O1 segment, we have studied the function of At-Antp by RNA interference (RNAi). The At-Antp RNAi larvae possess 10 walking legs: In addition to the normal eight walking legs they develop an additional pair of legs on the O1 segment (Fig. 2 and Figs. S2 and S3). The knockdown of At-Antp by RNAi thus leads to the formation of an ectopic pair of legs on O1. Morphologically, these legs look like wild-type legs (Fig. 2 A and C), but are slightly shorter and thinner (Fig. 2 B and D). The following O2 segment is morphologically normal as evidenced by the proper development of the book lungs (compare Fig. 2 C and D), and the rest of the opisthosoma also appears normal. Expression of the appendage patterning genes Distal-less (At-Dll) (Fig. 3A), dachshund (At-dac) (Fig. 3B), and extradenticle-1 (At-exd-1) (Fig. 3C) that are markers for distal, medial, and proximal leg portions (30, 31), respectively, is virtually identical in the normal legs and in the ectopic legs on O1. In addition, the gene homothorax-1 (At-hth-1), which encodes a putative cofactor of At-exd-1, is expressed throughout the ectopic leg except for the distal leg tip and, thus, in a pattern identical to the normal walking legs (Fig. 3D). These data show that the ectopic legs on the O1 segment have a normal proximal-distal axis comprising proximal, medial, and distal portions. To establish the segmental identity of the ectopic legs on O1, we have examined the expression of At-Dfd and At-Scr at the late inversion stage. At this stage, At-Dfd is expressed strongly in the distal tip of all legs, but in L1 and L2, there is also weaker expression further proximal in the presumptive metatarsal region (Fig. 3E). The ectopic leg pair of At-Antp RNAi animals expresses At-Dfd only at the tip similar to L3 and L4 legs, thus excluding a L1 or L2 identity. At-Scr is expressed differentially in all legs: It is not expressed in L1 but shows a pattern of repeated rings in L2 to L4 (Fig. 3F). The expression level of these rings is highest in L3, lowest in L4, and intermediate in L2. Intriguingly, the ectopic leg pair of At-Antp RNAi animals shows a unique expression pattern of At-Scr. There are very faint rings only in the distal part, and there is a strong distal tip domain, which is not present in any of the normal legs. The At-Dfd and At-Scr expression suggests that the ectopic legs on O1 are not simply a homeotic duplication of one of the normal legs on L1 to L4, but rather represent derepressed legs with a possibly genuine O1 identity. Taken together, these results show that At-Antp represses leg formation in the O1 segment and does not perform this function in conjunction with other Hox genes, because it is the only Hox gene expressed in O1.
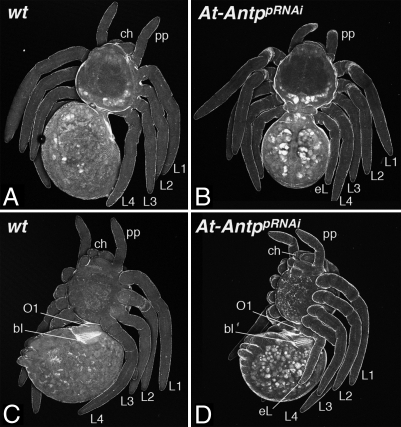
Fig. 2.
Ten-legged spiders result from Antp RNAi. (A and C) Wild-type larvae (A, dorsal view; C, ventral view). (B and D) Parental injection of At-Antp double-stranded RNA leads to the development of spiders with an extra pair of legs (B, dorsal view; D, ventral view) on opisthosomal segment O1. All other structures appear normal compared with the wild type; note also the normal book lungs on O2. bl, book lung; ch, chelicera; eL, ectopic leg; L, walking leg; O, opisthosomal segment; pp, pedipalp.
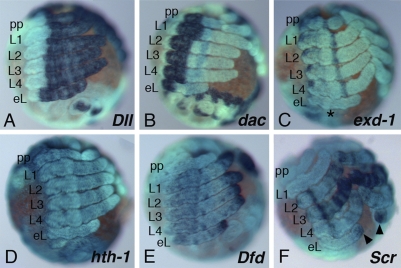
Fig. 3.
The additional legs are genuine walking legs. Expression of leg marker genes in At-Antp RNAi embryos at late stage 11 (shortly before dorsal closure). (A) At-Dll is expressed in the distal portion of the ectopic legs on O1 similar to the walking legs. (B) Expression of At-dac in the ectopic legs shows the usual medial expression as in the normal legs. (C) At-exd-1 is expressed in a proximal domain in the ectopic legs and in L1–L4, including the medial ring of expression (albeit faint) (star). (D) At-hth-1 is expressed throughout the entire leg apart from the tip in the legs and in the ectopic legs. (E) At-Dfd expression is similar in the ectopic legs and the normal legs. Note that expression in L1 and L2 reaches further proximal than in L3 and L4, where expression is restricted to the tip. (F) At-Scr marks each of the legs with a unique pattern. The expression pattern in the ectopic legs differs from the patterns of the normal legs and shows faint distal rings and a strong expression domain in the tip (arrowhead). bl, book lung; ch, chelicera; eL, ectopic leg; L, walking leg; O, opisthosomal segment; pp, pedipalp.
Dll-Positive Limb Rudiments on the O2 Segment After Double RNAi.
The question remains why At-Antp RNAi does not lead to the derepression of legs in other opisthosomal segments. One explanation could be the restriction of the leg-repression function to the area of its strongest expression, the O1 segment. Alternatively, other posterior Hox genes could have evolved a leg-repressing role as well (analogous to Ubx in insects; refs. 24 and 25) and compensate for the loss of Antp function. A potential RNAi effect would then only be observed if the expression of those Hox genes was knocked down along with At-Antp. We have therefore performed single and double RNAi with At-Ubx or At-abd-A (Fig. S3). No externally visible phenotypes were observed after either At-Ubx or At-abd-A RNAi, although the level of mRNA expression as examined by in situ hybridization was visibly reduced (Fig. S4). However, after double RNAi with At-Antp and At-Ubx (Fig. 4 and Fig. S3) the larvae showed the ectopic leg on O1 and, in addition, a small appendage rudiment growing on O2 (Fig. 4A). Normally, the appendages of the O2 segment are the book lungs, and these form in the embryo as small limb buds, which are later internalized and, thus, do not protrude from the body wall in larvae (see, e.g., Fig. 2C). In addition, the embryonic book lungs do not express Dll (Fig. 4E). By contrast, the O2 appendage rudiment in At-AntpUbx double RNAi animals protrudes from the body in larvae (Fig. 4A), and in embryos, has a distal outgrowth that expresses Dll (Fig. 4 D and F). The embryonic O2 appendage rudiment also expresses Dfd (Fig. 4C) and Scr (only in its proximal part) (Fig. 4B). Because these genes are normally only expressed in the leg segments (Fig. 1 and Fig. S1), these data strongly suggest a leg identity of this appendage and imply that At-Antp represses the formation of legs not only in O1, but also in O2, but this function is only revealed when the redundant function of At-Ubx is impaired as well.
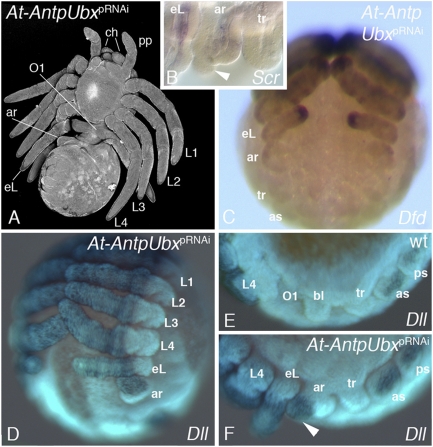
Fig. 4.
Double RNAi with At-Antp and At-Ubx. (A) At-AntpUbx larva in ventral view. Note the ectopic leg (eL) on O1 and the additional appendage rudiment (ar) on O2. (B and C) Expression of At-Scr (B) and At-Dfd (C) in the O2 appendage rudiment at late stage 11. (B) Preparation of the opisthosomal appendages (lateral view, anterior to the left) to show the expression of Scr in the proximal O2 rudiment; Scr is not expressed in the distal outgrowth of the O2 limb (arrowhead) and the tracheal bud (tr). (C) Ventral view. Dfd is expressed in the legs, and the eL on O1 and the O2 rudiment. (D–F) Expression of Dll at late stage 11. (D) Ventral view of At-AntpUbx RNAi embryo; note Dll expression in the O2 rudiment. (E and F) Comparison of Dll expression in the opisthosoma of wild-type (E) and At-AntpUbx RNAi embryos (F). Lateral view, anterior to the left. Note that in the wild type, the book lung buds on O2 do not express Dll, whereas Dll is expressed in the distal outgrowth of the O2 appendage rudiment (arrowhead). as, anterior spinneret bud; ch, chelicera; L, walking leg; pp, pedipalp; ps, posterior spinneret bud; tr, trachea bud.
At-Antp Leads to Antenna-to-Leg Transformations of the Drosophila Antenna.
We asked whether this appendage repression role of At-Antp can be reproduced in Drosophila by expressing At-Antp in the Drosophila antenna imaginal disk. The antenna is the only Drosophila appendage that develops without input from the Hox genes. Thus, expression of At-Antp in the antenna of Drosophila creates a situation similar to the spider O1 segment in the sense that an appendage-bearing segment is produced that expresses At-Antp as the only Hox gene. For ectopic expression of At-Antp in the Drosophila antenna disk, we used the dpp-Gal4 driver that has been used for the misexpression of Hox genes in the antenna disk (32) (Fig. 5 A–C). Contrary to our expectation, the expression of At-Antp transformed the distal portion (mainly the arista) of the antenna toward leg identity (Fig. 5C), very similar to the phenotype of the Drosophila Antp misexpression (Fig. 5B). Thus, in contrast to its role in the spider O1 segment, At-Antp does not repress appendage development when expressed in the Drosophila antennal appendage.
Fig. 5.
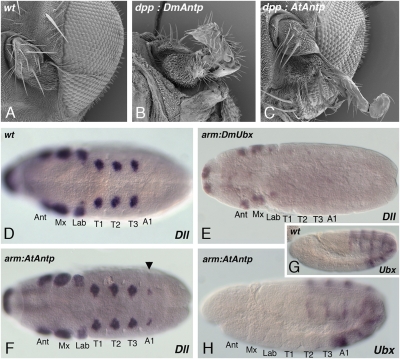
Misexpression of At-Antp in Drosophila melanogaster. (A–C) Misexpression of At-Antp in the antenna imaginal disk leads to arista-to-tarsus transformation. (A) The wildtype antenna consists of 3 antennal segments and the arista. (C) Misexpression of At-Antp using the dpp-Gal4 driver transforms the arista into tarsal identity, similar to the phenotype of Dm-Antp misexpression (B). (D–H) Ubiquitous expression of At-Antp in Drosophila melanogaster embryos does not repress Dll expression but leads to ectopic Dll expression in the first abdominal segment. (D) In wild-type stage 11 embryos, Dll is expressed in the thoracic leg primordia and in antennal, maxillary and labial segments and in the primordium of the labrum. (E) arm-Gal4 driven ubiquitous misexpression of Dm-Ubx leads to repression of Dll in the thorax. (F) arm-Gal4 driven ubiquitous misexpression of At-Antp shows no repression of Dll, but extra patches of Dll expression appear in the A1 segment (arrowhead). (G and H) Embryos misexpressing At-Antp (H) show normal expression of Dm-Ubx, compared with the wild type (G). This indicates that the derepression of Dll in A1 in arm:At-Antp embryos is not due to At-Antp mediated repression of Ubx. A1, first abdominal segment; Ant, antennal segment; Lab, labial segment; Mx, maxillary segment; T, thoracic segment.
At-Antp Derepresses Dll in Drosophila Embryos.
Unlike the O1 segment in spider embryos, the Drosophila antenna disk represents imaginal tissue and, perhaps, the limb-repressive function of At-Antp is restricted to embryonic tissue. Therefore, we asked whether At-Antp can repress limb formation in Drosophila embryos. Leg repression by Antp in the O1 segment of the spider is reminiscent of the leg-repressing role of Ubx in the abdomen of insects. Insect Ubx performs its limb-repression function by repressing the limb-inducing gene Dll in the abdomen (11). When Ubx is misexpressed in the thorax of Drosophila embryos, it is also able to repress Dll expression there (24, 25). We therefore reasoned that At-Antp might function similar to Drosophila Ubx and repress Dll expression when ectopically expressed in the thorax of Drosophila embryos. For ubiquitous At-Antp expression in Drosophila embryos, we used the arm-Gal4 driver that has been used for the misexpression of Ubx, and as a control, we repeated this Ubx misexpression experiment (24, 25). The ubiquitous misexpression of the Drosophila Ubx gene showed a strong repressive effect on Dll expression in the thorax of Drosophila embryos also in our hands (Fig. 5 D and E). In contrast, the ubiquitous expression of spider At-Antp showed no repression of Dll expression in the Drosophila embryo (Fig. 5F). Surprisingly, At-Antp overexpression even showed the opposite effect, because it led to a partial derepression of Dll expression in the first abdominal segment of the Drosophila embryos (Fig. 5F). This derepression of Dll in the first abdominal segment is reminiscent of the effect of Ubx loss-of-function mutants, which also leads to a derepression of Dll in the first abdominal segment (11, 33, 34). However, we could not detect any differences in endogenous Ubx expression between control embryos and arm-Gal4:UAS-At-Antp embryos (Fig. 5 G and H). This observation indicates that the derepression of Dll in the first abdominal segment is not the result of At-Antp repressing Ubx expression.
Discussion
Antp Represses Limb Development in the Anterior Spider Abdomen.
The Antp gene in insects is expressed in the thoracic segments and, thus, correlates with the formation of walking legs in the thorax (13). Previous expression studies in the spider C. salei have indicated that this role of Antp is unlikely to be conserved in spiders, because the legs do not develop in the expression domain of Antp (26). Antp is expressed in the legless opisthosoma of spiders (ref. 26 and the present study), and this expression pattern suggests a role in leg repression rather than in leg development. We show that RNAi with Antp in A. tepidariorum leads to the development of an ectopic pair of legs on the O1 segment. Because the ectopic legs are smaller than the normal walking legs, their unique Scr expression pattern might reflect their rudimentary nature rather than genuine differences in Scr patterning. Alternatively, the unique Scr expression could indicate that these legs represent a derepressed leg pair on the O1 segment rather than a homeotic copy of any of the normal leg pairs. This role is reminiscent of Ubx loss in the waterstrider Gerris buenoi that also leads to a novel identity of the legs L2 and L3 rather than a homeotic transformation (20). These data support the current view of Hox genes as managers of cell identity at multiple levels, rather than as global “master-switches” that determine segmental identity in an “all-or-nothing” fashion (35–37).
In summary, in contrast to insect Antp, the role of spider Antp is the repression of leg development. This role of At-Antp appears to extend to other opisthosomal segments as well. Double RNAi with At-Antp and At-Ubx leads to the formation of a short Dll-positive limb on O2 that also expresses the leg-typical Hox genes At-Scr and At-Dfd and that replaces the book lungs that are normally present on this segment. Neither single At-Antp nor single At-Ubx RNAi are capable of producing this effect, implying that At-Antp and At-Ubx have a redundant leg-repressive function in O2. Our results can be explained with the idea that all three genes, At-Antp, At-Ubx, and At-abd-A, serve as redundant leg repressors that work together to keep the opisthosoma leg-free. Only when all Hox genes in a given opisthosomal segment are impaired, then leg derepression occurs. Derepressing leg formation posterior to O2 would require the interference with At-Antp, At-Ubx, and At-abd-A simultaneously. Unfortunately, this last hypothesis could not be tested, because triple RNAi with At-Antp, At-Ubx, and At-abd-A did not yield conclusive results (Fig. S5 and SI Methods).
Evolutionary Changes in the Action of Hox Genes in Arthropods.
The role of At-Antp in abdominal leg repression is surprising, because all available data from insects on abdomen formation so far suggested that abdominal leg repression is performed by the posterior Hox genes Ubx and abd-A, but not by Antp (11, 38). It appears that spiders and insects have evolved partially different strategies to produce a legless abdomen independently by convergence. Indeed, recent studies have shown that the leg-repressive role of Ubx in the insect abdomen evolved relatively late in the insect lineage (39, 40), and the original function of Ubx seems not to be leg repression, because more basal arthropods show normal leg development within the Ubx expression domain (13, 14, 41, 42). Recent landmark studies have shown that insect Ubx proteins have evolved special sequence motifs that turn them into leg repressors and that are not present in the Ubx proteins of other arthropods including spiders (24, 25, 38). We therefore reasoned that spider Antp could have convergently evolved a special motif in the protein similar to Ubx from insects. Indeed, apart from the homeodomain, arthropod Antp proteins are very divergent, especially at the N terminus (Fig. S6), and this portion of the A. tepidariorum Antp protein might thus contain a newly evolved region responsible for leg repression. N-terminal changes in Antp leading to leg repression are not unprecedented in the arthropods. The appendages of the water flea Daphnia appear to be partially repressed by Antp correlating with changes in the N terminus of the Daphnia Antp protein (43). However, when misexpressed in Drosophila, spider Antp was not able to repress Dll and even had a derepressive effect on Dll in the first abdominal segment. Misexpression experiments must be interpreted with caution, because there is no guarantee that the produced mRNA is also translated into a functional and stable protein (44). The Dll derepression, however, is evidence that functional At-Antp protein is produced in the Drosophila embryos, but the concentration is unknown and thus could be too high or too low to repress Dll. However, the current data provide no evidence to suggest that the leg repressive function of At-Antp is caused by the evolution of a special motif in the Antp protein itself.
Instead, the fact that At-Antp is leg-repressive in its native context (i.e., in the spider), but is limb-permissive when misexpressed in Drosophila strongly suggests that At-Antp requires additional factors or specific target genes for its limb-repressing function that are normally present in the spider context, but are lacking when At-Antp is expressed in the Drosophila context. This observation points to evolutionary changes in cofactors, Hox collaborators or target genes. This conclusion is also strongly supported by the misexpression of At-Antp in the Drosophila antenna disk. Although the basic setting in the spider O1 segment and the Drosophila antenna disk is similar (i.e., At-Antp is the only Hox gene expressed in these segments), the effect of At-Antp differs substantially: In the context of the O1 segment, At-Antp represses leg formation, whereas in the context of the antenna disk, At-Antp transforms the antenna toward leg identity similar to Drosophila Antp. One possibility is that cofactors of the At-Antp protein do not act in Drosophila, either because they have a different structure in the two species (differential evolution of cofactors), are not present in Drosophila (spider specific genes), or are not available (different spatiotemporal expression profiles in the spider and the fly). Another possibility is that target genes of the At-Antp protein in the spider cannot be bound by At-Antp in Drosophila, either because their enhancer sequence is different, or because they are specific to spiders and do not exist in Drosophila. However, so far we have not been able to identify At-Antp cofactors or target genes in A. tepidariorum and substantial future work will be necessary to identify candidates for these genes in this spider.
In summary, our data provide evidence for a unique mode of Antp-mediated appendage diversification. In contrast to Antp protein changes reported in the crustacean Daphnia (43), our results suggest that changes in the availability of target genes, cofactors, or collaborators are responsible for differences of Antp function in spiders and flies. We thus propose that the convergent evolution in insects and spiders of an abdomen with suppressed limbs has involved evolutionary changes from limb-permissive to limb-repressive function on separate levels in the Hox gene pathway.
Methods
Animal Culture and Gene Cloning.
A. tepidariorum were obtained from our laboratory stocks in Göttingen. Isolation of gene fragments from A. tepidariorum was performed according to standard molecular cloning techniques. Sequences of primers and accession numbers are included in SI Methods.
RNAi.
Parental RNAi was performed as described (45) with minor modifications. To exclude off-target effects different fragments were injected separately (Fig. S2). A full documentation of the injections is available in SI Methods and Figs. S3–S5.
In Situ Hybridization, DNA Labeling, and Imaging.
In situ hybridization and nuclear staining with Sytox Green were performed as described (46, 47) with minor modifications. Images were captured with a Zeiss Axioplan-2 microscope or with a Leica dissection microscope equipped with an Intas digital camera and UV light. Confocal z-stacks of larvae were captured by using a Zeiss LSM 510 microscope. Before scanning, the animals were heat fixed and embedded in Voltalef H10S oil. Scanning electron micrographs of Drosophila heads have been recorded by using standard techniques.
Drosophila Strains.
The arm-Gal4 (w[*]; P{w[+mW.hs]=GAL4-arm.S}11), dpp-Gal4 (w[*]; wg[Sp-1]/CyO; P{w[+mW.hs]=GAL4-dpp.blk1}40C.6/TM6B, Tb[1]) and the UAS-Ubx1a line (w[1]; P{w[+mC]=UAS-Ubx.Ia.C}36.2/TM3, Ser[1]) were obtained from the Bloomington Stock center. UAS-At-Antp lines were generated via germ-line transformation according to standard procedures, constructs were injected into w- preblastoderm embryos, and four independent transgenic lines were established.
Supplementary Material
Acknowledgments
We thank Marco Winkler and Maja Gere for technical assistance; Beate Preitz for help with confocal microscopy; Sabine Sommer and Matthias Hahn for assistance with scanning electron microscopy; the following persons and institutions for providing fly stocks, materials, and technical expertise: The Bloomington Drosophila Stock Center at Indiana University, the Drosophila Genetic Research Center in the Kyoto Institute of Technology, Thom Kaufman, Annette Parks, Henry Sun, Ulrike Löhr, and Michael Krahn; and Andreas Wodarz, Michael Kessel, and Ulrike Löhr for discussion and advice. Two anonymous reviewers provided helpful suggestions to improve the manuscipt. This work was funded by the Göttingen Graduate School for Neurosciences and Molecular Biosciences, the Deutsche Forschungsgemeinschaft, and the University of Göttingen.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HE608680–HE608682).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116421109/-/DCSupplemental.
References
- 1.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 2.Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. doi: 10.1038/292635a0. [DOI] [PubMed] [Google Scholar]
- 3.Regulski M, et al. Homeo box genes of the Antennapedia and bithorax complexes of Drosophila. Cell. 1985;43:71–80. doi: 10.1016/0092-8674(85)90013-3. [DOI] [PubMed] [Google Scholar]
- 4.Akam M, Dawson I, Tear G. Homeotic genes and the control of segment diversity. Development. 1988;104:123–133. [Google Scholar]
- 5.Carroll SB. Homeotic genes and the evolution of arthropods and chordates. Nature. 1995;376:479–485. doi: 10.1038/376479a0. [DOI] [PubMed] [Google Scholar]
- 6.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 7.Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci USA. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine M, Hafen E, Garber RL, Gehring WJ. Spatial distribution of Antennapedia transcripts during Drosophila development. EMBO J. 1983;2:2037–2046. doi: 10.1002/j.1460-2075.1983.tb01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll SB, Laymon RA, McCutcheon MA, Riley PD, Scott MP. The localization and regulation of Antennapedia protein expression in Drosophila embryos. Cell. 1986;47:113–122. doi: 10.1016/0092-8674(86)90372-7. [DOI] [PubMed] [Google Scholar]
- 10.Gibson G, Gehring WJ. Head and thoracic transformations caused by ectopic expression of Antennapedia during Drosophila development. Development. 1988;102:657–675. [Google Scholar]
- 11.Vachon G, et al. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 12.Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes CL, Kaufman TC. Hox genes and the evolution of the arthropod body plan. Evol Dev. 2002;4:459–499. doi: 10.1046/j.1525-142x.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- 14.Averof M, Akam M. Hox genes and the diversification of insect and crustacean body plans. Nature. 1995;376:420–423. doi: 10.1038/376420a0. [DOI] [PubMed] [Google Scholar]
- 15.Liubicich DM, et al. Knockdown of Parhyale Ultrabithorax recapitulates evolutionary changes in crustacean appendage morphology. Proc Natl Acad Sci USA. 2009;106:13892–13896. doi: 10.1073/pnas.0903105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlopoulos A, et al. Probing the evolution of appendage specialization by Hox gene misexpression in an emerging model crustacean. Proc Natl Acad Sci USA. 2009;106:13897–13902. doi: 10.1073/pnas.0902804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern DL. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern DL. The Hox gene Ultrabithorax modulates the shape and size of the third leg of Drosophila by influencing diverse mechanisms. Dev Biol. 2003;256:355–366. doi: 10.1016/s0012-1606(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 19.Mahfooz N, Turchyn N, Mihajlovic M, Hrycaj S, Popadić A. Ubx regulates differential enlargement and diversification of insect hind legs. PLoS ONE. 2007;2:e866. doi: 10.1371/journal.pone.0000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khila A, Abouheif E, Rowe L. Evolution of a novel appendage ground plan in water striders is driven by changes in the Hox gene Ultrabithorax. PLoS Genet. 2009;5:e1000583. doi: 10.1371/journal.pgen.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- 22.Tomoyasu Y, Wheeler SR, Denell RE. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature. 2005;433:643–647. doi: 10.1038/nature03272. [DOI] [PubMed] [Google Scholar]
- 23.Prud'homme B, et al. Body plan innovation in treehoppers through the evolution of an extra wing-like appendage. Nature. 2011;473:83–86. doi: 10.1038/nature09977. [DOI] [PubMed] [Google Scholar]
- 24.Galant R, Carroll SB. Evolution of a transcriptional repression domain in an insect Hox protein. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- 25.Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- 26.Damen WGM, Hausdorf M, Seyfarth E-A, Tautz D. A conserved mode of head segmentation in arthropods revealed by the expression pattern of Hox genes in a spider. Proc Natl Acad Sci USA. 1998;95:10665–10670. doi: 10.1073/pnas.95.18.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwager EE, Pechmann M, Feitosa NM, McGregor AP, Damen WGM. hunchback functions as a segmentation gene in the spider Achaearanea tepidariorum. Curr Biol. 2009;19:1333–1340. doi: 10.1016/j.cub.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 28.Abzhanov A, Popadic A, Kaufman TC. Chelicerate Hox genes and the homology of arthropod segments. Evol Dev. 1999;1:77–89. doi: 10.1046/j.1525-142x.1999.99014.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwager EE, Schoppmeier M, Pechmann M, Damen WGM. Duplicated Hox genes in the spider Cupiennius salei. Front Zool. 2007;4:10. doi: 10.1186/1742-9994-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prpic NM, Janssen R, Wigand B, Klingler M, Damen WGM. Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling. Dev Biol. 2003;264:119–140. doi: 10.1016/j.ydbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Prpic NM, Damen WGM. Expression patterns of leg genes in the mouthparts of the spider Cupiennius salei (Chelicerata: Arachnida) Dev Genes Evol. 2004;214:296–302. doi: 10.1007/s00427-004-0393-5. [DOI] [PubMed] [Google Scholar]
- 32.Yao LC, Liaw GJ, Pai CY, Sun YH. A common mechanism for antenna-to-Leg transformation in Drosophila: Suppression of homothorax transcription by four HOM-C genes. Dev Biol. 1999;211:268–276. doi: 10.1006/dbio.1999.9309. [DOI] [PubMed] [Google Scholar]
- 33.Cohen B, Wimmer EA, Cohen SM. Early development of leg and wing primordia in the Drosophila embryo. Mech Dev. 1991;33:229–240. doi: 10.1016/0925-4773(91)90030-a. [DOI] [PubMed] [Google Scholar]
- 34.Mann RS. Engrailed-mediated repression of Ultrabithorax is necessary for the parasegment 6 identity in Drosophila. Development. 1994;120:3205–3212. doi: 10.1242/dev.120.11.3205. [DOI] [PubMed] [Google Scholar]
- 35.Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- 36.Castelli-Gair J, Akam M. How the Hox gene Ultrabithorax specifies two different segments: The significance of spatial and temporal regulation within metameres. Development. 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- 37.Akam M, et al. The evolving role of Hox genes in arthropods. Development. 1994;215:209–215. [PubMed] [Google Scholar]
- 38.Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev Cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 39.Palopoli MF, Patel NH. Evolution of the interaction between Hox genes and a downstream target. Curr Biol. 1998;8:587–590. doi: 10.1016/s0960-9822(98)70228-3. [DOI] [PubMed] [Google Scholar]
- 40.Lewis DL, DeCamillis M, Bennett RL. Distinct roles of the homeotic genes Ubx and abd-A in beetle embryonic abdominal appendage development. Proc Natl Acad Sci USA. 2000;97:4504–4509. doi: 10.1073/pnas.97.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abzhanov A, Kaufman TC. Crustacean (malacostracan) Hox genes and the evolution of the arthropod trunk. Development. 2000;127:2239–2249. doi: 10.1242/dev.127.11.2239. [DOI] [PubMed] [Google Scholar]
- 42.Hughes CL, Kaufman TC. Exploring the myriapod body plan: Expression patterns of the ten Hox genes in a centipede. Development. 2002;129:1225–1238. doi: 10.1242/dev.129.5.1225. [DOI] [PubMed] [Google Scholar]
- 43.Shiga Y, Yasumoto R, Yamagata H, Hayashi S. Evolving role of Antennapedia protein in arthropod limb patterning. Development. 2002;129:3555–3561. doi: 10.1242/dev.129.15.3555. [DOI] [PubMed] [Google Scholar]
- 44.Hsia CC, Paré AC, Hannon M, Ronshaugen M, McGinnis W. Silencing of an abdominal Hox gene during early development is correlated with limb development in a crustacean trunk. Evol Dev. 2010;12:131–143. doi: 10.1111/j.1525-142X.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- 46.Pechmann M, Prpic NM. Appendage patterning in the South American bird spider Acanthoscurria geniculata (Araneae: Mygalomorphae) Dev Genes Evol. 2009;219:189–198. doi: 10.1007/s00427-009-0279-7. [DOI] [PubMed] [Google Scholar]
- 47.Prpic NM, Schoppmeier M, Damen WGM. Whole-mount in situ hybridization of spider embryos. CSH Protoc. 2008;2008:t5068. doi: 10.1101/pdb.prot5068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.