Evolution is a quest for innovation. Organisms keep inventing new phenotypes to adapt to changing environments. Intuitively, adaptive processes are captured by Sewall Wright's picture of a population moving up a fitness landscape (1). At the molecular level, adaptation is carried by mutations with a selective advantage, which expand in the entire population and push it up a step in the landscape. Models of evolution often assume that adaptive steps are rare events in a population's history. An increasing amount of data, however, provides evidence of rapid adaptive processes, which are driven by a large supply of beneficial mutations. Important data sources include laboratory evolution experiments with microbes (2) as well as wild populations; a well-known example is the evolution of the seasonal human influenza virus (3). Understanding these data requires better theories of adaptation, at least for asexually reproducing populations. (Sex sometimes makes things simpler but, often, more difficult.) For example, given a supply of beneficial mutations, can we predict the speed by which a population moves up the fitness landscape? This task is surprisingly difficult, especially for large populations, where several beneficial mutations can be present at any point in time. The source of the difficulty is that rapid adaptation is a process far from equilibrium: Change is continuously favored over the present, and beneficial mutations outweigh deleterious ones (4). The paper by Good et al. (5) in PNAS is a significant advance in the theory of these dynamics.
The key insight of Good et al. (5) is to sharpen the question. They ask about the long-term survival chance of an individual: What is the probability that its descendants live in the far future of the population? This chance is limited by the risk that the individual's lineage becomes extinct at some point in the future. For neutral evolution (i.e., in a flat fitness landscape), classical theory shows that long-term survival is determined by random fluctuations of the reproductive process, i.e., genetic drift (6). In a given generation, each individual incurs a certain risk of remaining without offspring and becoming a dead end of evolution. Over many generations, these fluctuations accumulate and have a dramatic effect: The descendants of only one of today's individuals will make up the entire population, whereas the lineages descending from all other contemporary individuals will have gone extinct. The chance that the lucky lineage is yours is simply one over the number of individuals in today's population. This analysis is much more complicated for adaptive evolution, because there is more than one risk to long-term survival. An individual's long-term chance clearly depends on its fitness relative to the other individuals of the same generation. However, this chance also depends on the future beneficial mutations occurring in its lineage, as well as on the beneficial mutations in all other lineages. In other words, beneficial mutations in different lineages compete for fixation in the population, a process that is called interference. For a given lineage, interference creates a second, long-term risk: being outcompeted by others. The idea of interference goes back to classic work by Fisher (7) and Muller (8). It applies primarily to asexually reproducing populations that are large enough to harbor multiple beneficial mutations at a given point in time. However, interference also occurs in sexual populations whenever recombination is too slow to distribute beneficial mutations among all competing lineages (9).
Models of these dynamics use two different pictures of the adapting population. Both are based on a clever simplification: Instead of looking at the complete genealogy of the population, one combines all individuals of similar fitness into one cohort. Traveling-wave models (10–15) describe populations consisting of fitness cohorts with a bell-shaped distribution of sizes (Fig. 1A). The bulk of this fitness wave is deterministic and advances at a nearly constant speed. The tip of the wave, however, is stochastic. This is where the important interference between lineages takes place: Beneficial mutations from within the fittest cohorts seed new, even fitter lineages. Daniel Fisher (16) has created a beautiful metaphor of this dynamics: A dog of selection is led by its mutational nose. A second class of models (17–19), which I call interference theory, describes populations with fewer cohorts of rapidly changing size, most of which can seed a new, high-fitness lineage (Fig. 1B). Such fully stochastic waves push toward higher fitness at a more irregular speed (19). They resemble an entire pack of dogs, each sniffing for itself and trying to outrun the others. Which of these pictures is more appropriate depends on the evolutionary parameters of the system and must be decided by measurements.
Fig. 1.
Fitness distributions of adapting populations. (A) Traveling wave with a deterministic, bell-shaped bulk and a stochastic tip. An interference threshold (dashed line) separates fitness cohorts dominated by genetic drift from cohorts dominated by interference. Beneficial mutations (arrows) from high-fitness cohorts seed new, even fitter cohorts. The wave moves at a nearly constant speed toward higher fitness. (B) Fully stochastic fitness wave with few cohorts of rapidly changing size. Beneficial mutations (arrows) seeding new, high-fitness cohorts can arise from anywhere in the population. The wave moves at an irregular speed toward higher fitness.
Good et al. (5) use a traveling-wave model for their analysis. They are able to compute the long-term chances of individuals in asexual populations, given the joint risk of interference and genetic drift. In cases where the actual wave is fully stochastic, their method can be understood as a “mean-field” approximation obtained by averaging over an ensemble of populations. Chances and risks turn out to be distributed in a very undemocratic way. Individuals in the highest fitness cohorts have the largest chances, which are limited only by genetic drift. An interference threshold (dashed line in Fig. 1A) separates them from the majority of individuals, which have much smaller chances set by interference interactions.
The experimentally most relevant outcome of this analysis is the fixation statistics of mutations. This result is in remarkable agreement with recent results from interference theory (19), indicating that it is a robust feature of interference processes independent of specific assumptions in the traveling-wave analysis. Only the strongest beneficial mutations have a fixation probability determined by genetic drift; this probability is proportional to their selection coefficient. All mutations with effect smaller than some threshold are effectively neutral: Their fixation probability is about one over the population size, independently of their effect. However, this probability emerges from interference interactions rather than from genetic drift. Thus, strong selection can generate neutral behavior, a conclusion that may seem paradoxical at first glance.
Depending on the strength of interference, effective neutrality may well affect a substantial part of the mutations, resulting in an overall substitution rate that depends only weakly on population size. This feature has often been taken as evidence for near-neutral evolution. However, at least in asexual populations, it can signal quite the contrary: fierce interference selection. The substitution rate of
Strong selection can generate neutral behavior, a conclusion that may seem paradoxical at first glance.
beneficial mutations with a given selection coefficient shows an even more striking dependence on population size: In smaller populations, this rate is limited by genetic drift and increases with population size, as predicted by classical theory (20). In larger populations, the same rate is limited by interference and decreases with increasing population size. Importantly, these effects may by testable by evolution experiments.
The strong pruning of mutations by interference leads Good et al. (5) to a further interesting conclusion. They argue for a degree of universality in the evolutionary process: Systems with different “microscopic” characteristics, such as the effect distribution of new mutations, 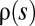 , can generate traveling fitness waves with the same width, speed, and interference threshold (Fig. 1A). They show, in particular, that these universal characteristics can be reproduced by an effective theory in which all new mutations have the same selection coefficient. Other “macroscopic” aspects are nonuniversal; that is, they depend on details of the distribution
, can generate traveling fitness waves with the same width, speed, and interference threshold (Fig. 1A). They show, in particular, that these universal characteristics can be reproduced by an effective theory in which all new mutations have the same selection coefficient. Other “macroscopic” aspects are nonuniversal; that is, they depend on details of the distribution 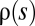 . For example, the effect distribution of fixed mutations is simply proportional to
. For example, the effect distribution of fixed mutations is simply proportional to 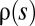 in the effective-neutrality regime. Universality is a principle from statistical physics. I believe its applicability in population genetics is a subtle issue, because there is no clear-cut separation between a microscopic level of interactions and a macroscopic level of observations. Hence, to determine what is and what is not universal in adaptive evolution is a challenge for future research.
in the effective-neutrality regime. Universality is a principle from statistical physics. I believe its applicability in population genetics is a subtle issue, because there is no clear-cut separation between a microscopic level of interactions and a macroscopic level of observations. Hence, to determine what is and what is not universal in adaptive evolution is a challenge for future research.
Footnotes
The author declares no conflict of interest.
See companion article on page 4950.
References
- 1.Wright S. The roles of mutation, inbreeding, crossbreeding, and selection in evolution. Proceedings of the Sixth International Congress on Genetics. 1932. pp. 355–366. Available at http://www.blackwellpublishing.com/ridley/classictexts/wright.pdf. Accessed March 2, 2012.
- 2.Barrick JE, et al. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature. 2009;461:1243–1247. doi: 10.1038/nature08480. [DOI] [PubMed] [Google Scholar]
- 3.Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999;286:1921–1925. doi: 10.1126/science.286.5446.1921. [DOI] [PubMed] [Google Scholar]
- 4.Mustonen V, Lässig M. From fitness landscapes to seascapes: Non-equilibrium dynamics of selection and adaptation. Trends Genet. 2009;25:111–119. doi: 10.1016/j.tig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Good BH, Rouzine IM, Balick DJ, Hallatschek O, Desai MM. Distribution of fixed beneficial mutations and the rate of adaptation in asexual populations. Proc Natl Acad Sci USA. 2012;109:4950–4955. doi: 10.1073/pnas.1119910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimura M. On the probability of fixation of mutant genes in a population. Genetics. 1962;47:713–719. doi: 10.1093/genetics/47.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher RA. In: The Genetical Theory of Natural Selection: A Complete Variorum Edition. Bennet H, editor. Oxford, UK: Oxford Univ Press; 2000. [Google Scholar]
- 8.Muller H. Some genetic aspects of sex. Am Nat. 1932;66:118–138. [Google Scholar]
- 9.Hill W, Robertson A. The effect of linkage on limits to artificial selection. Genet Res. 1966;8:269–294. [PubMed] [Google Scholar]
- 10.Tsimring LS, Levine H, Kessler DA. RNA virus evolution via a fitness-space model. Phys Rev Lett. 1996;76:4440–4443. doi: 10.1103/PhysRevLett.76.4440. [DOI] [PubMed] [Google Scholar]
- 11.Rouzine IM, Wakeley J, Coffin JM. The solitary wave of asexual evolution. Proc Natl Acad Sci USA. 2003;100:587–592. doi: 10.1073/pnas.242719299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen E, Kessler DA, Levine H. Front propagation up a reaction rate gradient. Phys Rev E. 2005;72:066126. doi: 10.1103/PhysRevE.72.066126. [DOI] [PubMed] [Google Scholar]
- 13.Desai MM, Fisher DS. Beneficial mutation selection balance and the effect of linkage on positive selection. Genetics. 2007;176:1759–1798. doi: 10.1534/genetics.106.067678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouzine IM, Brunet E, Wilke CO. The traveling-wave approach to asexual evolution: Muller's ratchet and speed of adaptation. Theor Popul Biol. 2008;73:24–46. doi: 10.1016/j.tpb.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallatschek O. The noisy edge of traveling waves. Proc Natl Acad Sci USA. 2011;108:1783–1787. doi: 10.1073/pnas.1013529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher DS. Leading the dog of selection by its mutational nose. Proc Natl Acad Sci USA. 2011;108:2633–2634. doi: 10.1073/pnas.1100339108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerrish PJ, Lenski RE. The fate of competing beneficial mutations in an asexual population. Genetica. 1998;102–103:127–144. [PubMed] [Google Scholar]
- 18.Wilke CO. The speed of adaptation in large asexual populations. Genetics. 2004;167:2045–2053. doi: 10.1534/genetics.104.027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffels S, Szöllosi GJ, Mustonen V, Lässig M. Emergent neutrality in adaptive asexual evolution. Genetics. 2011;189:1361–1375. doi: 10.1534/genetics.111.132027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haldane J. A mathematical theory of natural and artificial selection. Part V: Selection and mutation. Proc Camb Philos Soc. 1927;23:828–844. [Google Scholar]



