Abstract
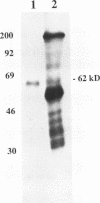
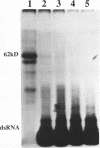
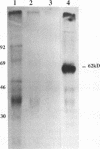
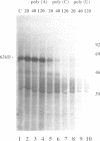
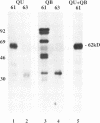
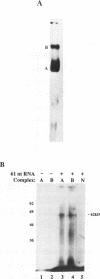
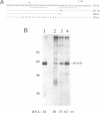
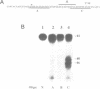
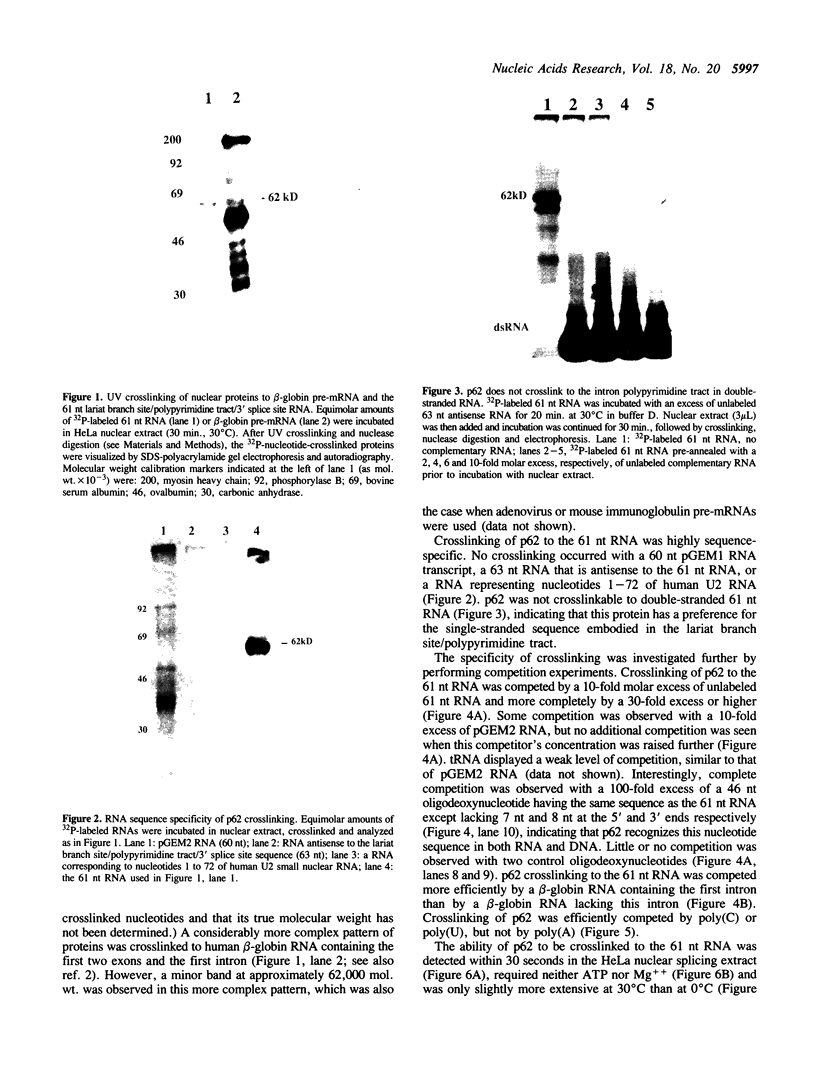
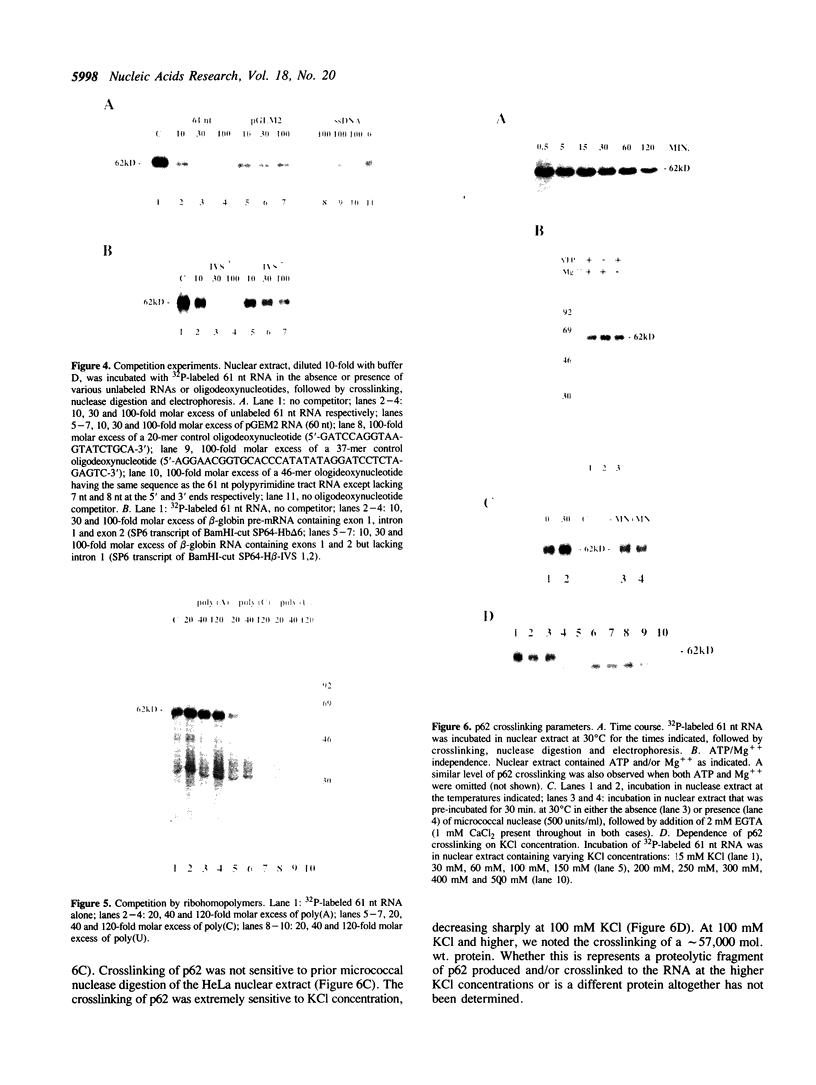
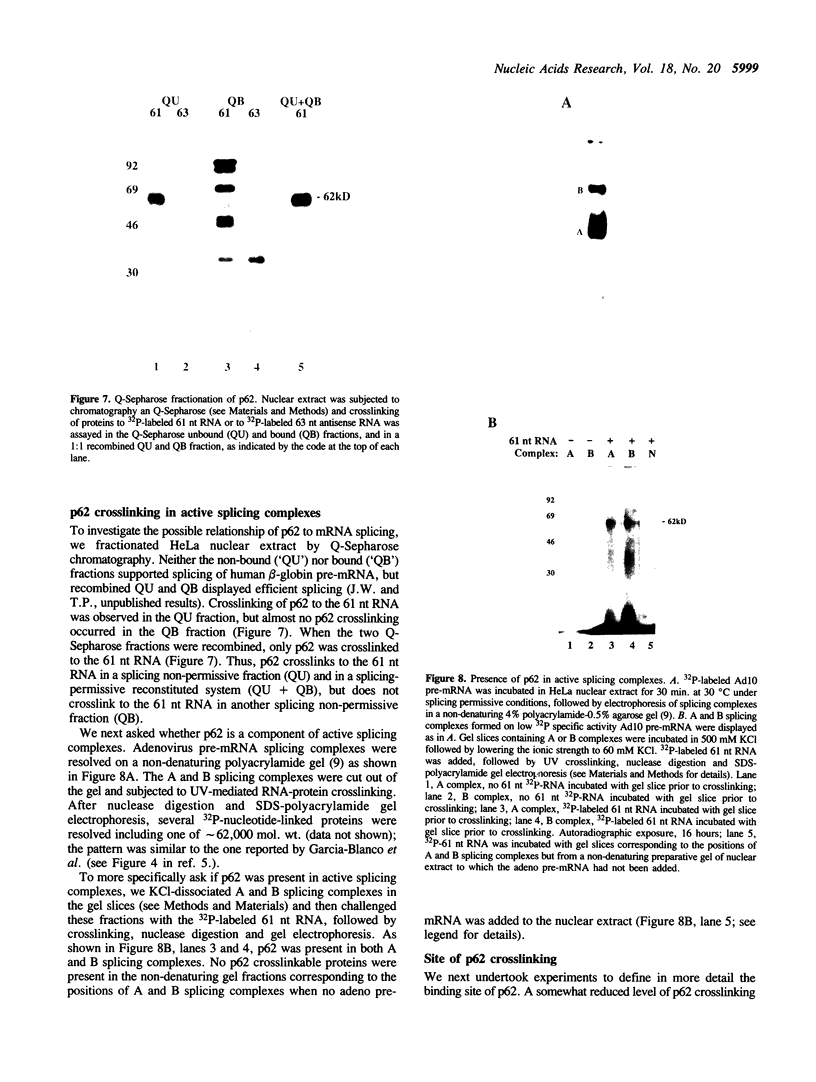
Incubation in HeLa nuclear extract of a 32P-labeled 61 nucleotide-long RNA corresponding to the lariat branch site/polypyrimidine tract/3' splice site of the first intron of human beta-globin pre-mRNA led to the crosslinking of a single protein of approximately 62,000 mol. wt. (p62). p62 corresponds to a polypyrimidine tract-binding protein recently described by Garcia-Blanco et al. (Genes & Dev. 3: 1874-1886, 1989). Crosslinking of p62 to the 61 nt RNA was highly sequence specific. No p62 crosslinking was observed with a 60 nt pGEM vector RNA, a 63 nt RNA antisense to the 61-mer or a 72 nt U2 RNA sequence. p62 crosslinking to the 61 nt RNA was competed by unlabeled 61 nt RNA, by beta-globin pre-mRNA containing intron 1, and by poly(U) and poly(C), but was competed to a lesser extent or not at all by pGEM RNA, a beta-globin RNA lacking intron 1, or poly(A). Experiments with mutated RNAs revealed that neither the lariat branch site adenosine nor the 3' splice site were required for p62 crosslinking to polypyrimidine tract-containing RNA. Elimination of the polypyrimidine tract reduced p62 crosslinking, as did mutation of a polypyrimidine tract UU dinucleotide to GA. However, replacement of a pyrimidine-rich tract immediately adjacent (3') to the lariat branch site with a 57% A + G pGEM vector RNA sequence also significantly reduced p62 crosslinking, indicating the involvement of both this pyrimidine-rich region and the classical polypyrimidine tract adjacent to the 3' splice site. The sites of protein interaction were further defined by RNase H protection experiments, the results of which confirmed the patterns of p62 crosslinking to mutant RNAs. p62 crosslinking was efficiently competed by a DNA oligonucleotide having the same sequence as the 61 nt RNA, showing that p62 requires neither ribose 2' OH groups nor uracil bases for its interaction with the polypyrimidine tract. p62 was not crosslinked to double-stranded 61 nt RNA. Q-Sepharose chromatography of HeLa nuclear extract yielded an unbound fraction (QU) in which p62 was the only polypyrimidine tract-crosslinkable protein and a bound fraction (QB) in which, surprisingly, several non-p62 proteins were crosslinkable to the polypyrimidine tract RNA. Yet, when the two Q-Sepharose fractions were combined, p62 strongly out-competed the otherwise-crosslinkable QB proteins for polypyrimidine tract RNA crosslinking. This indicates that p62 may have the highest affinity and/or crosslinking efficiency for the intron polypyrimidine tract of any HeLa nuclear protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvet J. P., Pederson T. Secondary structure of heterogeneous nuclear RNA: two classes of double-stranded RNA in native ribonucleoprotein. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3705–3709. doi: 10.1073/pnas.74.9.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. In vitro assembly of a pre-messenger ribonucleoprotein. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4296–4300. doi: 10.1073/pnas.80.14.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Blanco M. A., Jamison S. F., Sharp P. A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1989 Dec;3(12A):1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- Gerke V., Steitz J. A. A protein associated with small nuclear ribonucleoprotein particles recognizes the 3' splice site of premessenger RNA. Cell. 1986 Dec 26;47(6):973–984. doi: 10.1016/0092-8674(86)90812-3. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Marciniak R. A., Garcia-Blanco M. A., Sharp P. A. Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 May;87(9):3624–3628. doi: 10.1073/pnas.87.9.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S. H., Pedersen N., Pederson T. Identification of proteins that bind tightly to pre-mRNA during in vitro splicing. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3718–3722. doi: 10.1073/pnas.83.11.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S. H., Pederson T. Crosslinking of hnRNP proteins to pre-mRNA requires U1 and U2 snRNPs. Nucleic Acids Res. 1990 Jun 11;18(11):3307–3318. doi: 10.1093/nar/18.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Gotoh Y. Signals for the selection of a splice site in pre-mRNA. Computer analysis of splice junction sequences and like sequences. J Mol Biol. 1987 May 20;195(2):247–259. doi: 10.1016/0022-2836(87)90647-4. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Zamore P. D., Green M. R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988 Jan 29;52(2):207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. RNA binding specificity of hnRNP proteins: a subset bind to the 3' end of introns. EMBO J. 1988 Nov;7(11):3519–3529. doi: 10.1002/j.1460-2075.1988.tb03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J., Alibert C., Temsamani J., Reveillaud I., Cathala G., Brunel C., Jeanteur P. A protein that specifically recognizes the 3' splice site of mammalian pre-mRNA introns is associated with a small nuclear ribonucleoprotein. Cell. 1986 Dec 5;47(5):755–766. doi: 10.1016/0092-8674(86)90518-0. [DOI] [PubMed] [Google Scholar]
- Teare J., Wollenzien P. L. Structures of human and rabbit beta-globin precursor messenger RNAs in solution. Biochemistry. 1989 Jul 25;28(15):6208–6219. doi: 10.1021/bi00441a012. [DOI] [PubMed] [Google Scholar]
- Teare J., Wollenzien P. The structure of a pre-mRNA molecule in solution determined with a site directed cross-linking reagent. Nucleic Acids Res. 1990 Feb 25;18(4):855–864. doi: 10.1093/nar/18.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]