Abstract
Contextual representations serve to guide many aspects of behavior and influence the way stimuli or actions are encoded and interpreted. The medial prefrontal cortex (mPFC), including the anterior cingulate subregion, has been implicated in contextual encoding, yet the nature of contextual representations formed by the mPFC is unclear. Using multiple single-unit tetrode recordings in rats, we found that different activity patterns emerged in mPFC ensembles when animals moved between different environmental contexts. These differences in activity patterns were significantly larger than those observed for hippocampal ensembles. Whereas ≈11% of mPFC cells consistently preferred one environment over the other across multiple exposures to the same environments, optimal decoding (prediction) of the environmental setting occurred when the activity of up to ≈50% of all mPFC neurons was taken into account. On the other hand, population activity patterns were not identical upon repeated exposures to the very same environment. This was partly because the state of mPFC ensembles seemed to systematically shift with time, such that we could sometimes predict the change in ensemble state upon later reentry into one environment according to linear extrapolation from the time-dependent shifts observed during the first exposure. We also observed that many strongly action-selective mPFC neurons exhibited a significant degree of context-dependent modulation. These results highlight potential differences in contextual encoding schemes by the mPFC and hippocampus and suggest that the mPFC forms rich contextual representations that take into account not only sensory cues but also actions and time.
Keywords: electrophysiological recordings, population analysis, temporal encoding
Many aspects of behavior are contextually dependent. Contexts can be abstract, as in the case of human language, or can be more concrete and be defined by the constellation of spatial and sensory stimuli surrounding an individual. Contextual representations can form quickly and tend to remain stable despite local variations in perspective (1). Context can also help to inform the subject about potential changes in the meaning of stimuli and actions. The medial prefrontal cortex (mPFC) is involved in various forms of cognition that depend on spatial and contextual information, including context-based cognitive tasks (2–4), contextual fear conditioning (5), and context-induced drug relapse (6–8), and is believed to be an important node in the “context” network (1, 9–12). These functions likely depend on reciprocal interactions with the hippocampus (13, 14), an area that has a well-established role in spatial and contextual processing (15–20). Through the connection from the CA1 region, the hippocampus has multiple profound effects on mPFC neurons (21–28), and it has been proposed that during spatial navigation the mPFC may work in concert with the hippocampus to encode goal locations and aid in route planning (29, 30). However, the spatial representations formed by the mPFC are different from those formed by the hippocampus: mPFC neurons do not seem to have clear place fields and presumably do not form the same sort of spatial map that would inform the animal precisely where it is within an environmental context (31–34). Accordingly, lesions of the mPFC do not prevent rats from being able to navigate through even quite complex spatial environments, like mazes (35). However, mPFC lesions do make hippocampal unit place fields less stable over time and more reactive to changes in the local environment (36, 37). This result is consistent with the idea that the PFC might provide a more global representation of the spatial context (1, 38–40) that is independent of the subject's exact perspective.
The present study focused on how mPFC might encode whole contextual settings and how these types of representations may differ from those of the hippocampus. Toward these ends, we recorded multiple single units using 16 tetrodes implanted into the mPFC or hippocampus while animals were switched between two distinct environments. Several potential factors were considered that could contribute to differences in activity state patterns across contexts, including differences in sensory cues, differences in movement patterns, and the passage of time. Finally, given the important role of the mPFC in action monitoring and encoding (29, 32, 41, 42), we investigated whether context affected the way specific actions were represented.
Results
Different Environments Are Associated with Different Ensemble Activity Patterns in mPFC.
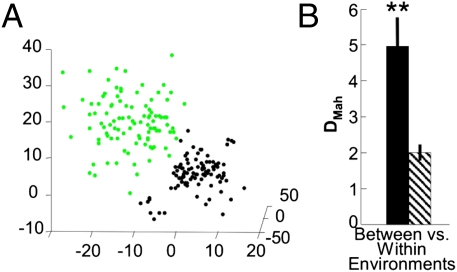
We first investigated how mPFC networks represented environmental context by recording the activity of 26–109 neurons per animal (total of five animals) in the anterior cingulate cortex subregion as animals were simply placed in two different novel environments (“A” and “B,” Fig. S1A) and allowed to freely explore. The instantaneous firing rates (iFR) of all recorded neurons at each 500-ms time bin were collected in a population vector, and the space populated by all these vectors is termed the multiple single-unit activity (MSUA) space (Methods). A 3D projection of the MSUA space, obtained by multidimensional scaling (MDS), in which each point represents the state of the entire recorded ensemble within one 500-ms bin, is shown in Fig. 1A. All points corresponding to different 500-ms bins within the same environment are shown in the same color. MDS was used purely for visualization, and all statistical analyses were performed in the full space of all recorded units (43). The MSUA space representation shown in Fig. 1A depicts the state of an mPFC ensemble for a 6 min, 40-s period when a rat was switched from one environment to another. The black dots represent the final 3 min and 20 s in environment A, and the green dots represent the first 3 min and 20 s in environment B. The differently colored dots corresponding to the times spent in each unique environment tightly clustered within distinct regions of MSUA space, indicating that different environments were associated with different patterns of activity across the population of recorded units. To confirm these visual observations statistically, the Mahalanobis distances between the two clusters of iFR vectors in the full MSUA space, corresponding to the times in each environment, were calculated. When Mahalanobis distances were computed between sets of iFR vectors from different environments, the differentiation between neural ensemble states was approximately two to three times larger compared with ensemble states taken from the same environment but separated by the same amount of time as for the between-environment comparison [t(9) = 4.98; P < 10−5; Wilcoxon signed rank = 0; P < 10−4; Fig. 1B; Fig. S1C is an illustration of the analysis]. The single-cell basis for these population effects will be investigated in greater detail below.
Fig. 1.
Representation of environmental context during free exploration. (A) 3D projection of MSUA spaces for free exploration sessions using novel environments. Dots (population vectors) are colored according to whether they were taken from environment A (black) or B (green). The axes of these 3D projections correspond to different combinations of the single-unit firing rates, as derived by MDS. (B) Mean Mahalanobis distances comparing groups of points within one environment vs. between two environments for mPFC ensembles. Mean values are from all subjects and sessions (paired t tests and Wilcoxon signed rank tests; **P < 10−5; error bars = SEM; n = 7 sessions from five animals).
These results were not due to a general, nonspecific dampening or enhancement of the overall population activity (SI Results). Nevertheless, movement paths were different (Fig. S2C) and could contribute to the ensemble separation, consistent with past studies (44, 45). However, we still observed a significant separation between the clouds of iFR points in the 3D projection of the MSUA space associated with two environments, even when movement effects were controlled by either restricting movement altogether (Fig. S3 A and B) or by enforcing similar movement patterns in the two environments (Fig. S3 C and D). Finally a significant differentiation between ensemble states was also observed if the contexts were familiar rather than novel (Fig. S4 A–C) or if the rats moved between environments by themselves rather than being carried over by hand (Fig. S4 D and E).
Contrasting mPFC with Hippocampal Representations.
Because hippocampal neurons are strongly involved in the representation of spatial context (8–13), we decided to contrast the results described above for mPFC ensembles with the results of similar experiments performed in rats with tetrode arrays implanted into the CA1 region of the hippocampus (n = 85 neurons from two rats during three sessions). For the mPFC ensemble shown in Fig. 2A (Lower), there was some separation between the clouds of iFR points in the 3D projection of the MSUA space corresponding to the two different spatial locations within one environment demarcated in Fig. 2A (Upper) (black vs. gray and dark green vs. light green). Fig. 2A also shows that both location representations seemed to shift in the MSUA space from one exposure to the next. Accordingly in the group data, a two-way ANOVA found significant main effects for both recording location [mPFC or HPC; F(1,23) = 114.38; P < 10−9] and exposure [between first and second exposure vs. within a single exposure; F(1,23) = 86.57; P < 10−7], as well as a significant interaction effect [F(1,23) = 221.81; P < 10−15]. Tukey's post hoc tests revealed that for mPFC ensembles the Mahalanobis distance between the clusters of points representing the same location on two subsequent exposures (black bars, Fig. 2C) was much larger than the distance between the clusters of points representing two locations during one exposure (striped bars, Fig. 2C). In stark contrast, post hoc tests indicated that for hippocampal ensembles (Fig. 2B) the Mahalanobis distance between the clusters of points representing two locations during one exposure (striped bars, Fig. 2C) was significantly larger than the distance between the clusters of points representing the same location on two subsequent exposures (black bars, Fig. 2C). When directly compared, mPFC ensembles exhibited significantly greater separation in their representation of a single location between two exposures to the same environment relative to hippocampal ensembles (black bars, Fig. 2C). In contrast, post hoc tests also confirmed that hippocampal ensembles exhibited significantly larger separation in their representation of two distinct locations within an environment on a single exposure relative to mPFC ensembles (striped bars, Fig. 2C). Thus, although hippocampal ensembles differentiated much more strongly between two spatial locations within a given environment than between two different exposures to the same environment, mPFC ensembles in contrast strongly differentiated between two successive exposures but much less between two spatial locations within the same exposure and setting (further analyses of mPFC–hippocampal differences in SI Results).
Fig. 2.
Comparison of mPFC and hippocampal ensemble representations and temporal context effects. (A) mPFC ensemble encoding of locations within a single environment on two different exposures. Upper: Animals were exposed to one environment on two occasions. Dots denote the location of the rat in the environment in 500-ms time bins. Black dots correspond to the time bins when the rat was in the NE corner during first exposure, and dark green dots correspond to the time bins when the rat was in the same corner of the same environment on the second exposure. Gray dots correspond to the time bins when the rat was in the SW corner during first exposure, and light green dots to those when the rat was in the same corner of the same environment on the second exposure. Red dots correspond to all other time bins for all other locations that were not considered in the present analysis. Lower: MSUA space representation for the ensemble of mPFC neurons recorded during the same session. Dots correspond to the state of the network in MSUA space in 500-ms bins and are colored according to the time bins in the location map above. (B) Hippocampal ensemble encoding of locations within a single environment on two different exposures. Upper: Dots denote the location of the rat in the environment in 500-ms time bins, based on the same color scheme as used in A. Lower: MSUA space for the ensemble of hippocampal neurons recorded during the same session. Dots correspond to the state of the network in MSUA space in 500-ms bins and are colored according to the time bins in the location map above, as in A. (C) Mean Mahalanobis distances in MSUA space comparing time bins when subjects were in the same location in the same environment on two different exposures (black bars) vs. different locations in the same environment on a single exposure (striped bars). Data for mPFC ensembles are shown at left, and data for hippocampal (HPC) ensembles at right (**P < 0.0001; error bars = SEM; n = 24 data points from 6 sessions from five animals). Note that mPFC population vectors differentiated more between the two exposures for the same location, whereas hippocampal ensembles differentiated more strongly between different locations during a single exposure.
Time-on-Task Affects mPFC Ensemble Activity.
The lack of consistency in the representation of spatial locations in mPFC activity across repeated exposures to the same environmental setting suggests that other factors, such as time, may underlie the strong environmental context effects. To examine the impact of time, we analyzed Mahalanobis distances between sets of iFR population vectors as a function of both temporal separation and according to whether these iFR vector sets were drawn from the same or different environments (Fig. 3A). As Fig. 3A shows, the MSUA space separation between any two sets of population vectors indeed increases as the temporal spacing between these two sets becomes larger. However, at the same time, along the whole time-axis in Fig. 3A there is a clear substantial increase in the MSUA space distances when comparing population vectors from the same vs. different environments even for the very same amount of temporal separation (i.e., when time is removed as a potential confound). To formally address the impact of time vs. environment, we fitted a simple linear model to the sets of MSUA space distances Dmah = β0 + β1Envt + β2Δt, where Δt is the amount of temporal separation between MSUA vector sets, and the dummy variable Envt ∈ {−1, +1} encodes whether environment A or B was presented. For the mPFC data sets we checked (via F ratios; SI Methods) whether each of the two factors (environment, time) on its own (with the other factor not included) explains a significant amount of variation in the MSUA space distances and whether it significantly adds to the amount of explained variance when the respective other factor was already in the model. A factor was considered significant only if it survived both of these statistical comparisons. In three of six data sets, both environment and time significantly contributed on their own and in conjunction with the respective other factor to the amount of Dmah variation (all P < 10−3). In one other data set only the environment factor reached significance, whereas in the remaining two only the time factor was significant (all P < 10−6).
Fig. 3.
Impact of temporal context on mPFC ensemble activity. (A) Mean Mahalanobis distance, from an example session, between sets of population vectors drawn from within the same (A-A, A′-A′, B-B, B′-B′; green line) or from between different (A-B, A′-B′; red line) environments (hyphen indicates repetition), and for different amounts of temporal separation (Δt). MSUA space distances increase with both temporal and environmental difference. Shaded areas indicate SEM. Transients at the start or end of environmental exposures were removed (SI Methods). (B) Example session illustrating the impact of time and environment on Mahalanobis distances between iFR sets taken from within the same (green) or between different (blue) repetitions of the same environment (see main text for explanation). Shaded areas = SEM. The green regression line indicates the predictions from the time/environment-model fit to the within-repetition distances (i.e., to the data in A).
We next used the model-based approach to determine whether the gradual time-dependent shifts could explain the shift in mPFC representations upon multiple repetitions of the same environment (Fig. 2A). To this end, the linear model above was fitted to the mean within-repetition distances and then applied to the mean between-repetition distances. That is, if A, B denote the two environments and A′, B′ their repetitions, the model was first fit to distances between vector sets taken from A only, B only, A′ only, or B′ only (within-environment), and from A-B or A′-B′ (between-environment). The parameters were then fixed, and it was determined how well this model predicted distances for comparisons A-A′, B-B′ (between-repetition but within-environment; Fig. 3B) and from A-B′, A′-B (between-repetition and between-environment). For three of the six data sets with repeated exposures, approximately 50–75% of the variance of the mean between-repetition distances (with transients removed) could be accounted for by the model obtained from the mean within-repetition distances (all P < 10−5). This confirms that for at least half of the data sets a considerable proportion of the iFR activity shifts across repetitions were due to timing effects.
Consistency of Single-Unit Responses upon Repeat Visits to the Same Environmental Context.
All of the analyses above involved population activity patterns. We next analyzed the environmental selectivity (Methods) of single mPFC neurons over multiple exposures to two different environments (A and B). To limit differences in movement across environments as much as possible, animals were placed in a small plastic chamber (Fig. S1B shows experimental details) that was transferred back and forth between the environments five times. When we examined the environmental selectivity across the five different A-B exposures we found that a small but significant number of neurons [11%, binomial P < 0.03; χ2(5) = 15.57, P < 0.007; Fig. S6F] consistently preferred one environment vs. the other across all five A-B switches. However, this approach of determining selectivity may be quite conservative because it uses only binary information (A > B or B > A) from each cell and exposure, with firing rates averaged across whole exposure periods. Thus, as a more sensitive approach that takes into account firing rate variations on a time-bin by time-bin basis within each environment, a decoding analysis based on linear classifiers was carried out. To these ends, for each data set a linear discriminant function was fitted to the first three environment A-B repetitions (the “training set”) to determine a hyperplane in MSUA space that optimally separated the two environments (Fig. S6G). Prediction performance of this classifier was then tested on population vectors from the two remaining (fourth and fifth) A-B repetitions (the “test set”), that is on trials that had not been used for fitting the classifier (see ref. 46). iFR population vectors from the test set falling onto the wrong side of the separating hyperplane obtained from the training set predict environmental setting incorrectly and thus contribute to the (predicted) separation error. Using this classification approach, neuronal samples were built starting with the most responsive units and then progressively adding neurons until all units were incorporated in the (sub)population used for prediction. Fig. S6H shows that decoding performance improved as more neurons were added, up to the point where approximately 50% of all units were included in the classifier, after which prediction performance started to degrade again (owing to the increase in noise as the sample is further expanded). At the point of optimal prediction performance, ≈84% of iFR vectors from the test trials were correctly assigned, and the largest Mahalanobis distances between context groups were observed. For this optimally predictive sample of size of ≈50%, the separation error was significantly lower than the one in nonparametric bootstraps (P < 0.01; constructed by randomly shuffling blocks of iFR vectors of length 10 s from different environments or exposures; details in SI Methods), and it was also significantly smaller than in samples consisting of ≈10% of the maximally responsive units [that is, about the relative proportion of units that was found to express consistent environmental selectivity in the binomial analysis above; t(4) = 5.13; P = 0.007, Bonferroni-corrected P < 0.027]. Thus, although many units may not consistently prefer one environment over the other according to firing rates averaged across whole exposures, many neurons may still contribute to environmental coding at specific time points during the exposures.
Context Affects the Way in Which Actions Are Encoded by mPFC Neurons and Networks.
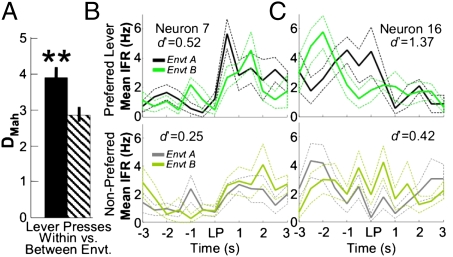
Finally we examined how contextual information affected action encoding by mPFC neurons. For this analysis we had rats perform an operant-based continuous alternation task in two environments (Fig. S1A). In this case, we found that there was a larger Mahalanobis distance between presses made on the same lever across the two environments than between left and right lever presses performed within the same environment [t(9) = 4.98, P < 0.01; Wilcoxon signed rank test, P < 0.01; Fig. 4A]. This result suggests that environmental context may have a strong impact on how lever press actions are encoded at the ensemble level.
Fig. 4.
Environmental context encoding by “lever press-responsive” neurons. (A) Mahalanobis distances between lever presses made on the same lever in two environments (black) vs. lever presses made on two different levers in one environment (striped) (n = 3 sessions from three animals). (B and C) Contextual modulation of lever press-related neuronal activity. Upper: Lever presses on the preferred lever are plotted for both environment A (black) and B (green). Lower: Nonpreferred lever responses in the two environments (A, gray and B, light green). Solid lines show mean iFR and dotted lines SEM. Inset numbers indicate cell number (corresponding to Fig. S7B) and |d′| values for these neurons.
At the single-neuron level, the selectivities of individual neurons for left vs. right lever presses in environment A vs. B were significantly correlated overall (r = 0.44, P < 0.001; Fig. S7A), yet the strength of the correlation was not too high, suggesting that some degree of contextual modulation may be present. Lever press-responsive neurons were defined as those that had an absolute selectivity score of at least |d′| > 0.5 for the lever press period (on either the right or left levers) vs. the intertrial interval. Of the 107 total neurons in this sample, 36 were deemed “lever-press responsive” using these criteria. In total, 33% (12 of 36) of lever press-selective neurons fired consistently more to lever presses in one environment vs. the other (SI Results). Responses to a given lever press could differ both in terms of magnitude (Fig. 4B and Fig. S7B) or timing (Fig. 4C and Fig. S7B) if performed in environment A vs. B. These analyses show that environmental context affects the profile of the lever press responses and that a significant proportion of neurons responsive to lever presses were also selective for one of the two environments.
Discussion
The present study showed that the activity of ensembles of mPFC neurons contains information about entire environmental contexts or changes in context, whereas hippocampal ensembles tended to provide more consistent and specific representations of locations within a given context. Contextual differentiation by mPFC ensembles was based on a number of factors, including differences in the sensory attributes that define an environment, time, and as we explain below, movement patterns. Context in turn affected the way in which lever press actions were encoded at the ensemble and single-neuron level.
Given the role of the PFC in spatially based cognition and the strong input the mPFC receives from the hippocampus (both directly and indirectly via the mediodorsal thalamus) (13, 14), it seemed logical to expect that mPFC neuronal responses would be at least in part affected by information about the rat's spatial context. In addition, the mPFC plays a key role in context-based cognitive tasks (2, 3) and context-induced drug relapse (6–8), and is believed to be an important node in the “context” network (1, 9–12). However, despite numerous attempts, studies have consistently failed to find any clear location-specific mPFC firing patterns that are independent of behavior (i.e., place cells) (31–34). The present data were largely consistent with these studies in that mPFC activity was more affected by changes in environmental context on a large scale rather than by the rat's precise spatial location. In contrast, the activity of hippocampal ensembles depended more on the unique constellation of place cells that provided location-specific information rather than on overall environmental context, although significant discrimination between whole contexts was also present at the hippocampal level (compare Fig. S5).
Various factors impacted the manner in which mPFC neurons and ensembles responded to context. First, ≈11% of individual mPFC neurons maintained a stable preference for a given environment across all five exposures in the restricted-movement sessions, indicating that they may be consistently responding to certain sensory features of a given environment. However, more sensitive decoding analysis revealed that in fact up to ≈50% of neurons may contribute to the encoding of environmental context. Second, although separations in MSUA space were still detectable in the restricted movement condition, the separations were smaller than those observed for the free exploration sessions (Fig. S3 A and B). Likewise, the enforced similarity in movement patterns created by the continuous alternation task reduced the separation in MSUA space (Fig. S3 C and D). Therefore, consistent with the conclusions of past studies (44, 45), different movement patterns associated with exploration of different environments also impacted mPFC activity. Finally, the activity state patterns were not static in a given environment but seemed to systematically drift over time, suggesting that mPFC ensembles may also encode “time-on-task” as part of their contextual representation (Fig. 3). In fact, by linearly extrapolating from the changes in MSUA space distances produced by changes in temporal separation, it was possible in some data sets to predict how far the network state would have moved when the animal returned to the same environment at a later point in time. This constant temporal movement of the network state could therefore explain to some degree why different exposures to the very same environment were associated with quite different population activity states. Several recent reports have shown similar time-related encoding by hippocampal cells and ensembles (47–49). Although we could also confirm significant temporal movement in our hippocampal ensembles within a given environment (compare Fig. S5 A and B) that was not significantly different from the one seen in mPFC (compare Fig. S5C), in contrast to the mPFC there seemed to be little or no temporal shift in network state across repetitions of the same environment (compare Fig. 2), and hence population representations tended to be more similar on repeated exposures in hippocampus. Collectively these data suggest that for the mPFC “context” may depend not only on sensory features of the environment but also the animal's actions and the passage of time.
Action representations were in turn influenced by the context in which they were performed. Of all of the neurons that responded to lever presses during the continuous alternation task, we found that ≈33% had a consistent preference for one environment over another. In the present study, these actions had the same meaning in both environmental contexts because the animals were performing the same continuous alternation task. Recently we found that a change in the task rule in the same physical environment induces a sudden and profound change in network activity state within the mPFC (50). Perhaps if the task context changed along with the environmental context (e.g., the same actions took on a different meaning in a different environment), a larger shift in the representations of actions might have been observed. In this case, the context information could have been exploited to trigger the appropriate actions, similar to many ecological situations in which the meaning of stimuli and actions depends on the surrounding context in which they are embedded.
Collectively, the present results suggest that mPFC may track contexts or contextual boundaries as well as alter the interpretation or meaning of stimuli and actions so they are consistent with the present context. A dysfunction in this or a related form of contextual processing at the level of the PFC may be an important factor in the cognitive deficits observed in patients with schizophrenia, as previously proposed (51).
Methods
Subjects, Surgery, and Behavior.
Details in SI Methods.
Data Analysis.
The present study used methods described previously by Lapish et al. (43) and Durstewitz et al. (50). To obtain an estimate of the neural firing rate for each isolated cell i as a function of time bin t, ri(t), all spike trains were convolved with Gaussian kernels (SD = 500/4 ms) and binned at 500 ms (approximately the inverse of the average firing rate of ≈2.4 Hz). Neurons with average firing rates below 0.1 Hz were excluded from further analysis. For population analysis, population vectors r(t) = [r1(t) … rN(t)] were formed (called iFR vectors), with N the number of single units isolated from a given recording session. The term MSUA space refers to the N-dimensional space spanned by all recorded units and populated by these vectors r(t). To quantify environmental effects on network activity, we computed the Mahalanobis distances (e.g., ref. 52) between the sets of N-dimensional vectors associated with the different environment epochs, with covariance matrices pooled for the two conditions compared. The (squared) Mahalanobis distance between two sets of points can be thought of as the Euclidean distance between the group means normalized by the covariances of the data along all MSUA dimensions (i.e., it quantifies the separation between two clouds of points in relation to the data scatter). Because the number of dimensions (neurons) could sometimes approach the number of data points (time bins), a regularized version of the covariance matrix was used (e.g., ref. 53) to avoid singularity and statistical reliability issues.
There was no selection of units, and all units were included in all analyses unless otherwise stated. In the present data set, ensembles ranging from 26 to 109 units per rat were recorded simultaneously, yielding MSUA spaces of dimensionality 26–109. To control for potential confounds in Mahalanobis distance comparisons, like differences in MSUA space dimensionality or in the sample size, we selected the same number of units and vector points for each distance calculation. We limited the number of dimensions to the smallest recorded ensemble size by randomly selecting only this number of neurons from the larger ensembles 1,000 times, and taking the averages across these 1,000 random drawings to still make full use of all units recorded. For all between- and within-environment context analyses sample sizes of the same number of vector points (200 time bins) were used for each set of vectors compared in all sessions.
To assess whether environmental switches affected ensemble activity states, we compared Mahalanobis distances between equivalent time periods from either one (within) or two (between) environments. For within-environment comparisons, we used two periods of 200 time bins from the last section within one environment, which were separated by the same amount of time that it took to transfer animals between the two environments. For between-environment comparisons we selected the final 200 time bins from the first environment and the first 200 time bins from the second environment (Fig. S1C). Thus, for these comparisons time was eliminated as a confounding factor. Paired t tests and nonparametric Wilcoxon signed rank tests were performed on within- and between-environment distances for each behavioral condition.
Supplementary Material
Acknowledgments
We thank Dr. Chris Lapish for helpful discussions on the manuscript. This work was funded by grants from the Canadian Institutes for Health Research and National Alliance for Research on Schizophrenia and Depression (to J.K.S.) and by Bundesministerium fuer Bildung und Forschung Grant 01GQ1003B and Deutsche Forschungsgemeinschaft Grant Du 354/6-1,7-2 (to D.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114415109/-/DCSupplemental.
References
- 1.Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- 2.Shidara M, Richmond BJ. Anterior cingulate: Single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- 3.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 4.Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn Mem. 2008;15:357–367. doi: 10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 7.Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossert JM, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JA. A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia. 2007;45:673–684. doi: 10.1016/j.neuropsychologia.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess N, Becker S, King JA, O'Keefe J. Memory for events and their spatial context: Models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalisch R, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters J, Daum I, Gizewski E, Forsting M, Suchan B. Associations evoked during memory encoding recruit the context-network. Hippocampus. 2009;19:141–151. doi: 10.1002/hipo.20490. [DOI] [PubMed] [Google Scholar]
- 13.Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114:184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 14.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 15.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 16.Olton DS, Branch M, Best PJ. Spatial correlates of hippocampal unit activity. Exp Neurol. 1978;58:387–409. doi: 10.1016/0014-4886(78)90096-1. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Niki H. Hippocampal unit activity and delayed response in the monkey. Brain Res. 1985;325:241–254. doi: 10.1016/0006-8993(85)90320-8. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7:716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolls ET, et al. Hippocampal neurons in the monkey with activity related to the place in which a stimulus is shown. J Neurosci. 1989;9:1835–1845. doi: 10.1523/JNEUROSCI.09-06-01835.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 21.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 22.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- 25.Sirota A, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benchenane K, et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 30.Poucet B, et al. Spatial navigation and hippocampal place cell firing: The problem of goal encoding. Rev Neurosci. 2004;15:89–107. doi: 10.1515/revneuro.2004.15.2.89. [DOI] [PubMed] [Google Scholar]
- 31.Poucet B. Searching for spatial unit firing in the prelimbic area of the rat medial prefrontal cortex. Behav Brain Res. 1997;84:151–159. doi: 10.1016/s0166-4328(96)00144-1. [DOI] [PubMed] [Google Scholar]
- 32.Isomura Y, Takada M. Neural mechanisms of versatile functions in primate anterior cingulate cortex. Rev Neurosci. 2004;15:279–291. doi: 10.1515/revneuro.2004.15.4.279. [DOI] [PubMed] [Google Scholar]
- 33.Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci USA. 2005;102:4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshi E, Sawamura H, Tanji J. Neurons in the rostral cingulate motor area monitor multiple phases of visuomotor behavior with modest parametric selectivity. J Neurophysiol. 2005;94:640–656. doi: 10.1152/jn.01201.2004. [DOI] [PubMed] [Google Scholar]
- 35.Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- 36.Kyd RJ, Bilkey DK. Prefrontal cortex lesions modify the spatial properties of hippocampal place cells. Cereb Cortex. 2003;13:444–451. doi: 10.1093/cercor/13.5.444. [DOI] [PubMed] [Google Scholar]
- 37.Kyd RJ, Bilkey DK. Hippocampal place cells show increased sensitivity to changes in the local environment following prefrontal cortex lesions. Cereb Cortex. 2005;15:720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- 38.Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- 39.Bar M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–289. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Ciaramelli E, Leo F, Del Viva MM, Burr DC, Ladavas E. The contribution of prefrontal cortex to global perception. Exp Brain Res. 2007;181:427–434. doi: 10.1007/s00221-007-0939-7. [DOI] [PubMed] [Google Scholar]
- 41.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- 43.Lapish CC, Durstewitz D, Chandler LJ, Seamans JK. Successful choice behavior is associated with distinct and coherent network states in anterior cingulate cortex. Proc Natl Acad Sci USA. 2008;105:11963–11968. doi: 10.1073/pnas.0804045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Euston DR, McNaughton BL. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci. 2006;26:13143–13155. doi: 10.1523/JNEUROSCI.3803-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: Contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balaguer-Ballester E, Lapish CC, Seamans JK, Durstewitz D. Attracting dynamics of frontal cortex ensembles during memory-guided decision-making. PLOS Comput Biol. 2011;7:e1002057. doi: 10.1371/journal.pcbi.1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 50.Durstewitz D, Vittoz NM, Floresco SB, Seamans JK. Abrupt transitions between prefrontal neural ensemble states accompany behavioral transitions during rule learning. Neuron. 2010;66:438–448. doi: 10.1016/j.neuron.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 52.Krzanowski WJ. Principles of Multivariate Analysis: A User's Perspective New York. New York: Oxford Univ Press; 2000. [Google Scholar]
- 53.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer Science; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.