Abstract
Neuroblastoma is a common childhood malignant tumor originated from the neural crest-derived sympathetic nervous system. A crucial early event in neuroblastoma pathogenesis is arrested differentiation of neuroblasts at various stages. Treatment of neuroblastoma with TPA and PDGF-BB leads to terminal differentiation of neuroblastoma cells. However, the signaling pathways that are involved in this process remain largely unknown. Here, we report that inhibition of endogenous FOXO proteins attenuated TPA/PDGF-BB mediated differentiation of neuroblastoma cells. Activated FOXO transcription factors acted on PDGFRA promoter to direct its basal mRNA expression as well as its induction upon serum deprivation. Depletion of endogenous PDGFRA in neuroblastoma cells significantly diminished neurite formation and extension under TPA/PDGF-BB treatment. Furthermore, ectopic expression of PDGFRA abolished the blockage of neuroblastoma differentiation by FOXOs inhibition. These findings define the FOXO–PDGFRA axis as crucial mechanistic components that govern TPA-induced neuroblastoma differentiation.
Neuroblastoma is a childhood tumor of the peripheral nervous system. This tumor is derived from immature sympathetic cells of the ganglionic lineage and is arrested at various stages of differentiation. Tremendous clinical heterogeneity, encompassing truly benign as well as extremely aggressive forms, is presented in neuroblastomas. In vivo as well as in vitro data have shown that the degree of sympathetic neuronal tumor cell differentiation influences patient outcome. Therefore, elucidation of the molecular basis governing neuroblastoma cell differentiation is crucial for identification of targets for effective therapeutic intervention in neuroblastoma (1). In vitro differentiation of neuroblastoma cell line in the presence of various agents and growth factors has been widely used to dissect the molecular mechanisms underlying neuroblastoma differentiation. A number of differentiation protocols have been published in recent years (1). Retinoic acid (RA) induces differentiation of neuroblastoma cells in vitro and is one of few targeted therapeutics currently used in the clinic for advanced neuroblastoma. However, the overall response rate to RA in neuroblastoma patients is low. In addition to RA, TPA showed very significant effects on cell growth and differentiation. When cultured in serum-free SHTE medium containing TPA and PDGF-BB (SHTE, TPA and PDGF-BB, STP in short), SH-SY5Y cells have been shown to differentiate into nonproliferative, neuron-like cells, characterized by outgrowth of varicosity-containing neuritis terminated by growth cones and by induction of neuronal sympathetic differentiation markers (i.e., GAP43, growth associated protein 43). In addition, differentiated SH-SY5Y cells become functional as they accumulate norepinephrine in dense core granules and build up an action potential, which is depolarized by acetylcholine, resulting in release of stored neurotransmitters (1). Protein kinase C (PKC) isoforms have been suggested to regulate TPA-mediated neuroblastoma cell differentiation (2). However, PKC family consists of at least 12 members, and each one of them has distinct biological functions. Therefore, the signaling pathways that govern STP-induced differentiation process remain to be elucidated.
The PI3K/AKT pathway is a central downstream effector of growth factor receptors and is often dysregulated in cancer. The PDGFRs exert many of their physiologic effects by activating PI3K/Akt/mTOR axis. Interestingly, mTOR signaling pathway has been shown to suppress the expression of PDGFR at transcriptional levels (3), but the responsive transcription factor is unclear. PDGFRs are expressed in brain throughout development. PDGF-BB stimulates survival and neurite outgrowth of hindbrain neuroblasts in culture, indicating that PDGF also influences survival and differentiation of neuroblasts from the central nervous system (CNS) (4). These findings, in conjunction with the effect of PDGF on glia cell development (5), suggest a major role for PDGF signaling in the development of the nervous system (6). However, the role of PDGFR in neuroblastoma differentiation is not clear.
The FOXO transcription factors have a wide range of biological functions, including the regulation of cell proliferation, apoptosis, and differentiation. They also regulate life span and energy metabolism (7–11). FOXO family members are widely expressed in the developing mammalian brain (12, 13). Recently, FOXO proteins have been shown to regulate neuronal polarity establishment (14). In this study, we identify a role for the FOXO transcription factors in neuroblastoma differentiation. We show that FOXO proteins are responsible for maintaining the basal expression levels of PDGFRA, as well as the accumulation of PDGFRA under serum starvation condition. Depletion of endogenous FOXO proteins in neuroblastoma cells results in dysregulated differentiation phenotype, which can be rescued by PDGFRA expression. Our data demonstrate that PDGFRA is a critical downstream target gene of the FOXO proteins in regulating STP-mediated neuroblastoma differentiation.
Results
Transcriptional Regulation of PDGFRA by FOXO Family Members.
To identify downstream targets of FOXO family members in mammalian cells, we introduced an inducible FOXO3-TM mutant in which the three Akt phosphorylation sites were mutated to alanines, fused in frame with the estrogen receptor (ER) ligand-binding domain (FOXO3-TMER). The activity of FOXO3-TMER is elevated upon addition of 4-hydroxy tamoxifen (4-OHT) (10, 11, 15). FOXO3-TMΔDBER (a FOXO3-TM form lacking DNA binding domain) was used as a negative control. Human neuroblastoma cell line SH-SY5Y cells were infected with FOXO3-TMER or FOXO3-TMΔDBER retroviruses. We performed microarray experiment (Affymetrix GeneChips) using RNA extracted from FOXO3-TMER cell lines treated or untreated with 4-OHT. Gene expression profiles were analyzed and differentially expressed candidate genes were obtained with the P value set at 0.01 and had at least twofold induction. PDGFRA was significantly induced from microarray analysis when 4-OHT was used to activate FOXO3-TMER. We then performed QRT-PCR and Western blotting to verify the microarray data. PDGFRA mRNA levels as well as its protein levels were induced upon FOXO3-TMER activation. Consistent with previous reports, p27 and Pink1 were also significantly up-regulated in FOXO3-TMER cells upon 4-OHT treatment (10, 11). Cells expressing FOXO3-TMΔDBER failed to induce PDGFRA when exposed to 4-OHT (Fig. S1A), suggesting that the elevation of PDGFRA mRNA levels by FOXO3 requires the DNA-binding activity of FOXO3. Using p53−/− mouse embryonic fibroblast cells (MEF) expressing FOXO3-TMER or TMΔDBER, we found robust induction of PDGFRA at both mRNA and protein levels (Fig. S1 B and C), indicating that regulation of PDGFRA by FOXO3 might be conserved between humans and mice.
To determine whether other FOXO family members can also induce PDGFRA transcription, we tested cells stably expressing FOXO1-TMER or FOXO4-TMER. Upon the addition of 4-OHT, PDGFRA was readily induced (Fig. S1D), suggesting it might be a common target of all 3 FOXO family members. To examine whether FOXO directly induces PDGFRA transcription, we treated the cells with cycloheximide (CHX) before the addition of 4-OHT to prevent de novo protein synthesis. As shown in Fig. S1E, CHX failed to inhibit FOXO3-TMER induction of PDGFRA expression, indicating that de novo protein synthesis is not required.
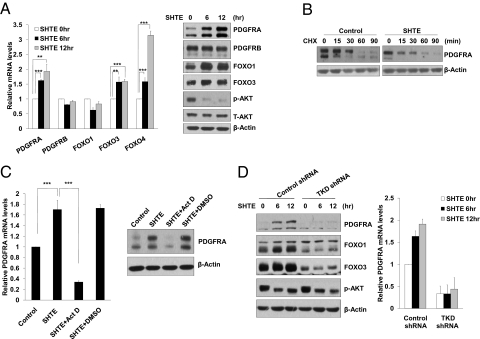
To test whether PDGFRA mRNA levels could be up-regulated upon activation of FOXO family members by serum starvation, we measured PDGFRA mRNA levels in SH-SY5Y cells cultured in serum-free SHTE medium. In the absence of serum, PDGFRA mRNA as well as its protein levels was significantly induced. By contrast, PDGFRB expression levels were not affected (Fig. 1A). Due to the lack of reliable commercial FOXO4 antibody, we checked FOXO4 expression by QRT-PCR and noticed a significant induction of FOXO4 transcription when cells were switched to serum-free SHTE medium (Fig. 1A). Up-regulation of PDGFRA upon serum withdrawal was also observed when depriving serum alone in MEFs or in SH-SY5Y cells without switching to SHTE medium (Fig. S1 C and F). The up-regulation of PDGFRA protein levels under serum-free SHTE treatment is not due to elevated protein stability, as the protein half-life of PDGFRA showed no significant difference before and after serum-free SHTE exposure (Fig. 1B). Furthermore, we confirmed that the induction of PDGFRA expression by serum-free SHTE was attributed to a transcriptional increase rather than a change in mRNA stability, because treatment of SH-SY5Y cells with actinomycin D (Act D), a known transcription inhibitor, drastically blocked serum-free SHTE-induced accumulation of PDGFRA mRNA (Fig. 1C).
Fig. 1.
FOXO induces PDGFRA. (A) Activation of FOXO signaling upon serum deprivation up-regulates PDGFRA. SH-SY5Y cells were exposed to serum-free SHTE medium for the indicated times. Total RNA and protein were then extracted. Relative levels of PDGFRA, PDGFRB, FOXO1, FOXO3, and FOXO4 mRNA were determined by QRT-PCR (Left). The protein lysate were subjected to Western blotting with antibodies against the indicated proteins (Right). (B) PDGFRA protein stability remains unchanged upon serum-free SHTE treatment. SH-SY5Y cells either untreated or treated with serum-free SHTE for 8 h were chased with CHX (100 μM). Total cell lysates were prepared at the indicated times after the addition of CHX and analyzed by Western blotting using anti-PDGFRA and β-Actin antibodies. (C) Transcription inhibitor Act D abolishes the induction of PDGFRA upon serum-free SHTE exposure. SH-SY5Y cells were pretreated with Act D (0.5 μg/mL) or DMSO for 30 min, and then cultured in serum-free SHTE medium in the presence of Act D or DMSO for 8 h. The levels of PDGFRA mRNA and protein were assayed by QRT-PCR (Left) or Western blotting (Right). (D) Depleting FOXO members attenuates PDGFRA expression. SH-SY5Y cells expressing control shRNA or TKD shRNA were treated with SHTE, and then harvested at the indicated time points for protein and RNA analyses.
To investigate whether the FOXO proteins might contribute to PDGFRA induction under serum-free SHTE treatment, we generated lentiviral-based shRNAs targeting the FOXO family members: FOXO1, FOXO3, and FOXO4. We confirmed that expression of FOXO shRNAs (FOXO-TKD) led to the knockdown of endogenous FOXO1, FOXO3, and FOXO4 in SH-SY5Y cells when delivered simultaneously (Fig. 1D and S1G). Intriguingly, FOXO-TKD shRNA largely abolished the basal expression of PDGFRA, as well as its accumulation under serum-free SHTE treatment (Fig. 1D). In contrast to FOXO TKD shRNA inducing the knockdown of FOXO1, FOXO3, and FOXO4, knocking down either FOXO4 or FOXO1 and -3 failed to impair PDGFRA induction in response to serum deprivation (Fig. S1G), suggesting that FOXO1, FOXO3, and FOXO4 may have redundant functions in directing the transcriptional up-regulation of PDGFRA expression. In addition to SH-SY5Y cells, we also tested whether FOXO members are responsible for PDGFRA induction in other neuroblastoma cell lines. In SK-N-SH cells, serum-free SHTE treatment resulted in accumulation of PDGFRA at both protein and mRNA levels. Depleting FOXOs profoundly attenuated basal expression as well as SHTE-mediated up-regulation of PDGFRA (Fig. S1H). FOXO members have been reported to regulate other receptor tyrosine kinases (RTKs) including insulin receptor (IR) (16), IGF1 receptor (IGF-1R) (17). We therefore compared the expression patterns of these two RTKs in our system. Interestingly, FOXO depletion had no significant impact on basal IR or IGF-1R expression levels. Serum-free SHTE treatment only induced IGF-1R, which is FOXO-dependent (Fig. S2). Taken all together, activation of FOXO proteins either by exogenous expression or upon serum deprivation exerts positive effect on the expression of PDGFRA. Importantly, FOXO members are responsible for basal transcriptional control of Pdgfra gene.
FOXOs Direct PDGFRA Expression by Acting on Its Promoter.
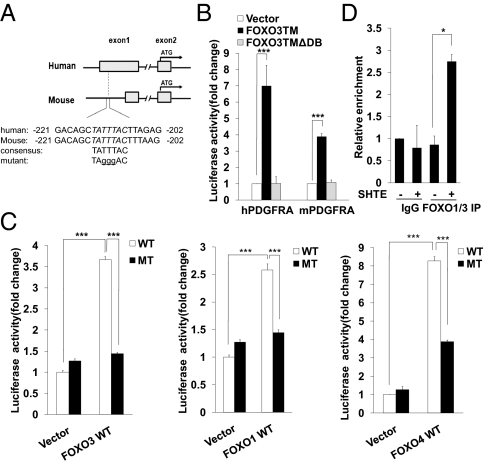
To identify the exact mechanism by which FOXO family members induce the expression of PDGFRA mRNA, we searched the promoter region of human Pdgfra gene and found a consensus FOXO binding element (FBE) in exon1 region, which is highly conserved in the human and the mouse genes (Fig. 2A). Cloning this region in a luciferase reporter plasmid shows that it only responds to active form of FOXO3 but not FOXO3-TMΔDB, which lacks the DNA binding region (Fig. 2B). All three FOXO family members, FOXO1, -3, and -4, were able to activate human PDGFRA luciferase reporter activity. Mutation of the core FBE site profoundly attenuated the luciferase reporter activity induced by exogenously expressed FOXO proteins (Fig. 2C). We also determined whether mouse pdgfra promoter-driven luciferase activity can be induced by FOXO proteins and found very robust activation of the reporter triggered by exogenous expressed FOXO members. Replacement of the same core consensus sequence abolished FOXO-induced luciferase activity (Fig. S3A), suggesting this FBE is functionally conserved across species. To assess whether FOXO proteins associate with Pdgfra gene promoter region under stress conditions that lead to activation of endogenous FOXO members, ChIP assay was performed to detect the interaction between endogenous FOXO members and the Pdgfra promoter using cells cultured in serum-free SHTE medium. Due to the lack of specific FOXO4 antibody, we can only determine the “protein-DNA” complex using FOXO1 and FOXO3 antibodies. Upon serum-free SHTE treatment, cells showed modest but significant FOXO1 and FOXO3 enrichment at the Pdgfra promoter (Fig. 2D). To determine whether ectopically expressed FOXO4 physically associates with Pdgfra promoter, we performed ChIP experiment using SH-SY5Y cells stably expressing FOXO4-TMER. We found that exogenous FOXO4 occupied the Pdgfra gene promoter in the presence of 4-OHT (Fig. S3B). Taken all together, these results indicate that FOXO family members bind directly to the promoter region of the Pdgfra gene and transcriptionally up-regulate PDGFRA mRNA expression under serum withdrawal condition.
Fig. 2.
PDGFRA is a direct FOXO target. (A) Identification of a conserved FBE site in the human and mouse pdgfra promoters. (B) FOXO3-TM activates luciferase reporter genes driven by pdgfra promoters. 293T cells were cotransfected with human or mouse pdgfra promoter luciferase reporter together with indicated plasmids. Results were represented the mean and error of three independent experiments conducted in triplicates. ***P < 0.001. (C) Transactivation of the human pdgfra promoter by FOXO family members. 293T cells were cotransfected with indicated plasmids, luciferase activity was measured in triplicates. Data shown are means ± SD from three independent experiments. ***P < 0.001. (D) Endogenous FOXOs directly bind to the promoter region of the pdgfra gene. SH-SY5Y cells either untreated or treated with serum-free SHTE medium were subjected to a ChIP assay with FOXO1 and FOXO3 antibodies. Promoter binding was analyzed by QRT-PCR using β-Actin as a control. Data shown are means ± SD from three independent experiments. *P < 0.05.
PDGFRA Is Required for Neuroblastoma Cell Differentiation.
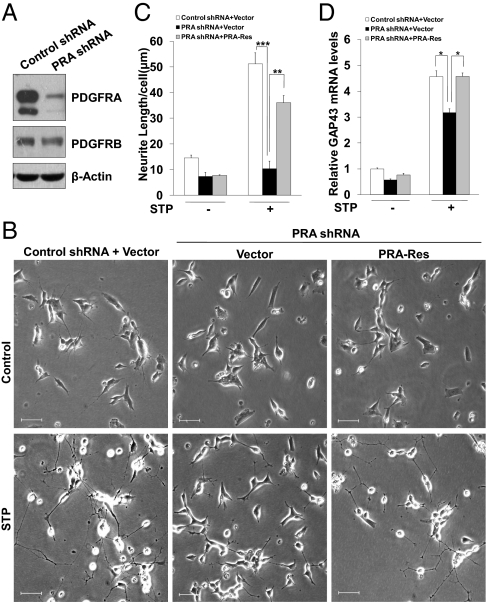
PDGF isoforms have been suggested to promote neuroblastoma cell growth, as well as to regulate neuronal cell migration, growth and differentiation during the development of human brain (18). To gain more insights into the role of PDGFR in regulating neuroblastoma cell differentiation, we depleted PDGFRA in SH-SY5Y cells and exposed cells to differentiation medium. We first confirmed endogenous PDGFRA levels were largely reduced by PDGFRA-shRNA (PRA-shRNA), whereas PDGFRB remained unchanged (Fig. 3A). When exposed to STP differentiation medium, cells expressing control shRNA showed a significant increase of neurite-bearing portions and increased neurite length (Fig. 3 B and C). The neurites have characteristics of growth axons, as judged from the outgrowth of varicosity-containing neurites that terminated in visible growth cones. We also measured the mRNA expression levels of GAP-43, a neuronal differentiation marker, and found a robust induction of GAP43 under STP treatment, suggesting the STP protocol successfully induced SH-SY5Y differentiation (Fig. 3D). Strikingly, knocking down PDGFRA in SH-SY5Y cells significantly abolished SH-SY5Y differentiation, as judged by the absence of neurites formation and elongation as well as impaired GAP43 induction (Fig. 3 B–D). Notably, PDGFRA knockdown only mildly affected S-phase entry (Fig. S4), but did not have adverse effects on cell survival, and thus the impairment of differentiation in PDGFRA knockdown cells was not associated with reduced cell survival. PDGFRA-depletion also abolished STP-induced SK-N-SH differentiation (Fig. S5 A–C). To further confirm PDGFRA is required for SH-SY5Y differentiation under STP treatment, we introduced a shRNA resistant form of PDGFRA (PRA-Res) into SH-SY5Y cells (Fig. S6 A and B). Re-expression of PDGFRA in PDGFRA-deficient cells largely restored the differentiation defect (Fig. 3 B–D), indicating PDGFRA is required for neuroblastoma differentiation under STP condition. Because serum deprivation under STP treatment has broad effects on many other signaling pathways, it would be interesting to test whether TPA plus PDGF-BB (TPA/PDGF-BB) in the presence of serum can potentiate the differentiation of SH-SY5Y cells stably expressing PDGFRA. To address this question, we constructed PDGFRA-expressing SH-SY5Y cells, and exposed them either to TPA/PDGF-BB (RPMI medium 1640 containing 10% FBS) or to STP medium. Exogenous PDGFRA significantly increased neurite formation and extension (Fig. S7 A and B) under TPA/PDGF-BB exposure. Notably, TPA/PDGF-BB is just as effective as STP in inducing differentiation in PDGFRA expressing cells (Fig. S7B). Together, these results demonstrate PDGFRA as a unique, key regulator in TPA-induced neuroblastoma differentiation.
Fig. 3.
PDGFRA depletion attenuates the differentiation ability of SH-SY5Y. (A) SH-SY5Y cells expressing either control shRNA or PRA shRNA were subjected to Western blotting to determine the knockdown efficiency. β-Actin, loading control. (B) PDGFRA-Res significantly rescued PDGFRA depletion-induced differentiation defect. SH-SY5Y cells expressing either vector or PRA-Res construct were infected with the indicated lentiviral shRNA. Cells were then cultured in STP medium for 72 h to induce differentiation, phase contrast micrographs were shown. (Scale bars, 50 μm.) (C) PDGFRA-Res profoundly rescued neurite outgrowth defect resulted from PDGFRA depletion. SH-SY5Y cells were treated as in (B). Average neurite length per cell was quantified as described in Materials and Methods. (D) SH-SY5Y cells were treated as described in B. RNA was then harvested, and GAP43 expression levels were analyzed by QRT-PCR.
FOXO Deficiency Attenuates Neuroblastoma Differentiation via PDGFRA.
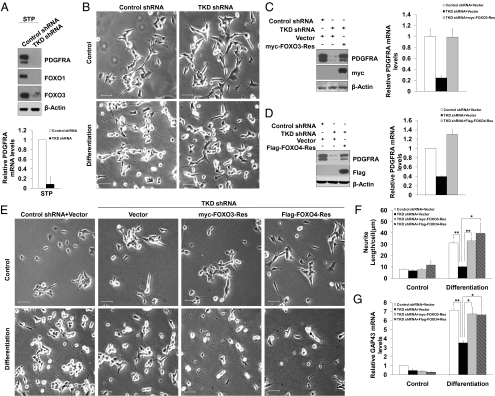
FOXO proteins have recently been found to regulate neuronal polarity (14). To examine if FOXO members regulate neuroblastoma differentiation, we depleted endogenous FOXO family members in SH-SY5Y cells and then exposed cells to STP differentiation medium (Fig. 4A). Intriguingly, knocking down FOXO proteins almost completely abolished STP-induced morphological changes indicative of neuronal differentiation (Fig. 4B). Consistent with the absence of morphological changes, the induction of GAP43 under STP treatment was significantly blocked in TKD shRNA expressing cells (Fig. 4G). Notably, knocking down FOXOs mildly attenuated G1/S transition under normal conditions (Fig. S4), but did not have adverse effects on cell survival. Importantly, STP treatment largely blocked S-phase entry in both control shRNA cells and cells depleted with FOXOs or PDGFRA (Fig. S4). These data exclude the possibilities that the differentiation defect caused by FOXO knockdown or PDGFRA depletion is a result of abnormal cell proliferation or decreased cell survival. FOXO TKD shRNA also abolished STP-induced SK-N-SH differentiation (Fig. S5 A–C). Given the weak effect of TPA in inducing neuroblastoma cell differentiation, a variety of other mitogenic factors have been combined with TPA to synergize to promote differentiation (6, 19). Therefore, we set out to examine whether FOXO–PDGFRA axis is also required for neuroblastoma cell differentiation triggered by TPA and serum. Morphologic differentiation of SH-SY5Y cells under TPA/serum treatment was observed (Fig. S8B), albeit to a lesser extent compared with that obtained with STP treatment. Intriguingly, FOXO TKD shRNA or PDGFRA shRNA markedly blocked cell differentiation as judged by the absence of neurites formation and elongation as well as impaired GAP43 induction (Fig. S8 A–D), indicating the function of FOXOs on TPA-induced SH-SY5Y differentiation is not strictly limited to the addition of PDGF-BB.
Fig. 4.
FOXO proteins are essential for SH-SY5Y cell differentiation. (A) Decreased PDGFRA expression levels in FOXO triple knockdown cells. Cells infected with control shRNA or TKD shRNA were treated with STP medium. Cell lysates and RNA were then collected and analyzed by Western blotting and QRT-PCR. (B) Knocking down FOXO proteins abolished SH-SY5Y cell differentiation. SH-SY5Y cells expressing indicated shRNA lentiviral vectors cultured in STP medium for 72 h. Cell morphology change was then analyzed by phase contrast microscopy. (Scale bars, 50 μm.) (C-G) shRNA-resistant FOXO3 or FOXO4 rescued SH-SY5Y cell differentiation defect due to FOXOs deficiency. FOXO3-Res (C) or FOXO4-Res (D) rescued PDGFRA protein and mRNA expression levels in the presence of TKD shRNA. (E) The same cells as in C and D were exposed to STP medium for 72 h. Cell morphology change was monitored by phase contrast microscopy. (F) Average neurite length per cell was analyzed. (Scale bars, 50 μm.) (G) RNA samples from the same cells as in E were collected and the expression levels of GAP43 were analyzed by QRT-PCR. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine the specificity of the FOXO shRNA-induced differentiation defect phenotype, we performed a rescue experiment. We generated expression plasmids encoding rescue forms of FOXO1, FOXO3, and FOXO4 by introducing silent mutations in the cDNA encoding the FOXO proteins designed to render them resistant to FOXO shRNA (FOXO-Res). We confirmed that expression of FOXO shRNAs failed to effectively induce knockdown of FOXO1-Res, FOXO3-Res, and FOXO4-Res (Fig. S6 C–E). We next tested whether expression of the rescue forms of FOXO proteins suppresses the FOXO shRNA-induced differentiation defect in SH-SY5Y cells. Expression of FOXO3-Res or FOXO4-Res significantly reversed the TKD shRNA-induced down-regulation of PDGFRA expression (Fig. 4 C and D) and neurite outgrowth defect in SH-SY5Y cells (Fig. 4 E and F). GAP43 induction was also restored upon the addition of shRNA-resistant FOXO3 or FOXO4 expression vectors (Fig. 4G). Expression of FOXO1-Res restored PDGFRA expression in FOXO TKD cells (Fig. S9 A and B). However, SH-SY5Y cells seemed to be more sensitive to the cytotoxicity induced by exogenously expressed FOXO1, which hampered our effort to rescue neurite outgrowth defect using FOXO1-Res construct. The fact that expression of FOXO3-Res or FOXO4-Res rescued neuroblastoma differentiation defect in cells depleted of endogenous FOXOs indicate that the FOXO TKD shRNA-induced phenotype is the result of specific knockdown of FOXO proteins, rather than off-target effects of shRNA or nonspecific activation of the shRNA machinery.
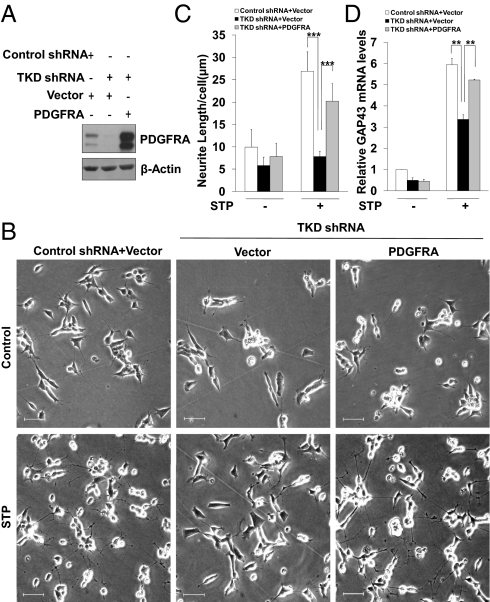
We next asked whether FOXO depletion-induced neuroblastoma differentiation defect phenotype could be rescued in the presence of exogenous PDGFRA. If the down-regulation of PDGFRA upon FOXO TKD shRNA delivery is essential for neurite outgrowth as well as the induction of GAP43, then exogenous expression of PDGFRA would be predicted to reverse the FOXO knockdown phenotype. In agreement with this prediction, we found that the ectopic expression of PDGFRA (Fig. 5A) significantly, albeit partially, restored neurite formation, extension (Fig. 5 B and C), and GAP43 accumulation (Fig. 5D) in FOXO TKD shRNA expressing cells. These data strongly suggest that PDGFRA is a critical direct target of the FOXO transcription factors in TPA-induced neuroblastoma differentiation.
Fig. 5.
FOXOs regulate SH-SY5Y differentiation via PDGFRA. Ectopic PDGFRA rescued the differentiation defect in TKD shRNA expressing cells. (A) SH-SY5Y cells expressing either vector or PDGFRA lentiviral vector were infected with indicated shRNA. PDGFRA expression levels were determined by Western blotting using anti-PDGFRA and anti-β-Actin antibodies. (B–D) The same cells as in A were exposed to STP medium for 72 h, differentiation phenotype was analyzed by phase contrast microscopy (B), and by measuring average neurite length per cell (C), and by examining GAP43 expression levels (D). (Scale bars, 50 μm.)
Discussion
PDGFRs belong to RTKs and are critical for PI3K/Akt activation (3). The PI3K/AKT pathway transmits signals from ligand-stimulated RTKs to effector molecules that control cell proliferation, energy metabolism and survival. PI3K/Akt activation leads to nuclear exclusion of FOXO proteins and thus the inactivation of their transcriptional activities. Here we provide evidence that FOXO proteins are direct upstream transcription factors responsible for PDGFRA expression both at basal levels as well as its induction under growth factor deprivation. We show that suppression of Akt signaling by serum deprivation recruited FOXO proteins to the Pdgfra promoter, which in turn led to the transcriptional up-regulation of PDGFRA levels. Conversely, FOXO1/3/4 knockdown suppressed the induction of PDGFRA mRNA in response to serum withdrawal. Importantly, FOXO depletion also profoundly attenuated the basal expression levels of PDGFRA mRNA and protein, indicating FOXO proteins are crucial for maintaining the basal expression levels of PDGFRA. It has been shown that, in many tumor models, inhibition of AKT induces a conserved set of RTKs, including HER3, IGF-1R, and insulin receptor. This is in part due to mTORC1 inhibition and in part secondary to a FOXO-dependent activation of receptor expression (17). Likewise, transcriptional induction of HER3 driven by FOXO3 and posttranslational up-regulation of HER3 were reported to compensate for inhibition of HER2 activity (20). Our findings on FOXO–PDGFRA axis may suggest novel functions for these proteins beyond neuroblastoma differentiation in diverse biological processes, for instance, in cell survival and tumorigenesis.
It has been shown that PDGFRA is crucial for CNS development, especially oligodendroglial lineage development. By contrast, a previous report suggested that brain-specific deletion of FOXO1, -3, and -4 (FOXO triple knockout, TKO) had only minimal effect on CNS development (21). Oligodendrocytes are generated from progenitors (oligodendrocyte progenitor cells, OPCs). PDGF can promote OPC proliferation, migration, survival and maturation via PDGFRA (5). It would be interesting to analyze PDGFRA levels in FOXO TKO mice to see if its expression levels are compromised in neuronal system. Because FOXO members function only as up-stream transcriptional factors of PDGFRA, it is conceivable that knocking down FOXO members only attenuates, but does not completely eliminate, basal PDGFRA expression. This possibility may account for the absence of obvious CNS developmental defect in FOXO TKO mice.
The exact mechanisms underlie how FOXO–PDGFRA axis regulates neuroblastoma differentiation remain to be elucidated. Neuritogenesis is guided by extracellular cues that control cytoskeleton rearrangements. The Rho GTPase subfamily, best known for their roles in regulating signaling pathways linking extracellular stimuli to the assembly and organization of the actin cytoskeleton (22), also control microtubule dynamics, cell polarity, and membrane trafficking (23). RA-dependent neuroblastoma differentiation has been suggested to require the activation of PI3K/Akt (24) and a subsequent activation of Rac1, as dominant negative Rac1 blunted RA-induced neurite outgrowth. In our experimental model system, in contrast to the reported RA-triggered signaling cascade, STP treatment leads to PI3K/Akt inhibition due to the absence of serum, as evidenced by less AKT phosphorylation even in the presence of TPA and PDGF-BB (Fig. S9C). It's plausible that distinct intracellular cascades are required for different experimental model systems (25). However, one has to be cautious when use dominant negative (DN) GTPase constructs to inhibit Rac1 and cdc42 activity. It has been shown that these DN constructs resulted in altered activities of multiple GTPase pathways and globe inhibition of various neurite functions (25). Recent studies on the regulation of neuronal polarity revealed an unrecognized role of FOXO proteins in neuronal polarity establishment (14). Pak1 was identified as a direct target of FOXO proteins, which allowed the FOXO proteins to control neuronal polarization. It is important to note that Pak1 plays a key role in regulating actin and microtubule cytoskeleton, events that are also crucial for neuroblastoma differentiation. Interestingly, primary cells from PDGFRA-null mice showed defects in Pak1 activation (26). It is likely that FOXO proteins may direct the expression of multiple genes whose protein products are intimately related. Characterization of the key effectors operating downstream of FOXO–PDGFRA will provide more insights into the biological functions of the FOXO proteins.
Materials and Methods
Luciferase Reporter Assay.
Luciferase reporter assay was performed as described (15, 16).
ChIP Assay.
ChIP was performed as described (15). PDGFRA ChIP primers: forward 5′-TTGAGCCCATTACTGTTGGA-3′; reverse 5′-ACGGCCTCCAATGATCTCTT-3′.
For description of materials and chemicals, plasmid constructs, cell culture, QRT-PCR, Western blotting, RNA interference, neurite length measurements, and statistical analysis, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Hongbing Zhang (Institute of Basic Medical Sciences and School of Basic Medicine, Peking Union Medical College and Chinese Academy of Medical Sciences) for his helpful discussion and reagents. We thank Jinhong Gao for excellent technical support. This work is supported by the National Basic Research Program of China 973 Program Grants 2009CB522202 (to H.Y.), National Natural Science Foundation of China Grant 31071195 (to H.Y.), and Ministry of Education of China 111 Project B06016.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119535109/-/DCSupplemental.
References
- 1.Edsjö A, Holmquist L, Påhlman S. Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin Cancer Biol. 2007;17:248–256. doi: 10.1016/j.semcancer.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Parrow V, Nånberg E, Heikkilä J, Hammerling U, Påhlman S. Protein kinase C remains functionally active during TPA induced neuronal differentiation of SH-SY5Y human neuroblastoma cells. J Cell Physiol. 1992;152:536–544. doi: 10.1002/jcp.1041520313. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smits A, et al. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci USA. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pringle N, et al. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J. 1989;8:1049–1056. doi: 10.1002/j.1460-2075.1989.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Påhlman S, Johansson I, Westermark B, Nistér M. Platelet-derived growth factor potentiates phorbol ester-induced neuronal differentiation of human neuroblastoma cells. Cell Growth Differ. 1992;3:783–790. [PubMed] [Google Scholar]
- 7.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Carter ME, Brunet A. FOXO transcription factors. Curr Biol. 2007;17:R113–R114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 10.You H, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei Y, et al. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc Natl Acad Sci USA. 2009;106:5153–5158. doi: 10.1073/pnas.0901104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 13.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre-Ubieta L, et al. A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes Dev. 2010;24:799–813. doi: 10.1101/gad.1880510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandarlapaty S, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui T, et al. Human neuroblastoma cells express alpha and beta platelet-derived growth factor receptors coupling with neurotrophic and chemotactic signaling. J Clin Invest. 1993;92:1153–1160. doi: 10.1172/JCI116684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Påhlman S, Meyerson G, Lindgren E, Schalling M, Johansson I. Insulin-like growth factor I shifts from promoting cell division to potentiating maturation during neuronal differentiation. Proc Natl Acad Sci USA. 1991;88:9994–9998. doi: 10.1073/pnas.88.22.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett JT, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci USA. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paik JH, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 24.López-Carballo G, Moreno L, Masiá S, Pérez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–25304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 25.Pertz OC, et al. Spatial mapping of the neurite and soma proteomes reveals a functional Cdc42/Rac regulatory network. Proc Natl Acad Sci USA. 2008;105:1931–1936. doi: 10.1073/pnas.0706545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickett EA, Olsen GS, Tallquist MD. Disruption of PDGFRalpha-initiated PI3K activation and migration of somite derivatives leads to spina bifida. Development. 2008;135:589–598. doi: 10.1242/dev.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.