Abstract
The superiority of spaced vs. massed training is a fundamental feature of learning. Here, we describe unanticipated timing rules for the production of long-term potentiation (LTP) in adult rat hippocampal slices that can account for one temporal segment of the spaced trials phenomenon. Successive bouts of naturalistic theta burst stimulation of field CA1 afferents markedly enhanced previously saturated LTP if spaced apart by 1 h or longer, but were without effect when shorter intervals were used. Analyses of F-actin-enriched spines to identify potentiated synapses indicated that the added LTP obtained with delayed theta trains involved recruitment of synapses that were “missed” by the first stimulation bout. Single spine glutamate-uncaging experiments confirmed that less than half of the spines in adult hippocampus are primed to undergo plasticity under baseline conditions, suggesting that intrinsic variability among individual synapses imposes a repetitive presentation requirement for maximizing the percentage of potentiated connections. We propose that a combination of local diffusion from initially modified spines coupled with much later membrane insertion events dictate that the repetitions be widely spaced. Thus, the synaptic mechanisms described here provide a neurobiological explanation for one component of a poorly understood, ubiquitous aspect of learning.
Keywords: polymerization, brefeldin A, memory
An extensive body of experimental work indicates that periodic exposure to the same material results in better retention than a single “cramming” session. Although this distributed practice effect was first recognized in late 19th century (1–3), and has since been the subject of a very large psychological literature (4), the neurobiological processes that give rise to the phenomenon are poorly understood. Activity-dependent synaptic plasticity, and, in particular, long-term potentiation (LTP) of glutamatergic transmission, is thought to underlie rapid storage of new information (5, 6). Therefore, it is surprising that little experimental attention has been given to the possibility that specialized features of LTP may contribute to the spaced trials (distributed practice) effect. This likely reflects the lack of data indicating that the substrates of the potentiation effect include properties that are engaged, or enhanced, only by widely spaced stimulation episodes. Specifically, several types of studies point to the conclusion that the elaborate processes yielding fully developed LTP reach completion within 10–15 min (5, 7, 8); these findings do not include results suggestive of a delayed capacity for triggering additional changes to already potentiated synapses. There is considerable evidence for a later LTP stabilization step involving protein synthesis (9), but the effects of this on subsequent plasticity involve inputs other than those already expressing potentiation (10).
Here, we describe a set of mechanisms and timing rules in hippocampus that result in widely spaced episodes of theta burst stimulation (TBS) generating a much greater degree of LTP than is obtained with shorter delays. Surprisingly, the added potentiation occurs in synapses that were left unchanged by the initial theta train and undergo enhancement only when a second round of TBS is applied to the same afferents after a long delay. This result strongly suggests that synapses with very different thresholds for stable potentiation are colocalized on individual hippocampal dendrites, a prediction we have confirmed using stimulation of single spines. We, therefore, propose mechanisms that impose a requirement for long delays between the delivery of learning-related afferent activity in order to produce maximal synaptic changes and thus to optimally encode new memory.
Results
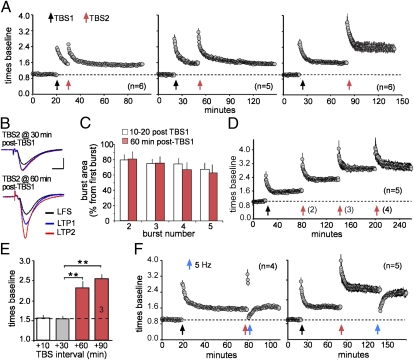
To test for a neurobiological analog of the spaced training effect, we used two trains of TBS (TBS1/TBS2), an afferent activity pattern that mimics neuronal firing during learning (11), separated by different intervals. TBS was applied to Schaffer–commissural afferents of field CA1b in adult rat hippocampal slices. TBS2 caused short-term, but not additional long-term, response facilitation when delivered at 10 or 30 min after TBS1. However, the same stimulation doubled the level of potentiation when delayed by 60 min (Fig. 1 A and B). Within-train facilitation of theta burst responses was not detectably different at the 10–20-min vs. 60–90-min delays, indicating that the renewed capacity for inducing potentiation found at the latter time point does not reflect slowly developing changes to release or receptor characteristics (Fig. 1C). A third train delayed by 60 min after TBS2 again increased potentiation, whereas a fourth and fifth had no effect (Fig. 1D). Three trains each separated by 1 h, thus, fully saturate LTP within a given population of synapses. Next, we increased the interval between TBS1 and TBS2 to test whether a “window of opportunity” exists regarding additional potentiation. TBS2 delivered 90 min after TBS1 resulted in the same degree of LTP as obtained with a 60-min delay (Fig. 1E). Other studies using variable stimulation intensities suggest that additional potentiation can be induced four hours after induction of LTP1 (10, 12). In all, the present results show that LTP has a previously undetected refractory period, lasting for 30–60 min, which creates an analog of the behavioral spaced trials effect (13).
Fig. 1.
Timing determines the efficacy of a second theta train in eliciting additional potentiation. (A) A second theta burst train (TBS2) does not produce additional potentiation when applied 10 min (Left) or 30 min (Center) post-TBS1 but is effective when applied after a 60 min delay (Right) [y axis: fold change in the slope of field (f)EPSP relative to the pre-TBS1 baseline: means ± SEMs]. (B) Traces for LTP1 and LTP2. (C) Percentage facilitation of the composite (four sequential fEPSPs) responses triggered by theta bursts 2–5 delivered at the indicated times after TBS1. The y axis summarizes the areas of the responses expressed as a percent increase above the area of the first burst response in the train (P = 0.92). (D) Using 60-min intertrain intervals, TBS3 elicits further potentiation, whereas TBS4 does not. (E) Mean potentiation at 60 min after TBS2 applied at indicated post-TBS1 intervals (**P < 0.01). (F) LTP2 is reversed by low-frequency (5-Hz) stimulation applied 60 s (Left), but not 60 min (Right), after TBS2.
The relevance of these spacing results to long-term memory depends on the extent to which the increments in potentiation are stable. Low-frequency (5-Hz) stimulation erases LTP1 if applied shortly after TBS but then becomes progressively less effective over the following 30 min (8, 14); similarly, 1-min trains of 5-Hz pulses fully reversed LTP2 when delivered 60 s, but not 60 min, after TBS2 (Fig. 1F).
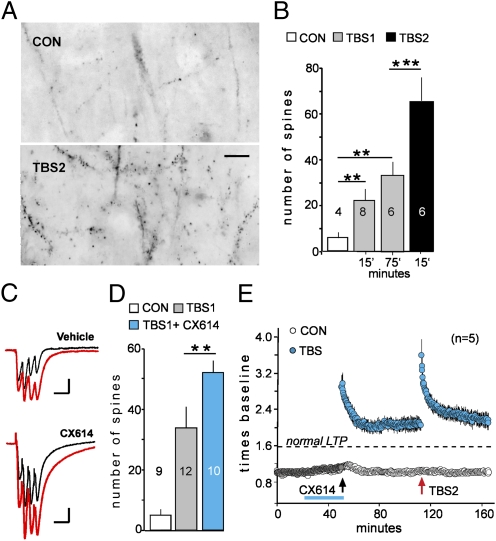
The LTP2 effect suggests that either (i) potentiation of individual synapses has multiple steps or (ii) TBS2 engages synapses that failed to reach LTP threshold during TBS1. The second hypothesis predicts that TBS2 will substantially increase the number of spines containing LTP-stabilizing processes. Actin polymerization is an essential step of this kind (9, 15–17), and we, therefore, used infusions of Alexa Fluor 568–phalloidin at 15 or 75 min post-TBS to label spines containing high concentrations of filamentous (F)-actin (Fig. 2A). Automated counting showed that TBS1 causes a marked increase in densely F-actin-positive spines that is of comparable size at 15 and 75 min after stimulation (Fig. 2 A and B). The persistence of the F-actin increase made it possible to compare the number of labeled spines at 75 min post-TBS1 with that found at the same time point after TBS1 plus TBS2. The second stimulation train doubled the population of densely F-actin positive spines above that caused by TBS1 alone, supporting the idea that a new population of synapses was potentiated by TBS2 (Fig. 2B). In all, these results suggest that (i) subpopulations of synapses have different thresholds for LTP and (ii) TBS1 lowers the threshold at synapses where it does not initially induce stable potentiation. If so, then amplifying theta burst responses could allow TBS1 to induce LTP machinery in the high threshold cases. We tested this using a positive allosteric modulator of AMPA-type glutamate receptors (18, 19) to increase the composite depolarization produced by TBS1 (Fig. 2C). TBS1 in the presence of the drug triggered actin polymerization in a much larger than normal population of spines (Fig. 2D) and, as expected from this, substantially increased the size of LTP1 at the expense of LTP2 (Fig. 2E).
Fig. 2.
A second theta burst train expands the pool of F-actin-enriched spines. (A) Fluorescent phalloidin labeling in CA1 stratum radiatum following control stimulation (CON) (three test pulses per min) or TBS2 (reverse contrast). (Scale bar = 10 μm). (B) Counts of densely phalloidin-positive spines in slices collected 15 or 75 min after TBS1 (gray bars) or 15 min after TBS2 delayed by 60 min (black bar). (C) Traces show responses to two successive bursts separated by 200 ms (red for second response) delivered in the presence or absence of AMPA receptor modulator CX614. (D) Counts of TBS1-induced phalloidin labeling for vehicle (gray) and CX614-treated (blue) slices. (E) Pretreatment with CX614 (blue line) caused an ∼70% increase in the magnitude of LTP induced by TBS1; this was accompanied by a loss of TBS2–induced potentiation. (***P < 0.001; **P < 0.01.)
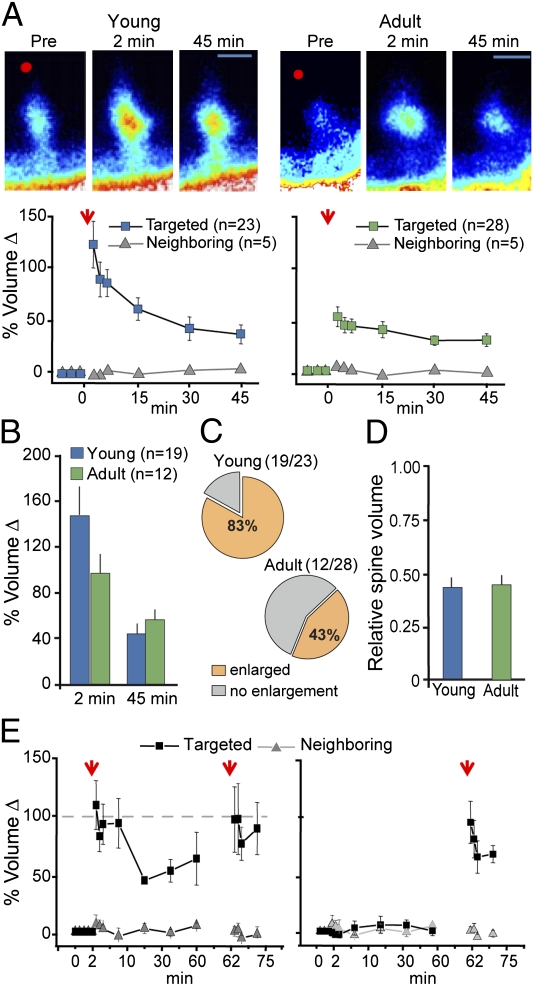
We next asked if properties of individual spines might contribute to a population of synapses being ‘missed’ by the first stimulus train. This involved a direct test of the hypothesis that dendritic spines in adult hippocampus exist in either a low or high induction threshold state. Uncaging glutamate near the tip of a single dendritic spine [single spine glutamate uncaging (SSGU)] results in a coordinated increase in spine volume and enhanced synaptic function lasting for more than an hour (20, 21). This form of plasticity shares many properties with Schaffer collateral LTP, including dependence on N-methyl-d-aspartate receptors (NMDARs), Rho GTPase signaling, Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation, F-actin polymerization and synaptic AMPAR insertion (20–24). Uncaging glutamate near small spines in young and adult slices caused an increase in head volume that remained elevated above baseline levels for at least 45 min (Fig. 3A). Head volume changes did not occur in neighboring spines, confirming that the stimulation protocol produces plasticity at a single synapse. Spine volume changes were similar between young and adult slices (Fig. 3B). However, only ∼40% of targeted spines enlarged after SSGU in adult slices (Fig. 3C), indicating that individual spines of the same general type do not respond to stereotyped postsynaptic stimulations in the same way. Note that the percentage of resistant cases (∼60%) is close to that for the densely F-actin positive spines added by TBS2 (Fig. 2B). The low success rate in adults was unlikely related to technical issues because we used a strong induction protocol (20) that caused structural plasticity in >80% of young spines (Fig. 3C), a value in good agreement with previous studies (20, 21). Indeed, the enlargement probability was significantly greater in young than in adult slices (P < 0.005) even though the targeted spines were of similar volume and the same induction protocol was used (Fig. 3D). We next tested the idea that individual spines from adult slices have a limited capacity for structural plasticity. Spines that enlarged after glutamate uncaging were refractory to successive attempts at induction, even when the timing between uncaging events was extended beyond one hour (Fig. 3E, Left). The induction probability was not dependent on the time that the slice was in the recording chamber (Fig. 3E, Right). These data support a model where only a minority of adult dendritic spines can respond to plasticity-inducing stimuli, and that once a synapse reaches threshold for stable plasticity, it does not contribute to further potentiation induced by subsequent stimulations.
Fig. 3.
Induction of single spine enlargement (SSE) in acute hippocampal slices. (A) All targeted spines from young and adult eGFP-expressing (47) mice showed significant enlargement following SSGU (arrow). Neighboring (nontargeted) spine volumes were stable for the entire experiment [young: F(1,27) = 12.6; P = 0.001 (n = 23 targeted, n = 5 neighboring); adult: F(1,32) = 6.8; P = 0.014 (n = 28 targeted, n = 5 neighboring)]. (Scale bar, 1 μm.) (B) Comparison of enlarged spine volumes at 2 and 45 min after uncaging. Spines were persistently enlarged relative to their preuncaging baseline in young (n = 19) and adult (n = 12) spines (P < 0.01; one-sample t test). The magnitude of enlargement between groups was not different at either time point. (C) Single spine enlargement occurs at a higher frequency in slices from younger animals (young: 19 spines enlarged out of 23 targeted spines; adult: 12 spines enlarged out of 28 targeted spines; P < 0.005; Fisher exact test). (D) Relative volumes were compared for young (n = 23) and adult (n = 28) spine populations by taking the spine head-to-shaft intensity for each targeted spine at the indicated time points and expressing this value relative to that obtained immediately preceding SSGU (P > 0.05). (E) Left, After initial induction, a second SSGU applied 60 min later does not produce additional enlargement (P > 0.05, paired t test; n = 12). Right, As a control, SSGU was performed following a 60-min delay. Error bars are SEMs.
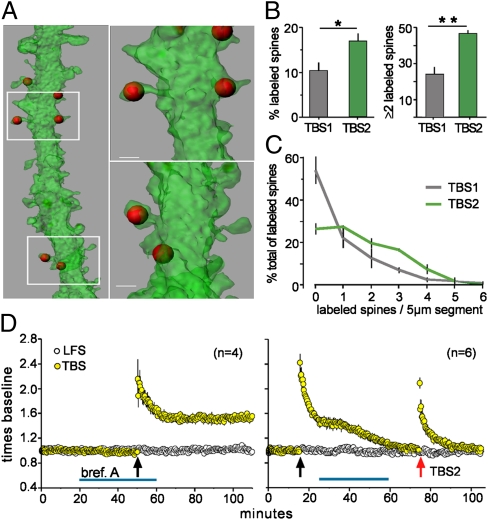
Collectively, the above findings suggest that TBS1 has two distinct effects: (i) induction of LTP in low threshold spines; and (ii) conversion of TBS1-resistant spines into the low threshold state, which provides a refreshed pool of synapses capable of responding to TBS2. What type of mechanism could implement the second proposition? Recent studies have shown that induction of plasticity at a single spine transiently lowers the plasticity threshold at adjacent spines (20). The mechanism underlying this phenomenon involves local spreading of signaling cascades from the activated spine to its neighbors, and some of these spreading signals are potent drivers of F-actin dynamics (24–26). Therefore, we asked if TBS2 induces F-actin polymerization in spines neighboring the synapses potentiated by TBS1. Alexa Fluor-tagged phalloidin was applied 75 min after delivery of TBS1 in slices prepared from GFP-expressing mice and sections were evaluated by confocal microscopy (Fig. 4A). Counts showed that after TBS1 about 10% of all spines had high concentrations of F-actin, whereas the value after TBS2 was 18% (P < 0.04) (Fig. 4B, Left). The percentage of 5-μm dendritic segments with two or more labeled spines after TBS2 was double that found after TBS1 (P < 0.005) (Fig. 4B, Right). Frequency distribution plots of the clustering data confirmed that (i) TBS1 “misses” spines on the majority of dendritic segments and (ii) most of the densely F-actin positive spines occur in clusters after TBS2 (Fig. 4C). These points indicate that the actin polymerization induced by TBS2 is largely restricted to dendritic branches that already have potentiated synapses. From this, it follows that promiscuous spreading of spine signaling events could contribute to the conversion of TBS1-resistant synapses into a state capable of responding to TBS2.
Fig. 4.
Spine actin polymerization after TBS2 occurs on the same dendritic segments as after TBS1. (A) 3D reconstructions of GFP-labeled dendrites (47) (green) show the localization of phalloidin-labeled F-actin aggregates (red) after TBS1. (B) Left, Percentage of all spines that were phalloidin-positive after TBS1 alone or after TBS2 applied 60 min after TBS1 (*P < 0.05). Right, Percentage of 5-μm dendritic segments that contained two or more phalloidin-positive spines was greater after TBS2 than after TBS1 (**P < 0.005). (C) Frequency distribution for dendritic segments containing zero to six labeled spines after TBS1 or TBS2; after TBS2, more of the labeling occurred in clusters. (D) Left, Brefeldin A had no effect on LTP induced by TBS1. Right, Brefeldin applied after TBS1 caused LTP1 to decay to baseline and completely blocked induction of LTP2.
Left unaddressed by the above experiments is the critical question of why a delay of ∼1 h is needed for TBS2 to become effective. One possibility is that high threshold spines lack elements necessary for LTP and that theta bursts trigger the production, transport, and/or insertion of these into synapses processes that might require considerable time. We tested this idea using brefeldin A, a selective toxin that targets a subset of Sec7-type GTP exchange factors, and thereby disrupts formation of transport vesicles (27) and protein delivery to the plasma membrane (28, 29). Forty-minute treatments that immediately preceded TBS1, or that ended 10 min before the theta bursts, had no effect on baseline physiology or the induction of LTP1 (Fig. 4D, Left). In striking contrast, post-TBS1 infusions completely blocked the production of additional LTP by TBS2 (Fig. 4D, Right). The differential effect of brefeldin agrees with the prediction that slowly developing membrane insertion events initiated by TBS1 prepare high threshold spines for the arrival of TBS2. However, as shown in Fig. 4D, brefeldin administered after TBS1 also produced a dramatic and unexpected result: it caused LTP1 to gradually decay back to baseline, again without affecting responses to a control input. The latter finding points to a hypothesis in which similarly delayed events set in motion by TBS1 serve to anchor LTP in one set of spines and to provide ingredients needed for potentiation in a second.
Discussion
The spaced-trial effect operates across multiple time frames, ranging from a few seconds to weeks (30). The work reported here describes LTP phenomenology, arising from previously undetected synaptic properties, that is logically related to those aspects of the effect occurring over the course of hours. Previous efforts to find neurobiological explanations for the advantages of distributed practice obtained evidence that spaced trials can offset memory defects in mice lacking two variants of the transcription factor CREB (31, 32). The genomic effects suggested by these kinds of results could provide another route whereby spacing in the hours domain facilitates strong encoding. Whether the transcriptional mechanisms interact with the synapse specific processes described above is an important issue for future research. Studies are also needed on the likelihood that cell biological variables of an unknown type contribute to the improved retention found with much longer (days, weeks) between trial intervals. More broadly, progress in defining brain systems mediating the competition between a conditioned stimulus and irrelevant cues provides network level insights into the spaced trials effect (33, 34) because learning theory (35, 36) predicts that spacing reduces the competitive strength of background signals.
The current findings provide evidence that adult spines have different thresholds for plasticity induction. Uncaging experiments indicated that intrinsic factors operating within individual spines of the same general morphological type are responsible for this distinction. Our results also describe an interaction between spines in which induction of LTP in low threshold cases primes their initially unresponsive neighbors so that later stimulation of the same afferents initiates actin polymerization and stable potentiation. Notably, induction of additional LTP only occurs when the second train is delayed by about one hour. We propose that events occurring within minutes after TBS1 combine with a much later membrane insertion step to produce this unique form of spine crosstalk.
Regarding the cellular bases for the distinction between low and high threshold spines, we found that brief treatments with a drug that selectively enhances AMPA receptor-gated synaptic currents markedly increases both the number of spines in which TBS1 triggers actin polymerization and the magnitude of LTP1. Under these conditions TBS2 produced little, if any, additional potentiation, as expected if the second theta episode modified contacts missed by TBS1. A plausible interpretation of the findings is that the high threshold cases have too small a population of AMPA receptors to produce the depolarization (during a theta burst) needed to generate an NMDAR response sufficient to trigger reorganization of the subsynaptic cytoskeleton. The number of AMPA receptors scales positively with the size of synapses (37), and so the above hypothesis can be reduced to an anatomical argument: the low vs. high threshold distinction reflects the area of the postsynaptic component of the apposition zone.
The above conclusion, although having the virtue of simplicity, faces two compelling problems. First, it implies that the delayed membrane insertion step indicated by the brefeldin experiments involves delivery of AMPA receptors to synapses. Such an event would necessarily increase excitatory postsynaptic potentials (EPSPs) at 30–60 min after TBS1 and this is not observed. Whereas a normally present mechanism that decreases LTP could, in principle, mask EPSP increases resulting from receptor insertion, the observed constant level of potentiation would require an almost-perfect balancing of the opposing processes. This seems unlikely. Second, the glutamate-uncaging experiments were designed to produce maximal stimulation of NMDARs and yet detected two classes of spines in the proportions expected from the LTP studies. Notably, uncaged glutamate is known from past work to produce robust, NMDAR-dependent excitatory responses at nearly all spines (20, 21) under the conditions used in our experiments. We, therefore, assume that the priming effect of TBS1 on nonresponsive spines occurs at a stage located between the transmitter receptors and the multiple steps leading to the assembly and stabilization of actin filaments. Prior work suggesting that strong stimulation of single synapses causes the spread of active GTPases to neighboring spines (24, 25) is of interest in this regard because these enzymes play critical roles in the production of stable LTP. It is also possible that TBS1 initiates a persistent change in nonresponsive spines that prepares them for the delayed arrival of TBS2. Reports that induction of LTP is accompanied by prolonged activation of CaMKII (38), a kinase localized to synapses, suggest a candidate for this role. These ideas can be tested using dual immunofluorescence microscopy to compare the number of synapses containing high levels of an active GTPase or phospho-CaMKII following TBS1 vs. TBS2.
Neither of the above proposals makes a clear prediction about the late step in priming. However, past research suggests some interesting possibilities. For example, several studies have shown that the kinase PKMζ is engaged at ≥1 h after induction and then contributes to maintenance of LTP (39). Although brefeldin is not likely to block the operation of PKMζ, it could serve to disrupt the delivery of the extrasynaptic AMPA receptors used by the enzyme. However, this would be expected to interfere with any contributions of the perisynaptic pool to the maintenance of baseline synaptic responses and we saw no evidence of this at control inputs. TBS, in addition to its actions on kinases, causes the breakdown of actin crosslinking proteins in the subsynaptic cytoskeleton; the relevant proteases also cleave transmembrane adhesion receptors that play a critical role in LTP-related actin polymerization (40–42). Replacing the degraded elements might well require the 45–60 min needed to prepare primed high threshold spines for the arrival of TBS2.
Finally, the demonstration that appropriately spaced, repeated stimulations of the same neural circuits markedly improves encoding over the time course of a classroom session has important implications for theories of why distributed practice produces better retention. One aspect of our data links the requirement for long delays to machinery that also serves to stabilize previously induced LTP and, thereby, supports consolidation explanations (43) for the behavioral effects. The spine threshold component of the present results implies that spaced trials capture a greater percentage of the information carried by activated afferent systems, something that would allow for easier cue recognition during retention tests. This idea accords with deficient processing and cue-variability theories of the distributed practice phenomenon (4, 43, 44). Thus, previously undetected cellular mechanisms impose temporal spacing requirements for synaptic plasticity and, thereby, provide a neurobiological explanation for one temporal segment of a ubiquitous feature of learning.
Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the local Institutional Animal Care and Use Committees.
Hippocampal Slice Electrophysiology and Treatments.
Acute hippocampal slices were prepared from young adult male Sprague–Dawley rat or C57BL/6 mouse hippocampus and established in an interface recording chamber maintained at 31 ± 1 °C with constant artificial cerebrospinal fluid (aCSF) perfusion (16). Electrodes placed in CA1a and CA1c were used to activate synapses in CA1b stratum radiatum (16). TBS consisted of a single train of 10 bursts (4 pulses at 100 Hz) separated by 200 ms. Baseline and test responses were collected at 3 pulses/min. Brefeldin A and the ampakine CX614 (45) were applied via the aCSF infusion line for bath concentrations of 20 μM for brefeldin and 20 μM for CX614. Alexa Fluor 568–phalloidin (Invitrogen) was locally applied, and punctate labeling within the CA1b stratum radiatum sample field (between the stimulating electrodes) was quantified using automated systems as described (16, 46).
Combined GFP–Phalloidin Labeling.
Slices from eGFP-expressing mice (47) received TBS1 or the combination of TBS1 followed 60 min later by TBS2; AlexaFluor 568-phalloidin was applied locally at 75 min after TBS1. Sections through the slices were processed for localization of phalloidin in GFP filled dendrites. A Zeiss LSM710 NLO confocal microscope was used to collect image z-stacks (63×; 0.13-μm z-steps through 25 μm, emitted light collected at 493–537 nm for EGFP and 591–621 nm for Alexa Fluor 568). Images were deconvolved in Volocity 5.0 and postacquisition processing and analysis was performed in Imaris (Bitplane).
Uncaged Glutamate Preparations.
Acute hippocampal brain slices (350-μm thick) from eGFP mice (30) (line M; 2–3 or 8–10 wk of age). Mice were killed by cervical dislocation, and following decapitation, the brain was rapidly removed and placed in choline chloride-based ice-cold cutting solution composed of the following (in mM): 110 choline-chloride, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 25 d-glucose, 11.6 ascorbic acid, and 3.1 pyruvic acid, equilibrated with 95% O2/5% CO2. The tissue was then mounted on a Vibrating microtome (Leica VT1200S), and 350-μm thick horizontal sections were cut. The slices were then warmed to 33 °C for 30 min in standard aCSF composed of the following (in mM): 124 NaCl, 3 KCl, 24 NaHCO3, 2 CaCl2, 1.25 NaH2PO4, 1 MgSO4, and 10 d-glucose, equilibrated with 95% O2 and 5% CO2. Then, slices were maintained in gassed aCSF at room temperature until being transferred to submerged-type recording chambers (volume, ∼1 mL). During experiments, slices were constantly superfused (2 mL/min) with room temperature gassed (95% O2 and 5% CO2) zero Mg2+ and high Ca2+ aCSF composed of the following (in mM): 119 NaCl, 2.5 KCl, 26.2 NaHCO3, 4 CaCl2, 1 NaH2PO4, 0 MgSO4, 11 d-glucose, 0.001 tetrodotoxin, and 0.2 Trolox. MNI-caged-l-glutamate was added to a final concentration of 2.5 mM. MNI-caged-l-glutamate and tetrodotoxin were purchased from Tocris; Trolox was purchased from Sigma.
Imaging and Glutamate Uncaging.
Two-photon imaging and glutamate uncaging were performed using an Olympus FV1000MPE-TWIN multiphoton microscope with two Ti:sapphire lasers [895 nm for imaging eGFP using a MaiTai DeepSee laser (Spectraphysics); 720 nm for uncaging MNI-Glutamate using a Chameleon Vision II laser (Coherent)]. In brief, coarse alignment of the two independently scanned beams was first performed to center the beams at the back focal plane of the objective (ULTRA 25×; 1.05 NA). For fine alignment at the sample plane, 1-μm fluorescent beads were imaged simultaneously with both beams until the images overlapped. Precise alignment of the stimulation scanner to the image scanner was confirmed by ablation of a 0.5-μm tetraspec bead (Invitrogen) with a high-energy pulse of 720-nm light. For glutamate uncaging, spines on secondary and tertiary dendrites that were a minimum distance of 4 μm from neighboring spines were selected. The depth of targeted spines in the slice was restricted to 25–50 μm from the surface. Individual spines were imaged while an uncaging laser was targeted ∼0.5 μm from the spine head. Imaging and uncaging parameters are based on a previous study (20). Imaging parameters were as follows: excitation, 895 nm; power at sample, ∼10 mW; pixel dwell time, 2.0 μs; and x-y scaling, 0.075 μm/pixel. Uncaging parameters were as follows: excitation, 720 nm; stimulus duration, 4 ms; frequency, 0.5 Hz for 1 min in 4 mM Ca2+, 0 mM Mg2+, and 1 μM TTX; and power at sample,∼18 mW. Before and following uncaging, z-stacks were acquired and spines were analyzed from z-projections for volume changes over time.
Spine Volume Analysis.
Relative spine volume was determined as described previously (20). Briefly, the fluorescence intensity of the spine head was normalized to shaft intensity. Volume changes at nearby spines were also analyzed to ensure that laser targeting of individual spines was specific. A spine was deemed “enlarged” if the volume change 2 min after uncaging was >50%. This threshold was chosen before the experiments were conducted and was based on prior studies (21).
Statistics.
For electrophysiological analyses, illustrations show group means ± SEM values and n indicates the number of slices tested. Two-tailed Student t tests were used to compare groups unless otherwise noted. The level of significance was assessed at P < 0.05. For the spine volume analysis (with glutamate uncaging), statistics were computed in SPSS and Microsoft Excel. P values for one- and two-tailed t test, one-way ANOVA, and repeated-measures ANOVA are specified per experiment. All data are presented as means ± SEM and n indicates the number of spines analyzed.
Acknowledgments
We thank Dr. Julie C. Lauterborn for support with spine analysis and Dr. Courtney A. Miller for comments on the manuscript. We are also grateful for receiving the CX614 compound as a gift from Cortex Pharmaceuticals. This research was supported by National Institute of Neurological Disorders and Stroke Grants NS045260 and NS064079, National Institute of Mental Health Grant MH083346, and Office of Naval Research Multidisciplinary University Research Initiative Grant N00014-10-1-0072.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Ebbinghaus H. Über das Gedächtnis. Untersuchungen zur experimentellen Psychologie. Leipzig: Duncker & Humblot; 1885. [Google Scholar]
- 2.Jost A. Die Assoziationsfestigkeit in ihrer Abhängigkeit von der Verteilung der Wiederholungen. Hamburg: Leopold Voss; 1897. [Google Scholar]
- 3.Thorndike EL. Education, a first book. New York: Macmillan; 1912. [Google Scholar]
- 4.Wickelgren WA. Single-trace fragility theory of memory dynamics. Mem Cognit. 1974;2:775–780. doi: 10.3758/BF03198154. [DOI] [PubMed] [Google Scholar]
- 5.Lynch G, Rex CS, Chen LY, Gall CM. Synaptic mechanisms for encoding memory. In: Koob G, Thompson RF, Le Moal M, editors. Encyclopedia of Behavioral Neuroscience. London: Academic; 2009. pp. 356–366. section ed Shors T. [Google Scholar]
- 6.Morris RG. Long-term potentiation and memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:643–647. doi: 10.1098/rstb.2002.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch G, Rex CS, Gall CM. LTP consolidation: Substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci. 1999;19:9728–9738. doi: 10.1523/JNEUROSCI.19-22-09728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Frey U, Morris RG. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 11.Teo JT, Swayne OB, Cheeran B, Greenwood RJ, Rothwell JC. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex. 2011;21:1627–1638. doi: 10.1093/cercor/bhq231. [DOI] [PubMed] [Google Scholar]
- 12.Frey U, Schollmeier K, Reymann KG, Seidenbecher T. Asymptotic hippocampal long-term potentiation in rats does not preclude additional potentiation at later phases. Neuroscience. 1995;67:799–807. doi: 10.1016/0306-4522(95)00117-2. [DOI] [PubMed] [Google Scholar]
- 13.Anderson MJ, Jablonski SA, Klimas DB. Spaced initial stimulus familiarization enhances novelty preference in Long-Evans rats. Behav Processes. 2008;78:481–486. doi: 10.1016/j.beproc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramár EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai AC, Kessler M. Pharmacology of ampakine modulators: From AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 19.Lynch G. AMPA receptor modulators as cognitive enhancers. Curr Opin Pharmacol. 2004;4:4–11. doi: 10.1016/j.coph.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honkura N, Matsuzaki M, Noguchi J, Ellis-Davies GC, Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex CS, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin C-Y, Lynch G, Gall CM. AMPA receptor stimulation increases α5β1 integrin surface expression, adhesive function and signaling. J Neurochem. 2005;94:531–546. doi: 10.1111/j.l471-4159.2005.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull. 2006;132:354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- 31.Kogan JH, et al. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- 32.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 34.Fanselow MS. Pavlovian conditioning, negative feedback, and blocking: Mechanisms that regulate association formation. Neuron. 1998;20:625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 35.Rescorla RA, Wagner AR. In: A theory of pavlovian conditioning: Variations in the effectiveness of reinforcement and non reinforcement. Classical Conditioning II: Current Theory and Research. Black AH, Prokasy WF, editors. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 36.Fanselow MS, Tighe TJ. Contextual conditioning with massed versus distributed unconditional stimuli in the absence of explicit conditional stimuli. J Exp Psychol Anim Behav Process. 1988;14:187–199. [PubMed] [Google Scholar]
- 37.Sassoé-Pognetto M, et al. Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 2003;463:237–248. doi: 10.1002/cne.10745. [DOI] [PubMed] [Google Scholar]
- 38.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 39.Sacktor TC. PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog Brain Res. 2008;169:27–40. doi: 10.1016/S0079-6123(07)00002-7. [DOI] [PubMed] [Google Scholar]
- 40.Vanderklish PW, et al. Marking synaptic activity in dendritic spines with a calpain substrate exhibiting fluorescence resonance energy transfer. Proc Natl Acad Sci USA. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomimatsu Y, Idemoto S, Moriguchi S, Watanabe S, Nakanishi H. Proteases involved in long-term potentiation. Life Sci. 2002;72:355–361. doi: 10.1016/s0024-3205(02)02285-3. [DOI] [PubMed] [Google Scholar]
- 42.Huttenlocher A, et al. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 43.Glenberg AM. Component-levels theory of the effects of spacing of repetitions on recall and recognition. Mem Cognit. 1979;7:95–112. doi: 10.3758/bf03197590. [DOI] [PubMed] [Google Scholar]
- 44.Hintzman DL. In: Repetition and memory. The Psychology of Learning and Motivation. Bower GH, editor. Vol 10. New York: Academic; 1976. pp. 47–91. [Google Scholar]
- 45.Arai AC, Kessler M, Rogers G, Lynch G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: Interactions with cyclothiazide and GYKI 52466. Mol Pharmacol. 2000;58:802–813. doi: 10.1124/mol.58.4.802. [DOI] [PubMed] [Google Scholar]
- 46.Rex CS, et al. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]