Abstract
Ankyrin-R/B/G (encoded by ANK1/2/3, respectively) are a family of very large scaffold proteins capable of anchoring numerous receptors and ion channels to specific, spectrin-containing membrane micro-domains. Hereditary mutations of ankyrins are known to be associated with diseases including spherocytosis, cardiac arrhythmia, and bipolar disorder in humans, although the underlying molecular bases are poorly understood. The middle spectrin-binding domain of ankyrins contains highly conserved ZU5-ZU5-UPA-DD domains arranged into the ZZUD tandem. Curiously, most of the disease-causing mutations in the tandem have no apparent impact on the spectrin binding of ankyrins. The high resolution structure of the ankyrin-B ZZUD tandem determined here reveals that the ZU5-ZU5-UPA domains form a tightly packed structural supramodule, whereas DD is freely accessible. Although the formation of the ZZU supramodule does not influence the spectrin binding of ankyrins, mutations altering the interdomain interfaces of ZZU impair the functions of ankyrin-B&G. Our structural analysis further indicates that the ZZU supramodule of ankyrins has two additional surfaces that may bind to targets other than spectrin. Finally, the structure of the ankyrin ZZUD provides mechanistic explanations to many disease-causing mutations identified in ankyrin-B&R.
Keywords: ankyrin-B syndrome, cytoskeleton, PIDD, UNC5
Ankyrins are a family of adaptor proteins required for the construction and maintenance of specialized membrane domains in various tissues via anchoring specific membrane proteins to spectrin-based cytoskeletons (see refs. 1 and 2 for reviews). Vertebrates contain three ankyrins: ankyrin-R, -B, and -G, all containing an N-terminal membrane-binding domain (MBD), a central spectrin-binding domain (SBD), a death domain (DD) with ill-defined functions, and a variable C-terminal regulatory domain (2) (Fig. 1C). Ankyrin-R, the first identified ankyrin from erythrocytes, is also expressed in other tissues (3, 4). Ankyrin-B, originally identified in brain, functions in many tissues including in the cardiac and skeletal muscles (2). Ankyrin-G, the most broadly expressed ankyrin, is critical for the formation and stabilization of axon initial segments of neurons, the biogenesis of lateral membrane domains of epithelia as well as other tissue functions (2, 5–8). Loss-of-function mutations in ankyrins are associated with hereditary diseases in humans. For example, a number of loss-of-function mutations of ankyrin-R are known to cause hereditary spherocytosis (9). Genetic variants of ankyrin-G are associated with bipolar disorder (10–14). Many missense mutations of ankyrin-B have been linked to inherited cardiac arrhythmia known as the “ankyrin-B syndrome” (15). Curiously, although MBD is responsible for binding to most of known ankyrin-association proteins, most of the ankyrin-B syndrome mutations are located in the SBD, DD, and regulatory domains, and these mutations do not seem to affect the binding of ankyrins to spectrin (16). These findings suggest that SBD is involved in functions other than binding to spectrin, although very little is known in this regard.
Fig. 1.
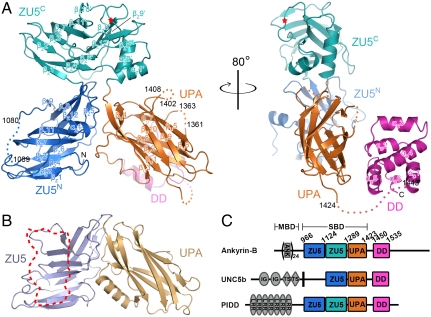
Overall structure of ankyrin-B ZZUD. (A) Ribbons diagram representation of the crystal structure of ankyrin-B ZZUD with ZU5N (blue), ZU5C (cyan), UPA (orange), and DD (purple) drawn in their specific colors. The same color code is used throughout the rest of the figures. The disordered regions are indicated by dotted lines with starting and end residues number labeled. The nine-residue deletion site is indicated by a red star. (B) Comparing with ankyrin-B, the ZU5 and UPA domains of UNC5b interact with each other following a similar manner. The DD binding region in ZU5 of UNC5b is highlighted with a dotted trace. (C) Domain architectures of the ZU5-UPA-DD containing proteins in the eukaryotes genomes. The domain boundaries of ankyrin-B ZZUD are defined based on the structure determined in this work.
Ankyrin SBD consists of three highly conserved domains, two ZU5 and a UPA domains arranged into a ZU5-ZU5-UPA (termed as ZZU) tandem (17). The high conservation nature of SBD in all ankyrins (across isoforms and species) strongly indicates that SBD plays critical roles in ankyrin’s functions. The best-documented function of SBD is to bind to spectrin (β-spectrin specifically), which requires the first ZU5 domain (ZU5N) only (16, 18). Thus, the second ZU5 domain (ZU5C) and the UPA domain of ankyrins are likely to be involved in functions other than binding to spectrin. Supporting this notion, a recent study of ankyrin-B and -G showed that a number of mutations in ZU5C and UPA had no impact on their binding to spectrin but led to functional impairment of ankyrins (8).
Our recent study of the ZU5-UPA-DD tandem of netrin receptor UNC5b revealed that its ZU5 interacts with both UPA and DD to form a supramodule with an auto-inhibited conformation (17). Because the ankyrin ZZU tandem is also followed by a DD, we proposed that ZU5C, UPA, and DD of ankyrins may also form a similar auto-inhibited conformation. PIDD, a scaffold protein that acts as a molecular switch in controlling programmed cell death (19), contains a similar ZZUD tandem (Fig. 1C). It remains to be established why ankyrins and PIDD contain two ZU5 domains arranged next to each other.
Here, we determined the high resolution crystal structure of the ZU5-ZU5-UPA-DD tandem (termed as ZZUD) of ankyrin-B, which reveals that the ZZU tandem indeed forms a structural supramodule. Within the supramodule, ZU5N but not ZU5C interacts with UPA. The ZU5N/UPA and the ZU5N/ZU5C interactions do not interfere ankyrin’s binding to spectrin. Further structural analysis of the ZZU tandem suggests that it contains two additional target binding sites, which may allow ankyrins to bind target proteins other than spectrin. Unexpectedly, unlike in UNC5b, DD in ankyrin-B ZZUD is not sequestered by the ZZU tandem. The structure of the ankyrin-B ZZUD tandem provides mechanistic explanations to a number of disease-causing mutations of ankyrins.
Results and Discussion
The Overall Structure of Ankyrin-B ZZUD.
To understand how the ZU5N, ZU5C, UPA, and DD domains of ankyrins assemble together to perform their functions, we attempted to solve the structure of ankyrin-B ZZUD by X-ray crystallography. After extensive trials, we discovered that the deletion of a nine-residue fragment in a solvent-exposed loop of ZU5C (Figs. 1A and 2) enabled us to obtain high diffraction-quality crystals of ZZUD. The ankyrin-B ZZUD structure was determined at a 2.2-Å resolution by molecular replacement (Table S1). The final structural model contains most of residues from ankyrin-B ZZUD except for a few flexible loops (Fig. 1A).
Fig. 2.
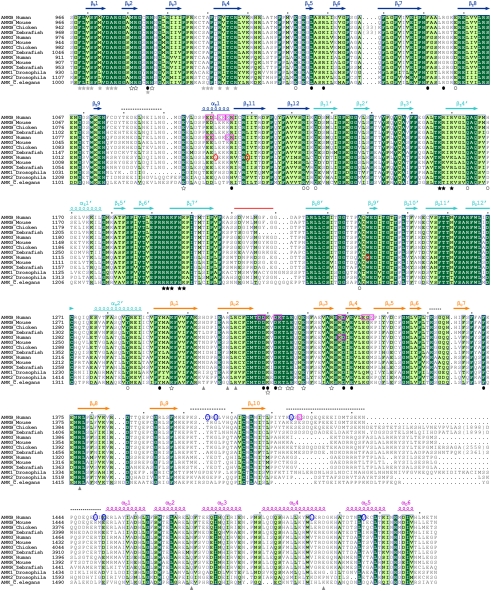
Sequence alignment of the ZZUD tandems from the ankyrin family members. In this alignment, residues that are absolutely conserved and highly conserved are highlighted in dark and light green, respectively. The secondary structural elements are indicated above the alignment and the coloring scheme matches with the structure of the protein shown in Fig. 1A. The nine deleted residues in the crystal structure are marked by a red line. The disordered regions (Fig. 1A) are indicated by dashed lines. The amino acid residues involved in the formation of the ZU5N/UPA and ZU5N/ZU5C interfaces are indicated with solid and open circles, respectively. The residues involved in the UPA/DD interaction are indicated by gray triangles. The residues involved in formation of the conserved surface across ZU5N and UPA and formation of the highly conserved, positively charged surface of ZU5C are indicated by hollow and solid stars, respectively. The residues involved in spectrin binding are indicated by gray stars. The identified loss-of-function mutations in ankyrin-B and -G (8, 21) are highlighted by pink open boxes. These mutations do not affect the spectrin binding of the ankyrins. The mutations of amino acids that cause ankyrin-B syndrome and hereditary spherocytosis are labeled with blue and red circles, respectively.
The four domains in the ankyrin-B ZZUD structure are well-defined (Fig. 1A). Despite of a lower than 20% amino acid sequence identity, the two ZU5 domains share a similar overall fold with an rmsd of approximately 2 Å among approximately 110 aligned residues. Each ZU5 domain contains approximately 150 residues and folds into a β-strand rich structure with two antiparallel sheets interacting with each other in parallel to form a β-sandwich. One obvious difference between the two ZU5 domains is that ZU5C contains an additional C-terminal α-helix (αZ2′), which covers the hydrophobic surface of the β-sheet and presumably stabilizes the domain (Fig. 1A). UPA also contains two antiparallel β-sheets but with a distinct topology from those of ZU5. Extensive interactions between the two ZU5 domains and between ZU5N and UPA domain stabilize the overall structure of the ZZU tandem, which adopts a cloverleaf-like architecture (Fig. 1A, Left). We note that ZU5N and UPA interact with each other mainly through their loop regions (Fig. 1A), and such ZU5/UPA interaction mode has also been observed in UNC5b (17) (Fig. 1B). Although being right next to each other in their primary sequences, ZU5C makes few contacts with UPA (Fig. 1A). The two ZU5 domains are separated by only one residue, and ZU5C and UPA are connected by a three-residue loop. Such short interdomain linkers also physically restrain the mobility of these three domains. Ankyrin-B DD adopts a typical death domain fold consisting of six helices, which is essentially identical to the solution structure of the isolated ankyrin-B DD with an rmsd value < 1.0 Å. In contrast to the extensive interactions between ZU5N and ZU5C and between ZU5N and UPA that lead to the formation of a stable ZZU supramodule, DD only loosely interacts with UPA (Fig. 1A; see below for details).
The ZZU Tandem Forms a Structural Supramodule.
The ZZU structure of ankyrin-B is mainly stabilized by the interactions of ZU5N with ZU5C and UPA, respectively. The buried surface areas of the ZU5N/UPA and ZU5N/ZU5C interfaces are approximately 1,600 Å2 and approximately 1,200 Å2, respectively. The residues forming the two interfaces are highly conserved among all ankyrins (Fig. 2), indicating that the ZU5N/UPA and ZU5N/ZU5C interactions and the overall architecture of the ZZU tandem observed in ankyrin-B are shared by other ankyrins as well.
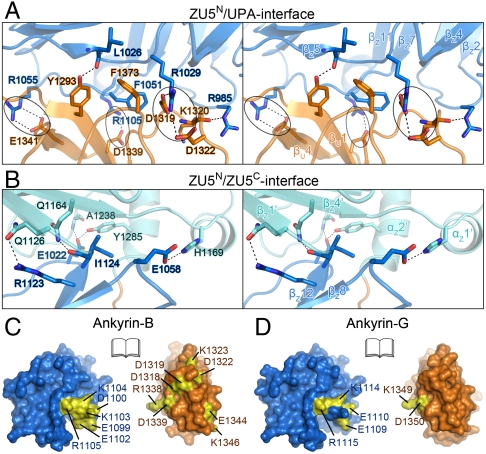
The ZU5N/UPA interface is primarily formed by residues from the loops of the two domains as well as two residues from β1 (Y1293) and β4 (E1341) of UPA (Fig. 3A). Three pairs of charge–charge interactions, formed by R1055ZU5N and E1341UPA, R1105ZU5C and D1339UPA, R1029ZU5N and D1319UPA/D1322UPA, are situated across the entire interface and thus are likely to be critical for the interdomain stability (Fig. 3A). Of the three pairs of the charged interactions, the R1029ZU5N/D1319UPA pair is buried in the interface and is likely to be particularly important for the ZU5N/UPA interaction. The ZU5N/UPA interface is further stabilized by several hydrogen bonds as well as hydrophobic interactions (Fig. 3A).
Fig. 3.
The ZU5N/UPA and ZU5N/ZU5C interfaces of ankyrin-B ZZUD. The molecular details are shown for the ZU5N/UPA (A) and ZU5N/ZU5C (B) interfaces. The salt bridges and hydrogen bonds are indicated as dashed lines. The three charge pairs in the ZU5N/UPA interface are highlighted by circles. The open-book views of the surfaces of the ZU5N/UPA interfaces in ankyrin-B (C) and -G (D) demonstrate that the identified loss-of-function mutations (colored in yellow) in ankyrin-B and -G (8, 21) are concentrated within or in the vicinity of the ZU5N/UPA interfaces of both ankyrins. The ankyrin-G ZZU structures are modeled by using the ankyrin-B ZZU structure as the template.
The ZU5N/ZU5C interface consists of residues from the β4/β5 loop, the β7/β8-loop, and β12 of ZU5N and β1′, β4′, the β4′/α1′-loop, and α2′ of ZU5C (Fig. 3B). These residues bridge the two domains together through both sidechain–sidechain and sidechain–main-chain hydrogen bonds (Fig. 3B). Compared with the ZU5N/UPA interface, the ZU5N/ZU5C interface contains much less residues and thus may be less rigid. However, because the two ZU5 domains are linked by only one residue, the degree of inter-ZU5 flexibility is limited.
We note with interest that the isolated ZU5N is well-folded (20), whereas the isolated ZU5C and UPA were always expressed as inclusion bodies, suggesting that ZU5C and UPA are not properly folded when expressed individually and may be stabilized in the ZZU supramodule via the interactions with ZU5N. Together, our structure strongly indicates that the ZZU tandem forms an integral structural supramodule, which is likely to be required for ankyrin’s functions.
The Intramolecular Interactions in the ZZU Tandem Do Not Affect the β-Spectrin Binding of ZU5N.
Ankyrin SBD interacts with β-spectrin via its ZU5N (16, 18, 20). Because ZU5N interacts with both UPA and ZU5C, one must wonder whether such intramolecular interactions might have any impact on the ankyrin/spectrin interaction. To answer this question, we built an ankyrin-B ZZUD/β-spectrin repeats 13–15 (R13-15) complex structure model by superimposing the ZU5N structure of ankyrin-B ZZUD with that of the ankyrin-R ZU5N/spectrin R13-15 complex (18) (Fig. 4). It is clear from the model that ZU5C is far away from the ZU5N/β-spectrin interface, thus is not expected to have any impact on the complex formation. Although UPA is physically close to β-spectrin R13-14, the contact between UPA and β-spectrin is minimal (Fig. 4). Thus, it is predicted that UPA is unlikely to have appreciable impact on the ZU5N/β-spectrin interaction either. Fully consistent with the above structure-based prediction, the Kd values of the ZZUD/β-spectrin and ZZUD_ΔUPA/β-spectrin complexes are essentially identical (Table S2). In addition, mutations of residues in the interdomain interfaces that are expected to disrupt the ZU5N/UPA and ZU5N/ZU5C interactions do not affect the ZU5N/spectrin interaction either (Table S2). Together, our biochemical and structural analysis demonstrates that the formation of the ZZU supramodule does not affect the spectrin binding of ZU5N. Our finding is fully consistent with the previously reported observations showing that ZU5N is necessary and sufficient for ankyrins to bind to spectrin (16, 18, 20).
Fig. 4.
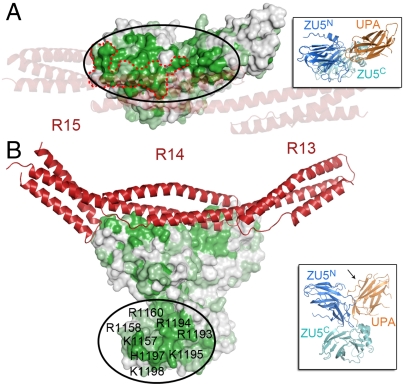
The ZZU tandem in ankyrins contains two highly conserved potential target protein-interacting surfaces. The structure of the ankyrin-B ZZU/β-spectrin R13-15 complex was modeled by superimposing ZU5N in the ankyrin-B ZZUD structure and that in the ankyrin-R ZU5N/β-spectrin R13-15 complex structure and showed with the top view (A) and the lateral view (B). In this modeled complex structure, the ZZU tandem is drawn in surface representation. Surface residues that are absolutely conserved, highly conserved, and not conserved according to the sequence alignment in Fig. 2, are colored in green, light green, and white, respectively. The two large conserved regions on the ZZU tandem are highlighted by circles. In A, the spectrin-binding surface on ZU5N is highlighted by a red dot trace; and for displaying the covered surface of the ZZU tandem, the β-spectrin R13-15 structure is shown as transparent ribbons. The highly conserved and positively charged surface residues of ZU5C are labeled. The insets show the orientations of the ankyrin-B ZZUD in the complex structure model in A and B, respectively. The β2/β3-loop of UPA is indicated by an arrow.
The ZU5N/UPA Interaction Is Required for Ankyrins’ Function Other than Binding to Spectrin.
Although ZU5N is necessary and sufficient for binding to spectrin, the extremely high amino acid sequence conservation of the entire ZZU tandem among ankyrins throughout evolution argues for indispensable functional roles of their ZU5C and UPA domains. Recently, Kizhatil et al. used an alanine-scanning-based approach and identified a number of residues within the ZZU tandems that are functionally important for ankyrin-B and -G (8). A number of point mutations were found to be unable to rescue incorrect localization of the InsP3 receptor in cardiomyocytes (the ankyrin-B mutants) or misassemble of the lateral membranes of epithelia (ankyrin-G mutants) induced by “knockdown” of endogenous ankyrins, but interestingly these mutations do not disrupt the ankyrin/spectrin interaction (8). Strikingly, by mapping the mutations in ankyrin-B to the structure, we found that almost all the mutated residues are located within or in the vicinity of the ZU5N/UPA interface (Fig. 3C). Three of such mutation sites of ankyrin-B, R1105ZU5N, D1322UPA, and D1339UPA, are involved in forming the three charge pairs in the ZU5N/UPA interface (Fig. 3A). A more recent study found that ankyrin-B interacts with dynactin-4 (21). Substitution of D1319UPA with alanine impairs the ankyrin-B/dynactin-4 interaction but does not interfere with the spectrin-binding ability of ankyrin-B (21). D1319UPA forms the buried salt bridge with R1029ZU5N in the ZU5N/UPA interface (Fig. 3A), and the mutation is expected to disrupt the ZU5N/UPA interaction of ankyrin-B. Like the mutations in ankyrin-B, the mutations in ankyrin-G are also localized in the ZU5N/UPA interface (Fig. 3D). Thus, the structure of the ZZUD tandem, together with these mutagenesis data, reveals that the ZU5N/UPA interaction formed by the conserved charged residues is critical for the non-spectrin-binding functions of SBD, and that the ZZU tandem acts as a structural as well as a functional supramodule for binding to target proteins other than spectrin (e.g. dynactin-4).
Ankyrin-B ZZU Contains Two Additional Potential Target Binding Sites.
To gain clues on possible additional target binding, we mapped the degree of amino acid sequence conservation (based on the sequence alignment shown in Fig. 2) to the surface of the ankyrin-B ZZU structure. We found that the highly conserved residues are clustered into two separated regions on the surface of ZZU (Fig. 4). The first one is on ZU5N and UPA, mainly composed of highly conserved residues from β1, β2, β4, and the loops connecting β1/β2, β2/β3, and β3/β4 of ZU5N, as well as the C-terminal part of long β2/β3-loop of UPA (Figs. 2 and 4A). Part of this region is involved in the binding to spectrin (Fig. 4A), substantiating the conclusion that spectrin binding is an evolutionally conserved property for all ankyrins. However, a large part of this region, including the β2/β3-loop of ZU5N and the β2/β3-loop of UPA, is not involved in the spectrin binding. The solvent-exposed residues in these two loops are not expected to play a structural role for ZU5N or UPA. Thus, this conserved surface crossing both ZU5N and UPA may interact with still unknown target(s), and such interaction(s) is likely to be a common property of all ankyrins. Interestingly, this conserved surface on UPA is partially blocked by the ZU5N-bound spectrin (Fig. 4A), suggesting that the hypothetical binding protein may regulate spectrin binding to ankyrins or vice versa. The second surface is composed of highly conserved residues from β4′, β7′, the β3′/β4′-loop, and the β6′/β7′-loop of ZU5C (Fig. 4B). Most of these conserved surface residues are positively charged, such as K1157, R1158, R1160, R1193, R1194, K1195, H1197, and K1198 (Figs. 2 and 4B). It is highly likely that this large, positively charged surface on ZU5C may function to bind to negatively charged target protein(s). Although the second region does not involve ZU5N and UPA, the formation of the ZZU supramodule fixes the orientation of ZU5C in the ZZU supramodule. Obviously, further work is required to test the structure-based predictions of the two additional potential target binding sites common to all ankyrins.
DD is not sequestered by ZZU in ankyrin-B.
In the UNC5b ZU5-UPA-DD structure, ZU5 tightly sequesters DD through hydrophobic interactions and prevents the domain from forming homodimer or from interacting with other death domains (17). Because ankryin-B DD and UNC5b DD are similar in both sequence and structure, ankyrin-B DD was originally predicted to be sequestered by its ZU5 domains too. Unexpectedly, the ankyrin-B ZZUD structure reveals that neither ZU5N nor ZU5C binds to DD. Compared to the ZU5/DD interface in UNC5b, the corresponding surface in ankyin-B ZU5N is covered by a loop connecting α1 and β9 (Fig. 1 A and B and Fig. S1) and thus is not accessible to DD. Although the corresponding surface of ZU5C is accessible, the corresponding hydrophobic residues critical for the UNC5b ZU5/DD interaction (17) are lacking in ankyrin-B ZU5C. Therefore, ankyrin-B ZU5C does not bind to its DD either. Instead, we observe the direct interaction between UPA and DD in the ankyrin-B ZZUD crystal (Fig. 1A). This UPA/DD interaction is mediated by several salt bridges formed by residues that are not conserved in other ankyrins (Fig. 2 and Fig. S2A), indicating that the UPA/DD interaction is likely to be an artifact of the crystal packing. To test the above prediction, we inserted the human rhinovirus (HRV) 3C protease cleavage site in the long flexible loop between UPA and DD of ankyrin-B. The insertion of the protease cleavage site does not change the elution volume of ZZUD on an analytical gel filtration column (Fig. S2B). Protease 3C cleavage of the insertion mutant produced two fragments corresponding to the ZZU tandem and the isolated DD (Fig. S2C), indicating that protease released DD does not associate with ZZU in solution. Additionally, we found that the ZZUD tandem and the ZZU tandem binding to spectrin with the same affinity (Table S2), suggesting that the DD/UPA interaction found in the ZZUD crystal does not exist in solution, as otherwise the spectrin R13-14 would physically crash into DD in the ZZUD/spectrin complex (Fig. S3). Therefore, unlike what is found in UNC5b, ankyrin DD is not sequestered by its ZZU tandem, and the domain is free to interact with its potential targets such as Fas DD (22). It is noted that a number of ankyrin-G and -B splicing variants contain large insertions (> 1,000 residues) between SBD and DD (23, 24), which may further separate DD from the ZZU tandem.
The Functional Implications of the Disease-Causing Mutations in ZZUD.
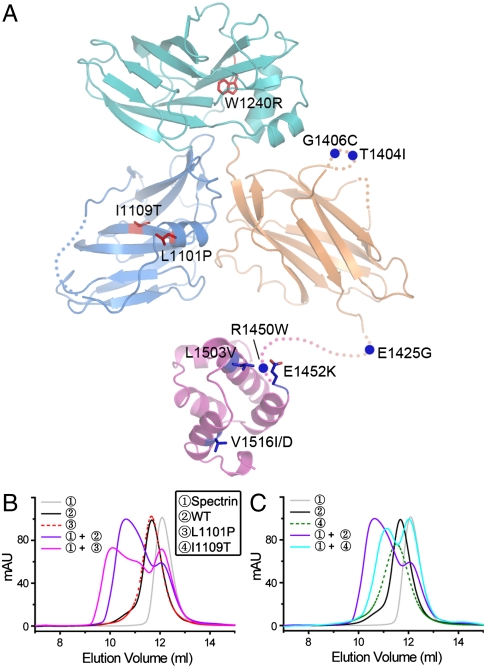
A total of eight inherited missense mutations involving seven residues in ankyrin-B ZZUD have been reported in patients with the ankyrin-B syndrome (25–29), and three loss-of-function mutations in ankyrin-R ZZUD were found to cause hereditary spherocytosis (9). Our structure provides insights into the mechanistic bases of the diseases caused by these mutations. The eight ankyrin-B syndrome-causing mutations are T1404I, G1406C, E1425G, R1450W, E1452K, L1503V, V1516I, and V1516D. Four out of the seven mutation sites are located in two disordered loops (T1404 and G1406 in the β2/β3-loop of UPA; E1425 and R1450 in the loop connecting UPA and DD, Fig. 5). The other three mutation sites (E1452, L1503, and V1516) involve residues on the solvent-exposed surface of DD (Fig. 5). Curiously, most of these seven residues are not conserved among the ankyrin family members (Fig. 2), and none of these mutation sites is involved in the binding of ankyrin to spectrin. Our structural and sequence analysis suggest that these eight ankyrin-B syndrome-causing mutations are not likely to cause overall folding alterations of the domains in the ZZUD tandem, but instead these mutations may change ankyrins’ binding to nonspectrin targets. We characterized biochemical properties of two loss-of-function mutations (G1406C and E1415G) of ankyrin-B ZZUD. As predicted, the G1406C and E1425G mutants of ankyrin-B ZZUD showed no observable defects in folding or in the binding to spectrin (Fig. S4 and Table S2). We note with interest that the E1425G mutant of ankyrin-B, which causes human cardiac arrhythmia (25, 26), can directly affect the N-terminal ankyrin repeats-mediated binding of Na/K ATPase, Na/Ca exchanger, and InsP3 receptor (30). These findings suggest that the ankyrin-B ZZUD tandem is likely to bind to other proteins, thereby to regulate its N-terminal ankyrin repeats-mediated membrane binding. The three disease-causing mutations of ankyrin-R (L1046P, I1054T, and W1185R) involve three highly conserved residues, corresponding to L1101 and I1109 in ZU5N and W1240 in ZU5C in ankyrin-B, indicating that these three residues play common roles for all ankyrins. Based on the ankyrin-B ZZUD structure, all these residues, W1240 in particular, are involved in the formation of the hydrophobic cores of either ZU5N or ZU5C (Fig. 5 and Fig. S5). The mutations of any one of these three residues are expected to alter the conformation of ZU5N or ZU5C and therefore impair the function of ankyrin-R. To test the above hypothesis, we characterized all three mutants of ankyrin-B ZZUD. The W1240R mutant displayed rapid degradation during protein purification, and we were not able to obtain any purified protein for biochemical characterization. Because W1240 forms the folding core of ZU5C (Fig. S5B), and substitution of the bulky tryptophan with positively charged arginine is expected to disrupt the folding and thus the function of the protein. Circular dichroism-based protein stability studies showed that the L1101P or the I1109T substitutions only displayed marginal impact on the overall folding of the ZZUD tandem (Fig. S4). Because L1101 and I1109 are both localized in ZU5N (Fig. S5A), we tested possible impact of each mutation on ZZUD’s binding to spectrin. Although the L1101P mutant can still bind to spectrin (Fig. S7), the complex formed is heterogeneous (Fig. 5B), indicating that the L1101P mutation can cause misassembly of the ankyrin/spectrin complex. The ZZUD_I1109T mutant showed a weakened spectrin binding (Fig. 5C). Therefore, the ZZUD structure provides a mechanistic basis for understanding ankyrin-R mutation-induced spherocytosis in humans. It is noted that the mutation of W1185 in ankyrin-R, which is located in ZU5C and not involved in spectrin binding, can cause hereditary spherocytosis, further indicating that ZU5C-mediated binding(s) to nonspectrin target is important functional property of ankyrins.
Fig. 5.
The disease-causing mutations in the ankyrin ZZUD tandem. (A) The disease-causing mutations in ankyrins are mapped onto the structures of the ZZU tandem and DD. The ankyrin-B syndrome mutations and hereditary spherocytosis mutations in ankyrin-R are colored in blue and red, respectively. The disordered loops are indicated by dotted lines. (B) Analytical gel filtration chromatography comparing the interactions of the wild-type (WT) ankyrin-B ZZUD and the L1101P mutant with β-spectrin R14-15. The L1101P mutant formed multiple complex peaks with β-spectrin R14-15. (C) The interactions of the WT ankyrin-B ZZUD and the I1109T mutant with β-spectrin R14-15. The I1109T mutant/β-spectrin R14-15 complex was eluted at a larger volume compared to the WT ZZUD, indicating that the mutant has a somewhat weaker binding to β-spectrin.
The PIDDosome organization center PIDD also contains two ZU5 domains in its ZZUD tandem (Fig. 1C). A structure-based analysis of the PIDD amino acid sequence suggests that the ZZU tandem of PIDD may adopt a similar supramodular conformation to that of ankyrin-B shown in the current study rather than that of UNC5b (Fig. S6). The ankyrin-B ZZUD structure can be used as a valuable template for exploring the molecular basis underlying apoptosis mediated by the PIDD-assembled cell death machinery (31).
In conclusion, we have solved the crystal structure of ankyrin-B ZZUD. The intramolecular interactions of ZU5N/UPA and ZU5N/ZU5C stabilize the ZZU architecture. The extensive ZU5N/UPA interaction provides a mechanistic explanation for previously identified functional deficient mutations found in ankyrin-B&G. The structure further indicates that the previously coined spectrin-binding domain of ankyrins binds to target proteins in addition to spectrin. Unlike UNC5b ZU5-UPA-DD, ankyrin-B DD is not sequestered by its own ZZU. Finally, the ankyrin-B ZZUD structure provides mechanistic explanations to many disease-causing mutations identified in ankyrins.
Materials and Methods
The ankyrin-B ZZUD protein was expressed in Escherichia coli BL21(DE3) and purified by Ni2+-NTA affinity chromatography followed by size-exclusion chromatography. Crystals were obtained by hanging drop vapor diffusion method at 16 °C in 18% w/v PEG3350 and 0.2 M ammonium acetate. An extended method describing protein preparation, crystallization, structure determination, and biochemical assays can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Vann Bennett for providing the ankyrin-B cDNA construct and the BL17U beamline of the Shanghai Synchrotron Radiation Facility for the beamline time. This work was supported by grants from the Research Grants Council of Hong Kong to M.Z. (663808, 664009, 660709, 663610, 663811, HKUST6/CRF/10, and SEG_HKUST06) and to Z.W. (662710). Z.W. is a fellow of the Tin Ka Ping Foundation under IAS of HKUST.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pbd.org (PDB ID code 4D8O).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200613109/-/DCSupplemental.
References
- 1.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol. 2009;1:a003012. doi: 10.1101/cshperspect.a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett V, Stenbuck PJ. Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1979;254:2533–2541. [PubMed] [Google Scholar]
- 4.Bennett V, Healy J. Organizing the fluid membrane bilayer: Diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, et al. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedstrom KL, et al. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kizhatil K, Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. J Biol Chem. 2004;279:16706–16714. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 8.Kizhatil K, et al. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher PG. Hematologically important mutations: Ankyrin variants in hereditary spherocytosis. Blood Cells Mol Dis. 2005;35:345–347. doi: 10.1016/j.bcmd.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira MA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze TG, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2009;14:487–491. doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott LJ, et al. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci USA. 2009;106:7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EN, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MT, et al. Genome-wide association study of bipolar I disorder in the Han Chinese population. Mol Psychiatry. 2011;16:548–556. doi: 10.1038/mp.2010.43. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009;47:203–209. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 17.Wang R, et al. Autoinhibition of UNC5b revealed by the cytoplasmic domain structure of the receptor. Mol Cell. 2009;33:692–703. doi: 10.1016/j.molcel.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ipsaro JJ, Mondragon A. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. doi: 10.1182/blood-2009-11-255604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Ipsaro JJ, Huang L, Gutierrez L, MacDonald RI. Molecular epitopes of the ankyrin-spectrin interaction. Biochemistry. 2008;47:7452–7464. doi: 10.1021/bi702525z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayalon G, et al. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem. 2011;286:7370–7378. doi: 10.1074/jbc.M110.187831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Rio M, et al. The death domain of kidney ankyrin interacts with Fas and promotes Fas-mediated cell death in renal epithelia. J Am Soc Nephrol. 2004;15:41–51. doi: 10.1097/01.asn.0000104840.04124.5c. [DOI] [PubMed] [Google Scholar]
- 23.Chan W, Kordeli E, Bennett V. 440-kD ankyrinB: Structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J Cell Biol. 1993;123:1463–1473. doi: 10.1083/jcb.123.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 25.Mohler PJ, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 26.Mohler PJ, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohler PJ, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: Human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 28.Sherman J, Tester DJ, Ackerman MJ. Targeted mutational analysis of ankyrin-B in 541 consecutive, unrelated patients referred for long QT syndrome genetic testing and 200 healthy subjects. Heart Rhythm. 2005;2:1218–1223. doi: 10.1016/j.hrthm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Mank-Seymour AR, et al. Association of torsades de pointes with novel and known single nucleotide polymorphisms in long QT syndrome genes. Am Heart J. 2006;152:1116–1122. doi: 10.1016/j.ahj.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinel A, et al. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-kappaB pathway. EMBO J. 2007;26:197–208. doi: 10.1038/sj.emboj.7601473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.