Abstract
Atopic dermatitis (AD) skin lesions exhibit epidermal and dermal thickening, eosinophil infiltration, and increased levels of the cysteinyl leukotriene (cys-LT) leukotriene C4 (LTC4). Epicutaneous sensitization with ovalbumin of WT mice but not ΔdblGATA mice, the latter of which lack eosinophils, caused skin thickening, collagen deposition, and increased mRNA expression of the cys-LT generating enzyme LTC4 synthase (LTC4S). Skin thickening and collagen deposition were significantly reduced in ovalbumin-sensitized skin of LTC4S-deficient and type 2 cys-LT receptor (CysLT2R)–deficient mice but not type 1 cys-LT receptor (CysLT1R)-deficient mice. Adoptive transfer of bone marrow-derived eosinophils from WT but not LTC4S-deficient mice restored skin thickening and collagen deposition in epicutaneous-sensitized skin of ΔdblGATA recipients. LTC4 stimulation caused increased collagen synthesis by human skin fibroblasts, which was blocked by CysLT2R antagonism but not CysLT1R antagonism. Furthermore, LTC4 stimulated skin fibroblasts to secrete factors that elicit keratinocyte proliferation. These findings establish a role for eosinophil-derived cys-LTs and the CysLT2R in the hyperkeratosis and fibrosis of allergic skin inflammation. Strategies that block eosinophil infiltration, cys-LT production, or the CysLT2R might be useful in the treatment of AD.
Keywords: murine model of atopic dermatitis, eicosanoid
Skin thickening with hyperkeratosis and increased type I collagen deposition is an important feature of chronic atopic dermatitis (AD) (1). Eosinophil infiltration of target tissues is an important feature of allergic diseases, including AD. Tissue eosinophilia has long been associated with fibrosis because eosinophils and their derived products are commonly present in inflammatory fibrotic lesions (2). In addition to the release of cytotoxic granule proteins, which include major basic protein and eosinophil cationic protein, eosinophils secrete an array of inflammatory and fibrogenic mediators, including lipid mediators, chemokines, and cytokines (3). Accumulating evidence has suggested a potential role for eosinophils in airway remodeling in asthma. Genetic ablation of eosinophils, or reduction of pulmonary eosinophilia by targeting IL-5, significantly reduces subepithelial deposition of ECM proteins in a mouse model of chronic pulmonary inflammation (4). In mild asthmatics treated with anti–IL-5, reduction of infiltrating numbers of eosinophils in the bronchial mucosa was associated with a significant decrease in the expression of ECM proteins (5).
Cysteinyl leukotrienes (cys-LTs) include leukotriene C4 (LTC4), which is synthesized by a variety of cells and enzymatically converted into leukotriene D4 and then leukotriene E4 by cleavage of its peptide side chain. LTC4 is formed by LTC4 synthase (LTC4S) through the conjugation of glutathione to the unstable intermediate leukotriene A4 (LTA4) which is generated by the action of 5-lipoxygenase (5-LO) on released arachidonic acid in the presence of the 5-LO activating protein. LTA4 can also be converted to a dihydroxy leukotriene, leukotriene B4 (LTB4), by LTA4 hydrolase (LTA4H). Eosinophils express LTC4S but not LTA4H, and they are a main source of cys-LTs but not of LTB4 (6). The cys-LTs are important for antigen-induced airway eosinophilic inflammation and hyperresponsiveness in mice (7) and have been reported to play a critical role in lung tissue fibrosis induced by repetitive airway challenge with antigen. The cys-LTs are also involved in a pulmonary fibrosis model elicited by bleomycin (8, 9).
AD is characterized by eosinophil infiltration and fibrosis in chronic skin lesions (10). Levels of LTC4 are increased in extracts of the skin lesions and in serum of patients with AD, and they decrease with amelioration of the disease (11). The role of eosinophils and cys-LTs in skin thickening and collagen deposition in AD is unknown. We have developed a mouse model of allergic skin inflammation using repeated epicutaneous (EC) sensitization with ovalbumin (OVA) to tape-stripped skin (12). This model has many similarities to human AD. It includes elevated total and antigen-specific blood IgE levels, as well as dermatitis characterized by epidermal and dermal thickening; infiltration of CD4+ T cells and eosinophils; and local expression of mRNA for the T-helper 2 (Th2) cytokines Il4, Il5, and Il13. Using this model, we demonstrate that eosinophil-derived LTC4 and the type 2 cys-LT receptor (CysLT2R) are critical for skin thickening and increased collagen deposition.
Results and Discussion
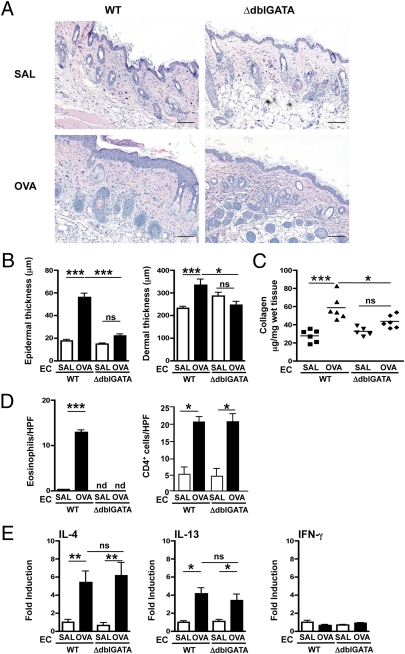
Impaired Thickening and Collagen Deposition in OVA-Sensitized Skin of Eosinophil-Deficient ΔdblGATA Mice.
We initiated these studies of allergic skin inflammation with ΔdblGATA mice because they are selectively deficient in eosinophils but have normal development of all other hematopoietic cell lineages, including mast cells, neutrophils, and macrophages (13). EC sensitization of WT BALB/c mice with OVA caused epidermal and dermal thickening as previously reported (12), as well as collagen deposition in the skin (Fig. 1 A–C). In contrast, EC sensitization with OVA caused no increase in epidermal and dermal thickness or collagen deposition in the skin of ΔdblGATA mice. Eosinophils were increased in OVA-sensitized skin of WT mice, as previously reported (12). Eosinophils were not detectable in the skin of ΔdblGATA mice, as expected (Fig. 1D). Dermal infiltration by CD4+ cells in OVA-sensitized skin and expression of mRNA for the Th2 cytokines Il4 and Il13 were comparably increased in OVA-sensitized skin of ΔdblGATA mice and WT controls (Fig. 1E). Expression of mRNA for Ifnγ did not increase in OVA-sensitized skin of WT or ΔdblGATA mice. The systemic immune response to EC sensitization with OVA was comparable in ΔdblGATA mice and WT controls, as evidenced by levels of OVA-specific serum IgE and IgG antibodies and of cytokines produced by OVA stimulation of cultured splenocytes (Fig. S1 A and B). These results suggest that eosinophils are important for skin thickening and collagen deposition in allergic skin inflammation. The dependence of skin thickening and collagen deposition on eosinophils in our model is consistent with the significant correlation observed between the number of infiltrating eosinophils and collagen deposition in patients with AD skin lesions (1).
Fig. 1.
Impaired epidermal and dermal thickening and collagen deposition in OVA-sensitized skin of ΔdblGATA mice. (A) Representative photomicrographs of H&E-stained sections. (Scale bars: 100 μm.) (B) Epidermal and dermal thickness. (C) Collagen content. (D) Numbers per high-power field (HPF) of eosinophils and CD4+ cells infiltrating the dermis. (E) Th2 and Th1 cytokine mRNA expression relative to saline (SAL)-sensitized skin of WT mice. Similar results were obtained in two other independent experiments with five mice per group. Columns and error bars represent the mean and SEM (n = 5–6 per group). *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant; nd, not detectable.
IL-4 and IL-13 have been reported to up-regulate collagen synthesis in fibroblasts (14, 15) and to play an important role in eosinophil recruitment to tissues (16). The comparable expression of Il4 and Il13 mRNA in OVA-sensitized skin of ΔdblGATA and WT mice suggests that these two cytokines do not play a direct role in skin thickening and collagen deposition in our model. Others have reported that collagen deposition in the lungs of mice that express an IL-13–inducible transgene is abrogated when these mice are crossed with ΔdblGATA mice (17). Thus, IL-4 and IL-13 could play a more proximal supporting role in collagen deposition in the inflamed skin.
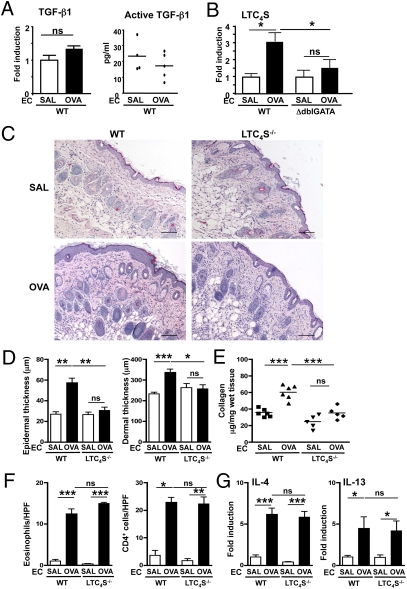
Impaired Thickening and Collagen Deposition in OVA-Sensitized Skin of LTC4S-Deficient Mice.
Eosinophils produce the profibrotic cytokine TGF-β1 (3). EC sensitization with OVA caused no significant change in local Tgfβ1 mRNA expression or active TGF-β1 protein in WT mice (Fig. 2 A and B). This finding suggests that TGF-β1 is unlikely to account for the increased collagen deposition in our model. It is also consistent with the observations that Tgfβ1 mRNA levels are not increased in AD skin lesions (18) and that collagen deposition in the lung in a mouse model of antigen-induced chronic airway inflammation is not associated with increased expression of active TGF-β1 (4).
Fig. 2.
Impaired epidermal and dermal thickening and collagen deposition in OVA-sensitized skin of Ltc4s−/− mice. (A) Tgfβ1 mRNA expression and TGF-β1 protein levels. (B) Ltc4s mRNA expression in EC-sensitized skin. (C) Representative photomicrographs of H&E-stained sections. (Scale bars: 100 μm.) (D) Epidermal and dermal thickness. (E) Collagen content. (F) Numbers per high-power field (HPF) of eosinophils and CD4+ cells infiltrating the dermis. (G) Il4 and Il13 mRNA expression relative to saline (SAL)-sensitized skin of WT mice. Columns and error bars represent the mean and SEM (n = 5–6 per group). Similar results were obtained in two other independent experiments with five mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
EC sensitization with OVA caused a significant increase in local Ltc4s mRNA expression in WT mice but not in ΔdblGATA mice (Fig. 2B). This result suggests that eosinophils are the main source of the increased Ltc4s mRNA expression in OVA-sensitized skin. Although macrophages and mast cells generate LTC4 with activation and their numbers are increased in OVA-sensitized mouse skin sites (12), as well as in AD skin lesions (19), these two cell types do not appear to contribute to the increase in Ltc4s mRNA expression in OVA-sensitized skin.
To examine the potential role of cys-LTs in skin thickening and collagen deposition in allergic skin inflammation, we examined the response of LTC4S-deficient (Ltc4s−/−) mice to EC sensitization. In contrast to genetically matched BALB/c WT controls, Ltc4s−/− mice failed to exhibit epidermal and dermal thickening or increased collagen deposition at sites of EC sensitization with OVA (Fig. 2 C–E). Dermal infiltration by CD4+ cells and eosinophils and mRNA expression of Il4 and Il13 were comparable in OVA-sensitized skin of Ltc4s−/− mice and WT controls (Fig. 2 F and G). The systemic immune response of the two groups to EC sensitization with OVA was also comparable in the levels of OVA-specific serum IgE and IgG antibodies and of cytokines produced by OVA-stimulated cultured splenocytes (Fig. S1 C and D). The data obtained in Ltc4s−/− mice on BALB/c background was similar for Ltc4s−/− mice on C57BL/6 background (Fig. S2). The normal Th2 response to EC sensitization in Ltc4s−/− mice is in contrast to the reported decreased Th2 response of Ltc4s−/− mice to i.p. immunization with OVA without alum and aerosol challenge (7). In the lung model, the function of the cys-LT pathway is directed to the antigen-presenting dendritic cells (20), whereas in the EC-initiated allergic skin model, its role is downstream. These results indicate that cys-LTs play an important role in skin thickening and collagen deposition in our model, independent of Th2 cytokines.
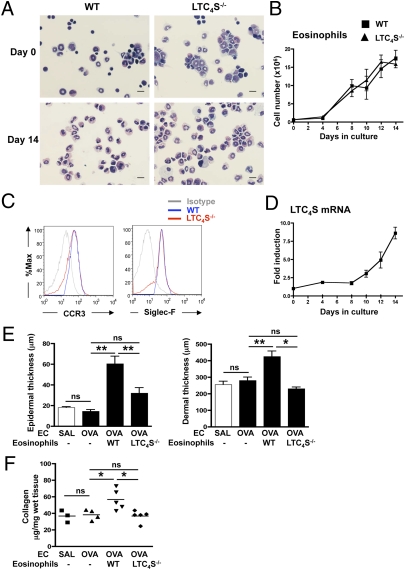
Eosinophil LTC4S Is Important for Thickening and Collagen Deposition in OVA-Sensitized Mouse Skin.
We next examined whether cys-LTs produced by eosinophils are important for the development of skin thickening and collagen deposition at sites of allergic skin inflammation. To this purpose, we reconstituted ΔdblGATA mice with eosinophils differentiated ex vivo from the bone marrow (BM) of WT and Ltc4s−/− mice by culture for 14 d with recombinant mouse stem cell factor (SCF), Flt-3 ligand, and IL-5 (21). At the initiation of culture, BM cells from WT mice contained ∼6% eosinophils (6.21 ± 0.16%, n = 3) as determined by modified Giemsa staining (Fig. 3A). Following ex vivo differentiation of WT BM cells, the total cell number increased ∼18-fold (Fig. 3B) and >90% (91.47 ± 3.7%, n = 3) of the cells exhibited the bilobed nucleus and eosinophilic granulated cytoplasm characteristic of mature eosinophils (Fig. 3A). FACS analysis revealed that the vast majority of the cells expressed the eosinophil surface markers CCR3 and Siglec-F (BD Bioscience) (Fig. 3C). Quantitative PCR (qPCR) analysis demonstrated that the levels of Ltc4s mRNA increased almost ninefold in the course of differentiation of WT BM cells (Fig. 3D). BM cells differentiated from Ltc4s−/− mice exhibited comparable expansion, percentage of eosinophils, and surface marker expression (Fig. 3 A–C). As expected, Ltc4s mRNA was undetectable in cells differentiated from the BM of Ltc4s−/− mice.
Fig. 3.
Adoptively transferred WT but not Ltc4s−/− BMeos restore epidermal and dermal thickening and collagen deposition in OVA-sensitized skin of ΔdblGATA mice. Ex vivo differentiation of mouse BMeos: representative light microscopic image of cultured BM cells at day 0 and day 14 (A), total cell number during culture to day 14 (B), representative FACS analysis of CCR3 and Siglec-F expression on cultured BM cells at day 14 (C), and Ltc4s mRNA expression levels during culture to day 14 for BM cells from WT mice (D). (Scale bars: 25 μm.) Expression levels were normalized to GAPDH and expressed relative to day 0. Epidermal and dermal thickness (E) and collagen content (F) in EC-sensitized skin of ΔdblGATA recipients of BMeos derived from WT and Ltc4s−/− donors. Columns and error bars represent the mean and SEM (n = 4–5 per group). Similar results were obtained in another independent experiment with three mice per group. *P < 0.05; **P < 0.01. %Max, maximum percentage; ns, not significant; SAL, saline.
BM-derived eosinophils (BMeos) were administered i.v. (3 × 106 per mouse) to ΔdblGATA recipients at days 43 and 46 of the 49-d EC sensitization protocol. Adoptive transfer of BMeos from WT but not Ltc4s−/− mice rescued the development of epidermal and dermal thickening as well as collagen deposition in the OVA-sensitized skin of ΔdblGATA recipients (Fig. 3 E and F). These results indicate that generation of cys-LTs by eosinophil-derived LTC4S is critical for the development of fibrosis at sites of allergic skin inflammation.
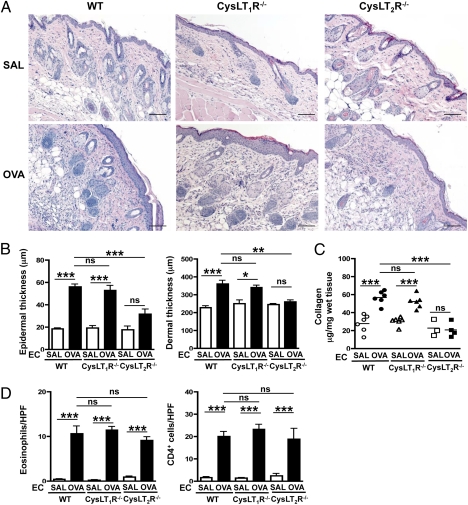
Expression of CysLT2R but Not CysLT1R Is Important for Thickening and Collagen Deposition in OVA-Sensitized Mouse Skin.
To examine the relative role of CysLT1R and CysLT2R in skin thickening and collagen deposition at sites of allergic skin inflammation, we examined the response of receptor-deficient strains. CysLT1R-deficient (Cysltr1−/−) mice EC-sensitized with OVA developed significant increases in epidermal and dermal thickening, collagen deposition, and numbers of eosinophils and CD4+ cells in the dermis, comparable to those in WT BALB/c controls (Fig. 4). In contrast, CysLT2R-deficient (Cysltr2−/−) mice on the same BALB/c background failed to manifest significant epidermal and dermal thickening or collagen deposition in OVA-sensitized skin sites (Fig. 4 A–C). However, dermal infiltration by CD4+ cells and eosinophils was comparable in Cysltr2−/− mice and WT controls (Fig. 4D). These results indicate that CysLT2R, rather than CysLT1R, is the receptor used by cys-LTs to mediate fibrosis at sites of allergic skin inflammation. This finding is in agreement with the results of our previous studies showing that Cysltr2−/− mice but not Cysltr1−/− mice are protected against bleomycin-induced lung fibrosis (9), in which, however, we did not identify the cell source of the cys-LTs. The finding in the cutaneous model of AD that eosinophil-derived cys-LTs and CysLT2R are in the path for induced collagen synthesis and fibrosis contrasts with the established role of CysLT1R in cys-LT–mediated vascular and airway smooth muscle responses (22).
Fig. 4.
Epidermal and dermal thickening and collagen deposition in OVA-sensitized skin of Cysltr1−/− and Cysltr2−/− mice. (A) Representative photomicrographs of H&E-stained sections. (Scale bars: 100 μm.) (B) Epidermal and dermal thickness. (C) Collagen content. (D) Numbers per high-power field (HPF) of eosinophils and CD4+ cells infiltrating the dermis. Columns and error bars represent the mean and SEM (n = 6 per group). Similar results were obtained in another independent experiment with four mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant; SAL, saline.
LTC4 Signaling via CysLT2R in Human Skin Fibroblasts Drives Collagen Synthesis and Secretion of Factors That Elicit Keratinocyte Proliferation.
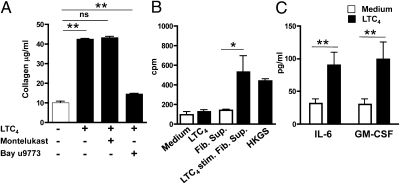
We examined whether cys-LTs act directly on skin-derived fibroblasts to increase their collagen production. We used primary human skin fibroblasts in an attempt to relate our findings to humans. Primary human skin fibroblasts expressed both receptors as determined by qPCR analysis (Fig. S3), and as observed by others in mouse skin fibroblasts (23). Stimulation of primary human skin fibroblasts for 24 h with LTC4 significantly increased their collagen production (Fig. 5A), as observed previously for rat lung fibroblasts (24). Only very low levels of collagen were detected in cell lysates of unstimulated and stimulated fibroblasts, suggesting that the majority of collagen measured in the supernatants was newly synthesized. These responses of human fibroblasts are unlikely to be attributable to increased cell survival or proliferation, because LTC4 has previously been shown to have no detectable effect on human skin fibroblast cell number or proliferation in culture (25). More importantly, LTC4 stimulation of collagen production by human skin fibroblasts was blocked by the dual CysLT1R and CysLT2R inhibitor BAY u9773 but not by the selective CysLT1R inhibitor montelukast (Fig. 5A). These results suggest that LTC4 acts directly on skin fibroblasts via CysLT2R to drive collagen production.
Fig. 5.
LTC4 signaling in human skin fibroblasts drives collagen synthesis and secretion of factors that elicit keratinocyte proliferation. (A) Collagen levels in culture supernatant of normal human skin fibroblasts stimulated for 24 h with 1 μM LTC4 in the absence of cys-LT receptor antagonists. (B) Effect of LTC4 and supernatants from LTC4 stimulated normal human skin fibroblasts on the proliferation of normal primary human keratinocytes. Stimulation with human keratinocyte growth supplement (HKGS) was used as a positive control. (C) Effect of 1 μM LTC4 on IL-6 and GM-CSF production by normal human skin fibroblasts. Columns and error bars represent the mean and SEM (n = 3 replicates per group). Similar results were obtained in another independent experiment with three replicates per group. *P < 0.05; **P < 0.01. Fib. Sup., fibroblast supernatant; ns, not significant.
AD skin lesions demonstrate increased proliferation of keratinocytes (26). The decreased epidermal skin thickening in Ltc4s−/− mice prompted us to seek a mechanism(s) by which cys-LTs might drive keratinocyte proliferation. Consistent with the failure to detect Cysltr1 or Cysltr2 mRNA expression in these cells by qPCR analysis, LTC4 treatment failed to cause proliferation of primary human keratinocytes (Fig. 5B). However, supernatants of skin fibroblasts that had been stimulated for 24 h with LTC4 caused a significant increase in keratinocyte proliferation, as evidenced by [3H]-thymidine incorporation (Fig. 5B). Several cytokines, including IL-6 and GM-CSF, are known to promote keratinocyte proliferation (27), and LTC4 stimulation over 24 h induced human skin fibroblasts to increase their production of IL-6 and GM-CSF (Fig. 5C). These results suggest that LTC4 promotes keratinocyte proliferation indirectly by causing fibroblasts to secrete keratinocyte growth factors.
Taken together, the findings reveal the eosinophil as the source of the cys-LTs in a mouse model of allergic skin inflammation and that LTC4 acts via the CysLT2R to induce the fibroblasts to lay down collagen and release factors that stimulate keratinocyte proliferation. Our results suggest that the combination of increased tissue eosinophils and LTC4 levels observed in AD skin lesions could be the basis of the keratinocyte proliferation and collagen deposition that results in skin thickening. Agents that block eosinophil migration into the skin, inhibit cys-LT synthesis, or antagonize CysLT2R may help ameliorate skin thickening in AD.
Materials and Methods
Mice and EC Sensitization.
ΔdblGATA, Ltc4s−/−, Cysltr1−/−, and Cysltr2−/− mice on BALB/c background were generated as previously described (4, 28–30) and bred for 10 or more generations to BALB/c background. WT BALB/c mice were obtained from the Charles River Laboratory. EC sensitization of 6- to 8-wk-old female mice was performed as previously described (12). Experimental procedures were in accordance with the Animal Care and Use Committee at the Children's Hospital Boston.
Histological and Immunohistochemical Analysis.
Skin specimens were prepared for histology and immunohistology as previously described (12). Eosinophils and CD4+ T cells were counted blinded in 10 high-power fields at a magnification of 400×. Dermal thickness was analyzed in H&E-stained sections viewed under a magnification of 100× by measuring the distance between the epidermal-dermal junction and the dermal-s.c. fat junction. Thickness was measured in five randomly selected fields from each sample.
Quantitation of Collagen in Skin.
Skin punch biopsies (6-mm in diameter) from sites of EC sensitization were collected and shaken at 4 °C overnight in 0.5 M acetic acid containing pepsin (1:10 ratio of pepsin/tissue wet weight) to extract pepsin-soluble protein. Skin samples then were centrifuged at 15,000 × g for 1 h. The supernatants were diluted 1:5, and collagen content was measured using the Sircol collagen dye-binding assay (Biocolor) according to the instructions of the manufacturer.
Antibodies and Flow Cytometry.
Fluorochrome-labeled anti-CCR3 (Biolegend) and Siglec-F were used for surface staining. Cells were analyzed on FACSCanto (BD Biosciences), and the data were analyzed using FlowJo (Tree Star, Inc.) software.
qPCR Analysis of Cytokines in the Skin.
Total RNA extraction, generation of cDNA, and real-time qPCR were done as previously described (31). Levels of active TGF-β1 protein were analyzed by ELISA according to manufacturer's protocol (R&D Systems).
Eosinophil Differentiation from BM and Adoptive Eosinophil Transfer.
Freshly isolated BM cells were grown as previously described (21). Briefly, freshly isolated single-cell suspensions from BM of WT or Ltc4s−/− mice were cultured at 106 cells/mL in complete RPMI 1640 supplemented with 20% (vol/vol) FCS, 0.05 mM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence of 100 ng/mL recombinant mouse SCF and 100 ng/mL rmFlt3-L (Peprotech) from days 0–4. On day 4, the medium containing SCF and Flt3-L was replaced with medium containing 10 ng/mL recombinant mouse (rm)IL-5 thereafter. WT or Ltc4s−/− BMeos differentiated for 14 d were administered i.v. (3 × 106 per mouse) to ΔdblGATA mice at days 43 and 46 at the third cycle of EC sensitization with OVA. At the end of the 7-wk sensitization period (day 49), mice were killed and analyzed for skin inflammation and collagen deposition.
Stimulation of Human Skin Fibroblasts with LTC4.
Frozen fibroblasts were previously grown from skin biopsies of normal donors after obtaining informed consent in accord with guidelines of the Institutional Review Board at the Children's Hospital Boston. Fibroblasts were grown to semiconfluence in DMEM supplemented with 15% (vol/vol) FCS. Cells were stimulated with 1 μM LTC4 (Cayman Chemical) in 2 mL of DMEM without FCS for 24 h with or without 10 μM montelukast or BAY u9773 (Cayman Chemical). Collagen levels in the culture supernatant were measured using the Sircol collagen dye-binding assay. Supernatants were assayed for IL-6 and GM-CSF by ELISA (eBioscience).
Human Epidermal Keratinocyte Proliferation.
Human epidermal keratinocytes (5 × 103 cells per well) grown from newborn foreskin (Invitrogen) were suspended in 200 μL of Medium 154 (Invitrogen) and stimulated with 1 μM LTC4, undiluted supernatants of LTC4-stimulated fibroblasts, or 1% human keratinocyte growth supplement (Invitrogen). Proliferation was assessed by measuring the incorporation of [3H]-thymidine during the last 16 h of 72-h cultures (1 μCi per well).
Statistical Analysis.
A two-tailed Student t test or one-way ANOVA was used to determine statistical differences between groups. A P value smaller than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Stuart Orkin for his kind gift of ΔdblGATA mice. We also thank Ms. Jacqueline Beaupre for her technical assistance. This work was supported by US Public Health Service Grant AR-047417 (to R.S.G.), US Public Health Service Grants U19AI095219 and R01HL090630 (to Y.K. and K.F.A.), and the Children's Hospital Boston Faculty Career Development Fellowship–Eleanor and Miles Shore Program for Scholars in Medicine at Harvard Medical School (to M.K.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203127109/-/DCSupplemental.
References
- 1.Toda M, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–881. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Schaffer F, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: A role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci USA. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 4.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 5.Flood-Page P, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostaglandins Leukot Essent Fatty Acids. 2003;69(2-3):135–143. doi: 10.1016/s0952-3278(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim DC, et al. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 8.Henderson WR, Jr, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165(1):108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 9.Beller TC, et al. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA. 2004;101:3047–3052. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua Z, Fei H, Mingming X. Evaluation and interference of serum and skin lesion levels of leukotrienes in patients with eczema. Prostaglandins Leukot Essent Fatty Acids. 2006;75(1):51–55. doi: 10.1016/j.plefa.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Spergel JM, et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fertin C, et al. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37:823–829. [PubMed] [Google Scholar]
- 15.Jinnin M, Ihn H, Yamane K, Tamaki K. Interleukin-13 stimulates the transcription of the human alpha2(I) collagen gene in human dermal fibroblasts. J Biol Chem. 2004;279:41783–41791. doi: 10.1074/jbc.M406951200. [DOI] [PubMed] [Google Scholar]
- 16.Kolodsick JE, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong CW, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33:1717–1724. doi: 10.1111/j.1365-2222.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 19.Leung DY. Immunopathology of atopic dermatitis. Springer Semin Immunopathol. 1992;13:427–440. doi: 10.1007/BF00200539. [DOI] [PubMed] [Google Scholar]
- 20.Barrett NA, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer KD, et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR., Jr Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. Journal Allergy Clinical Immunol. 2003;111(Suppl 1):S18–S34. doi: 10.1067/mai.2003.25. discussion S34–S36. [DOI] [PubMed] [Google Scholar]
- 23.Ogasawara H, et al. Characterization of mouse cysteinyl leukotriene receptors mCysLT1 and mCysLT2: Differential pharmacological properties and tissue distribution. J Biol Chem. 2002;277:18763–18768. doi: 10.1074/jbc.M109447200. [DOI] [PubMed] [Google Scholar]
- 24.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988;27:2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- 25.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: Eeffect of indomethacin. J Immunol. 1987;138:1190–1195. [PubMed] [Google Scholar]
- 26.Sapuntsova SG, Mel'nikova NP, Deigin VI, Kozulin EA, Timoshin SS. Proliferative processes in the epidermis of patients with atopic dermatitis treated with thymodepressin. Bull Exp Biol Med. 2002;133:488–490. doi: 10.1023/a:1019874023845. [DOI] [PubMed] [Google Scholar]
- 27.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 28.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 29.Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004;279:46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- 30.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 31.He R, et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.