Abstract
Invasive alien species are among the primary causes of biodiversity change globally, with the risks thereof broadly understood for most regions of the world. They are similarly thought to be among the most significant conservation threats to Antarctica, especially as climate change proceeds in the region. However, no comprehensive, continent-wide evaluation of the risks to Antarctica posed by such species has been undertaken. Here we do so by sampling, identifying, and mapping the vascular plant propagules carried by all categories of visitors to Antarctica during the International Polar Year's first season (2007–2008) and assessing propagule establishment likelihood based on their identity and origins and on spatial variation in Antarctica's climate. For an evaluation of the situation in 2100, we use modeled climates based on the Intergovernmental Panel on Climate Change's Special Report on Emissions Scenarios Scenario A1B [Nakićenović N, Swart R, eds (2000) Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK)]. Visitors carrying seeds average 9.5 seeds per person, although as vectors, scientists carry greater propagule loads than tourists. Annual tourist numbers (∼33,054) are higher than those of scientists (∼7,085), thus tempering these differences in propagule load. Alien species establishment is currently most likely for the Western Antarctic Peninsula. Recent founder populations of several alien species in this area corroborate these findings. With climate change, risks will grow in the Antarctic Peninsula, Ross Sea, and East Antarctic coastal regions. Our evidence-based assessment demonstrates which parts of Antarctica are at growing risk from alien species that may become invasive and provides the means to mitigate this threat now and into the future as the continent's climate changes.
Keywords: biological invasions, biosecurity, mitigation, propagule pressure, unintentional introductions
Terrestrial Antarctica remains one of the most pristine environments on Earth. However, much concern now exists that the combination of accelerating climate change and the rapidly growing scope and extent of scientific and tourist activities will lead to substantial environmental degradation (1–3). One of the primary drivers of this change is thought to be the increasing prospect of the establishment of terrestrial, invasive, nonindigenous (or alien) species (4–7). The likelihood of such invasions depends on the numbers of propagules of alien species entering the region, their probability of establishment, and the extent to which these established species are able to spread and alter local ecosystems (8–10). Understanding the initial phases of dispersal and establishment is especially significant for managing the risks posed by invasive alien species because the process of invasion is contingent (11); that is, a species cannot spread into a new area if its propagules have not arrived and become established.
Some evidence now exists that alien vascular plants and other taxa can successfully colonize both the maritime and continental Antarctic (4, 5, 12, 13), and it is clear from similar environments in the sub-Antarctic that, once established, such species can spread and have substantial impacts (5). However, no comprehensive, quantitative assessment of propagule pressure and the likelihood of establishment of alien species has been undertaken for Antarctica, despite the prominence that the potential threats posed by invasive alien species—and the steps required to mitigate them—have been accorded within the Antarctic Treaty System (14, 15).
Globally, most broad-scale assessments of the initial phases of biological invasions focus on intentionally introduced species because of the available data (16). In contrast, much less is known about inadvertent introductions, although they are just as significant a source of biological invasions (17). When such studies are undertaken, they are frequently based on vector numbers (such as human, shipping, or aircraft traffic) as a proxy for propagule pressure and establishment risk (18, 19), rather than on the spatially explicit quantification of the numbers of vectors, the propagule size of each individual vector, and the origins and establishment likelihood of the propagules carried. Thus, inadvertent introductions are far more poorly understood than others (10, 17, 20).
Therefore, in this study we provide a spatially differentiated risk assessment for the introduction and establishment of alien species to Antarctica. We do so based on an assessment of the following factors: (i) Vascular plant propagules (seeds) carried inadvertently by the main categories of visitors to the region—i.e., scientists, tourists, and their support personnel (4, 5, 21)—during the first summer season of the 2007–2008 International Polar Year (IPY); (ii) the characteristics of the species introduced; and (iii) spatial variation in the climate of the continent. Accidental introductions are most significant because intentional introductions are generally prohibited by the Protocol on Environmental Protection to the Antarctic Treaty (14). Previous work elsewhere in the broader Antarctic region, on a more limited scale, indicated that an assessment of vascular plant propagules carried by visitors provides a reasonable approximation of inadvertent introductions by other vectors, such as cargo, and of other terrestrial taxa, such as invertebrates (4, 5, 22).
The risk assessment undertaken here involves four major steps. First, the number of seeds on the clothing and bags of visitors traveling to the Antarctic must be quantified. Second, to provide a spatially explicit assessment of propagule pressure, the product of the number of seeds per visitor category and number of visitors from each category must be calculated for each of 81 grid cells representing ice-free areas of Antarctica in which landings occurred during the 2007–2008 IPY season. Third, to assess establishment probability, the proportion of propagules that are from species capable of surviving low-temperature environments must be determined. Here, the most conservative estimate is obtained by identifying the seeds collected from the visitors and estimating the proportion of species whose range includes the sub-Antarctic or Arctic. More liberal estimates of risk are obtained by calculating, from a larger, questionnaire-based survey of visitors, the numbers of visitors from each category that had in the previous 12 mo traveled to an alpine or polar location. The propagule pressure value must then be constrained downward by the median of these two values for each visitor category. Finally, the location of ice-free environments and data on terrestrial climates must be used to identify ice-free areas that might have temperatures suitable for cold-climate vascular plant species based on cumulative degree days (a measure of growing season length). The normalized product of the constrained propagule pressure and the environmental suitability provides a risk index for each grid cell. To estimate how this risk might change into the future, current climates in the preceding analysis are replaced with climates forecast for 2100 using the Intergovernmental Panel on Climate Change (IPCC) Special Report on Emissions Scenarios (SRES), Scenario A1B (7, 23, 24).
Results
Our sampling, which is representative of the range of visitors to the region, demonstrates that the likelihood of propagule transfer varies with category of visitor (Fig. 1A): Tourists and ships’ crews are less likely to transport propagules to the region than are scientists, science support personnel, and tourist support personnel. For those visitors carrying seeds, the number per visitor is similarly variable among categories and averages 9.5 seeds per person (Fig. 1B). In combination, the data show that the largest risk of propagule transfer per visitor is associated with science programs and tourist support personnel, rather than with tourists themselves (Fig. 1C).
Fig. 1.
Proportion of visitors carrying seeds, number of seeds per visitor carrying seeds, and number of seeds per visitor across all visitors. (A) Proportion of visitors (mean and 95% bootstrapped CI) carrying seeds within each of the visitor categories. (B) Mean (and 95% bootstrapped CI) number of seeds per visitor by category for those visitors carrying seeds. (C) Mean (and 95% bootstrapped CI) number of seeds per visitor by category for all visitors (i.e., those with and without seed loads). Sample sizes are given above all bars.
Differences in the numbers of science and tourist visitors temper this among-category variation. Simplifying visitors to these categories, the largest number of visitors to the Antarctic was tourists (including support personnel) (33,054), making 223,095 landings (on average 6.7 landings per visitor) on ice-free areas (Fig. 2A and Table S1). Over the same period, an estimated 7,085 scientists (including support personnel) landed at ice-free areas, concentrated primarily in the McMurdo Sound region of the Ross Sea and the Antarctic Peninsula (Fig. 2B and Table S1). Based on the proportion of visitors per category carrying propagules, the numbers of propagules per individual in each visitor category, and the visitor landings, the probability of propagule transport to the region is highest for the Antarctic Peninsula, followed by the Ross Sea region and then by several sites in East Antarctica. These data also indicate that an estimated 31,732 [95% confidence interval (CI): 8,885–51,021] seeds entered the Antarctic on tourists and 38,897 seeds (95% CI: 24,089–74,534) on scientists during the first summer of the IPY.
Fig. 2.
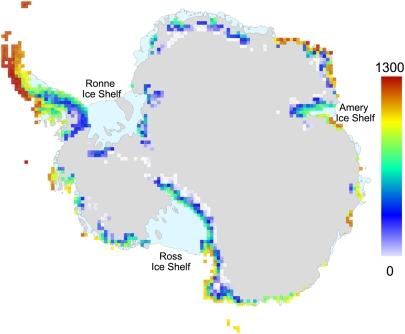
Distribution of cumulative annual degree days for 2007–2008 in the Antarctic. These were calculated by using a −5 °C threshold and mean daily near-surface temperature data from the Modern Era Retrospective-analysis for Research and Applications Reanalysis office (provided by the NASA Global Modeling and Assimilation) (50) on a 0.67° longitude × 0.5° latitude grid, interpolated to a 50-km square grid. Inset shows degree day detail on the Antarctic Peninsula. Ice-free ground is shown in gray. Ice-free landing data for tourists (A) and scientists (B) are shown.
Of the 2,686 seeds collected from sampled visitors, 88% were identified to family and 43% to species level. Species-level data show that these propagules include several species (among which are known invaders) from the sub-Antarctic or Arctic regions, similar in climate to parts of the Antarctic (Table S2). The questionnaire-based surveys demonstrate that 53% of the visitors had traveled to cold-climate areas—such as alpine, cold-temperate, or polar environments—in the year before their visit to Antarctica (Table S3). Thus, the seed- and survey-based data on propagule origins suggest that 49–61% (depending on visitor category; Table S4) of propagules reaching the Antarctic are from environments that include species capable of surviving the conditions likely to be encountered in the areas of Antarctica most commonly visited. Annual cumulative degree days for plant growth, a measure of environmental suitability, further indicate those areas where establishment of the cold-climate propagules is likely (Fig. 2). A risk index, based on propagule pressure and origins, and climatic suitability of the ice-free areas of the continent, indicates that the Western Antarctic Peninsula coast and the islands off the coast of the Peninsula have the highest current risk for the establishment of alien species (Fig. 3). Most other ice-free areas of Antarctica currently have low risk, with the possible exceptions of the western Ross Sea region and scattered sites around East Antarctica (Fig. 3).
Fig. 3.
Relative risk of alien vascular plants establishing in Antarctica. Visitor-free, ice-free areas are allocated a small value to represent the minor chance of establishment in the absence of visitor landings. Insets show risk index detail for the Antarctic Peninsula and the western Ross Sea. Ice-free areas are shown in dark gray, continental areas in light gray, and ice shelf/ice-tongue areas in light blue.
Cumulative degree days across ice-free Antarctica calculated by using climates forecast for 2100 suggest that the risk of alien species establishment continues to be highest in the Antarctic Peninsula area (Fig. 4). However, the number of degree days will also increase substantially in the coastal, ice-free areas to the west of the Amery Ice Shelf and to a lesser extent in the Ross Sea region.
Fig. 4.
Increase in annual cumulative degree days under SRES Scenario A1B (24) indicating increasing risk of alien species establishment. Increase in annual cumulative degree days, on ice-free areas of Antarctica, using 2090–2100 temperature means to estimate future degree days and annual cumulative degree days from 2007 to 2008 based on a lower temperature threshold of −5 °C for plant establishment. Ice-free areas are shown in dark gray, continental areas in light gray, and ice shelf areas in light blue.
Discussion
Despite the increased scientific interest in the Antarctic over the period of the IPY and a peak in tourist numbers, the total visitor number was not especially unusual (25), suggesting that the estimate for 2007–2008 is reasonable for calculating visitor-associated, annual anthropogenic propagule pressure on the continent for vascular plants. The propagule identifications also show that the seeds reaching Antarctica represent families known to contain the largest numbers of species that are invasive elsewhere on the planet (26) (Table S5), including species that are known invaders of cold-climate regions such as the Arctic and sub-Antarctic (5, 27). Visitors have also routinely traveled to cold-climate areas before their travel to the Antarctic. Given this propagule pressure, the provenance of many of the seeds, the geographic distribution of visitor landings, and current spatial variation in Antarctic climates, it is clear that several areas of Antarctica are at considerable risk from the establishment of nonindigenous species and that the highest risk sites are those along the Western Antarctic Peninsula.
Substantiation of this assessment is provided by several recent findings. The invasive grass species Poa annua is spreading at King George Island from the Arctowski research station to areas much less subject to human traffic (12). Its independent establishment has also recently been documented at three other research stations (namely, General Bernardo O'Higgins, Gabriel Gonzalez Videla, and Almirante Brown) along the western margin of the Peninsula.* These areas coincide with those predicted by our assessment to have the highest risk of alien establishment (Fig. 3). Experimental work has shown that P. annua can outcompete other temperate plant species (28), and it dominates lowland, disturbed areas on Southern Ocean islands (5). Consequently, it is recognized as an important invasive species in the broader region. Deception Island (included in the highest risk cell identified; Fig. 3) has also recently been colonized by two vascular plant species of South American origin (13), and two alien springtail species have established at the same site, at least one of which has substantial impacts on some sub-Antarctic systems (29).
In the case of the South American plants on Deception Island, both are wind-dispersed, cold temperate species but were found in an area that has a high visitor frequency. Determining whether the colonization was natural or a direct result of human activity therefore proved particularly problematic (13). Thus, it is clear that as climates change and visitor activity increases and diversifies across the continent, distinguishing natural colonization events associated with warming from inadvertent introductions will become progressively difficult, reflecting similar challenges elsewhere (30). Where such conservation challenges are most likely to grow in significance is made clear by the assessment of conditions in the future under SRES Scenario A1B (24).
Climate change over the next 100 y in Antarctica is expected to be spatially variable, with most regions of the continent cooling at first and then warming as the ozone hole recovers, but with areas of the Peninsula continuing to warm (7). Thus, although the risk of alien species establishment continues to be highest—and growing—along the Antarctic Peninsula, establishment risks will also rise substantially in the coastal, ice-free areas of the Ross Sea area and in parts of coastal East Antarctica (Fig. 4). The former already has relatively high visitor numbers, indicating that future risks to the area may prove to be considerable. Establishment of alien species will also be promoted by the exposure of new, disturbed ground following glacial retreat. Disturbance is a notable driver of the establishment of such species (31), and elsewhere in the broader Antarctic region, newly exposed glacial forelands are readily colonized by them (5, 12, 32). To some extent, these projections must be considered a current best, evidence-based estimate because they take no account of trends that are more difficult to forecast, such as those in visitor numbers to and areas visited in Antarctica, the range of activities science and tourist visitors might undertake in the future (1), and the impacts of further efforts by the Committee for Environmental Protection of the Antarctic Treaty System to reduce propagule pressure to the region (14). As information on the realized outcomes of these trends and actions becomes available, the assessments of risk posed by inadvertent introductions can be adapted.
By delivering comprehensive evaluations of human-associated propagule pressure and establishment likelihood, differentiated by spatial location and visitor category, our study offers an effective basis for management interventions to mitigate the risks of establishment of nonindigenous species across the entire Antarctic continent, a region of growing international political and biological interest (3, 6, 15, 33, 34). It indicates those visitor groups and areas for which biosecurity measures should be most stringent, those where controls might be less pronounced, and how the spatial arrangement of these areas is likely to change through time. The assessment also offers guidance for planning early detection surveys (35, 36) and support for management decisions about whether new species occurrences are the consequence of anthropogenic transport or natural colonization (30). Such decision-making can be further informed by identifying natural colonization paths at appropriate spatial and temporal resolutions. These include wind trajectories for wind-dispersed species (37), satellite tracks of seabirds (38) that are considered important natural dispersal agents (39), and genetic data on colonists and populations from elsewhere in the broader region (4, 40). Thus, our study provides an evidence-based, continent-wide risk assessment for the establishment of terrestrial alien species in Antarctica and the understanding required to mitigate this risk, one of the primary conservation goals of the Antarctic Treaty System (14). In so doing, it also demonstrates how a combination of information-rich and modeling approaches can be used to understand and moderate the risks of inadvertent introductions, which are among invasion biology's most significant challenges (10, 17, 20).
Materials and Methods
Ethics Statement.
Our study was not on humans but did involve anonymous questionnaires put to human visitors to Antarctica and/or sampling of their outer clothing and bags. In all cases, the intent of the work and onward use of the data were carefully explained by researchers or volunteers in advance of the distribution of the questionnaires and sampling. Any person then not wishing to participate could decline, and samples and/or questionnaires were only taken from individuals who consented fully by completing the questionnaires and/or presenting their gear for sampling. No data identifying individuals by name or any other means were collected at any point (i.e., all samples are anonymous), and none of the sampling was intrusive.
Propagule Pressure.
We estimated the number of propagules per visitor for ∼2% of all visitors (853 individual scientists, science-support personnel, tourists, tourist support personnel, and ships’ crew) to all major areas of the Antarctic during the first summer season of the IPY (2007–2008) by collecting seeds from their outer clothing, footwear, walking poles, day packs, and camera bags (21, 41) (Table S6) using Philips FC9154/01 vacuum cleaners. For most sampled visitors, the material collected from clothing and equipment was retained in a single bag, but for 349 visitors, the material from each item was kept separately (Table S6). Approximately half of the sampled visitors were involved in national Antarctic programs (14 ships/aircraft; 18 voyages) and half in tourist operations (13 ships; 37 voyages).
The plant seeds per sample were counted and sorted into morphologically similar groups (generally corresponding to species). Seeds were identified by comparing them with photographs of seeds in seed atlases (42–45) and online databases (e.g., the Seed Information Database, http://www.kew.org/msbp/scitech/SIDoverview.htm; Seedimages.com, www.seedimages.com). The proportion of visitors carrying seeds was estimated with 95% CI for each visitor category with bias-corrected and accelerated bootstrap methods by using GenStat 13 and R [library(boot); http://www.r-project.org]. Similarly, mean number of seeds per visitor was estimated, again with 95% CI, for each visitor category. Sampling was considered to capture the large majority of seeds, although this somewhat underestimated propagule pressure (21). The number of seeds that would drop off a visitor was considered proportional to the number of seeds found during sampling, and propagule viability was considered high (41, 46).
We mapped the numbers of visitors to Antarctica differentiated by their participation in either science (one category) or tourism (two categories: tourists and tourist support personnel), as reported by the International Association of Antarctica Tour Operators (http://iaato.org) and the Council of Managers of National Antarctic Programs (47) (Table S1) onto a regular spatial framework of 81 grid cells of 50 × 50 km representing ice-free areas of the continent where visitors have landed (ice-free land data provided by the Australian Antarctic Data Centre from the Antarctic Digital Database V5; © Scientific Committee on Antarctic Research 1993–2006) (48). Before doing so, duplicates, spurious records, and landings on ice-covered areas were removed from the dataset. Propagule pressure per 50 × 50-km grid cell was calculated per visitor category by multiplying the number of visitor landings (N) by the estimated probability of each visitor category carrying seeds (P) and by the mean numbers of seeds per visitor from this category [seed carriers only (X)] (Table S7).
Establishment Likelihood.
To estimate establishment likelihood, we used information on the origin of the seeds and on the environment they would experience on arrival. For the former, we adopted two approaches. First, for the seeds of species identified to species level that were collected from the visitors, we determined whether or not these species occur in the Arctic/sub-Antarctic (5, 27, 49) and are thus capable of growing in cold environments (Table S2). We assumed that the same proportion of species was able to establish in cold climate areas for identified seeds as for seeds that could not be identified to species level. We estimated that 47% of all seeds carried into the Antarctic by visitors can establish, forming a conservative estimate of establishment. A liberal estimate was determined from a larger, questionnaire-based survey (available in 10 languages) of 5,659 visitors (i.e., verified questionnaires) to determine what proportion thereof had visited regions with climates similar to those they might encounter in Antarctica (Arctic/alpine/sub-Antarctic/Antarctic) in the 12 mo before their travel to Antarctica (Table S3). A median was then calculated as the value lying between 0.47 (the proportion of viable seeds from the species-level seed analyses) and the proportion of each visitor category that had been to a cold-climate environment (range 0.49–0.61) (Table S4). This median risk proportion (Ri) was used to constrain the propagule pressure downward in calculating the risk index.
The most current information on the location of ice-free environments in Antarctica (Antarctic Digital Database V5) (48) and on Antarctic climate (http://gmao.gsfc.nasa.gov/research/merra/faq.php) (50) was used to identify ice-free areas that might have climates suitable for low-temperature vascular plant species based on cumulative degree days, assuming that these species can germinate, survive, and grow above −5 °C (51–54). We used mean daily 2-m air temperatures from the Modern Era Retrospective-analysis for Research and Applications Reanalysis (provided by the NASA Global Modeling and Assimilation Office) (50) on a 0.67° longitude × 0.5° latitude grid. These data were then spatially interpolated onto the 50 × 50-km grid.
Risk Index.
The risk index (RI) for each visitor class (i) for each 50 × 50-km cell (j) was calculated as:
where Nij is the number of landings of the ith visitor class in the jth cell; Pi is the proportion of the ith visitor class that is likely to be carrying seeds; Xi is the mean number of seeds (for seed carrying visitors) for the ith visitor class; Ri is the median of the proportion of seeds from Arctic or sub-Antarctic areas and proportion of visitors to Arctic, alpine, or sub-Antarctic sites for the ith visitor category; and DDj is the annual cumulative degree days in the jth cell.
Then, to calculate the overall risk index (ORI) for the jth 50 × 50 km cell the risk indices of each visitor class (T, tourist; TS, tourist support; S, scientist) were summed:
The ORI was then normalized to provide a probability of risk from 0 to 1. Ice-free areas with no visitor landings were assigned a very low risk.
Future Climate and Risks.
To estimate future risks based on a changing climate, we used the CSIRO Mk3.5 Climate Model (http://www.cawcr.gov.au/publications/technicalreports/CTR_021.pdf) (55) under Scenario A1B (IPCC SRES) (24) to arrive at a spatially explicit prediction of temperatures in 2100. The degree day values under this climate scenario were calculated as before. All spatial analyses and climate modeling were conducted by using MATLAB 7.12 (Mathworks, Natick, MA) and Manifold System Professional (Version 8.00, Manifold Software Limited, Hong Kong).
Supplementary Material
Acknowledgments
We thank the participants in the International Polar Year Project “Aliens in Antarctica” for their assistance; and Tim Blackburn, Phil Hulme, Mahlon Kennicutt, Melodie McGeoch, Dave Richardson, Justine Shaw, and two anonymous reviewers for commenting on a previous version of the manuscript. Randstad Data Management (Rotterdam, The Netherlands) assisted with questionnaire analysis. Philips Netherlands, Ltd. (Eindhoven, The Netherlands) donated the vacuum cleaners. This work was supported by our institutions, Netherlands Polar Program Grant 851.20.040, and the Scientific Committee on Antarctic Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The seed data reported in this paper have been deposited in the Antarctic Biodiversity database, Australian Antarctic Division, http://data.aad.gov.au/aadc/biodiversity/.
*Molina-Montenegro MA, Carrasco-Urra F, Rodrigo C, Valladares F, Poster, Scientific Committee on Antarctic Research Open Science Conference, August 3–6, 2010, Buenos Aires, Argentina.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119787109/-/DCSupplemental.
References
- 1.Tin T, et al. Impacts of local human activities on the Antarctic environment. Antarct Sci. 2009;21:3–33. [Google Scholar]
- 2.Walther G-R, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 3.Thatje S, Aronson RB. No future for the Antarctic Treaty? Front Ecol Environ. 2009;7:75. [Google Scholar]
- 4.Hughes KA, Convey P. The protection of Antarctic terrestrial ecosystems from inter- and intra-continental transfer of non-indigenous species by human activities: A review of current systems and practices. Glob Environ Change. 2010;20:96–112. [Google Scholar]
- 5.Frenot Y, et al. Biological invasions in the Antarctic: Extent, impacts and implications. Biol Rev Camb Philos Soc. 2005;80:45–72. doi: 10.1017/s1464793104006542. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardi A. Antarctica invaded. Science. 2008;319:409–410. doi: 10.1126/science.319.5862.409b. [DOI] [PubMed] [Google Scholar]
- 7.Turner J, et al., editors. Antarctic Climate Change and the Environment. Cambridge, UK: Scientific Committee on Antarctic Research; 2009. [Google Scholar]
- 8.Hulme PE. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J Appl Ecol. 2009;46:10–18. [Google Scholar]
- 9.Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: The role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib. 2009;15:904–910. [Google Scholar]
- 10.Pyšek P, Richardson DM. Invasive species, environmental change and management, and health. Annu Rev Environ Resour. 2010;35:25–55. [Google Scholar]
- 11.Puth LM, Post DM. Studying invasion: Have we missed the boat? Ecol Lett. 2005;8:715–721. [Google Scholar]
- 12.Olech M, Chwedorzewska KJ. The first appearance and establishment of an alien vascular plant in natural habitats on the forefield of a retreating glacier in Antarctica. Antarct Sci. 2011;23:153–154. [Google Scholar]
- 13.Smith RIL, Richardson M. Fuegian plants in Antarctica—natural or anthropogenically-assisted immigrants? Biol Invas. 2011;13:1–5. [Google Scholar]
- 14.Rogan-Finnemore M, editor. Non-Native Species in the Antarctic. Christchurch, New Zealand: Gateway Antarctica; 2008. [Google Scholar]
- 15.Berkman PA, Lang MA, Walton DWH, Young OR, editors. Science Diplomacy. Antarctica, Science and the Governance of International Spaces. Washington: Smithsonian Institution Press; 2011. [Google Scholar]
- 16.Blackburn TM, Lockwood JL, Cassey P. Avian Invasions. The Ecology and Evolution of Exotic Birds. Oxford: Oxford University Press; 2009. [Google Scholar]
- 17.Hulme PE, et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J Appl Ecol. 2008;45:403–414. [Google Scholar]
- 18.Drake JM, Lodge DM. Global hot spots of biological invasions: Evaluating options for ballast-water management. Proc Biol Sci. 2004;271:575–580. doi: 10.1098/rspb.2003.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatem AJ, Hay SI. Climatic similarity and biological exchange in the worldwide airline transportation network. Proc Biol Sci. 2007;274:1489–1496. doi: 10.1098/rspb.2007.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulme PE. Biosecurity and the politics of fear. Science. 2011;334:176–177. doi: 10.1126/science.334.6053.176-c. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Chown SL. Breaching the dispersal barrier to invasion: Quantification and management. Ecol Appl. 2009;19:1944–1959. doi: 10.1890/08-2157.1. [DOI] [PubMed] [Google Scholar]
- 22.Lee JE, Chown SL. Quantifying the propagule load associated with the construction of an Antarctic research station. Antarct Sci. 2009;21:471–475. [Google Scholar]
- 23.Bracegirdle TJ, Connelley WM, Turner J. Antarctic climate change over the twenty first century. J Geophys Res Atmosph. 2008;113:D03103. [Google Scholar]
- 24.Nakićenović N, Swart R, editors. Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 25.Jabour J. In: Health of Antarctic Wildlife. A Challenge for Science and Policy. Kerry KR, Riddle MJ, editors. Berlin: Springer; 2009. [Google Scholar]
- 26.Pyšek P. Is there a taxonomic pattern to plant invasions? Oikos. 1998;82:282–294. [Google Scholar]
- 27.Elven R. 2007. Checklist of the panarctic flora (PAF) vascular plants, Version May 2007. Available at http://www.binran.ru/infsys/paflist/index.htm.
- 28.Monzeglio U, Stoll P. Spatial patterns and species performances in experimental plant communities. Oecologia. 2005;145:619–628. doi: 10.1007/s00442-005-0168-3. [DOI] [PubMed] [Google Scholar]
- 29.Greenslade P, Convey P. Exotic Collembola on subantarctic islands: Pathways, origins and biology. Biol Inv. 2012;14:405–417. [Google Scholar]
- 30.Walther G-R, et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol Evol. 2009;24:686–693. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ. Are invasive species the drivers of ecological change? Trends Ecol Evol. 2005;20:470–474. doi: 10.1016/j.tree.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Frenot Y, Gloaguen J-C. Reproductive performance of native alien colonizing phanerogams on a glacier foreland, Îles Kerguelen. Polar Biol. 1994;14:473–481. [Google Scholar]
- 33.Aronson RB, Thatje S, McClintock JB, Hughes KA. Anthropogenic impacts on marine ecosystems in Antarctica. Ann N Y Acad Sci. 2011;1223:82–107. doi: 10.1111/j.1749-6632.2010.05926.x. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland WJ, et al. A horizon scan of global conservation issues for 2010. Trends Ecol Evol. 2010;25:1–7. doi: 10.1016/j.tree.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Hui C, Foxcroft LC, Richardson DM, MacFadyen S. Defining optimal sampling effort for large-scale monitoring of invasive alien plants: A Bayesian method for estimating abundance and distribution. J Appl Ecol. 2011;48:768–776. [Google Scholar]
- 36.Rout TM, Moore JL, Possingham HP, McCarthy MA. Allocating biosecurity resources between preventing, detecting, and eradicating island invasions. Ecol Econ. 2011;71:54–62. [Google Scholar]
- 37.Muñoz J, Felicísimo ÁM, Cabezas F, Burgaz AR, Martínez I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science. 2004;304:1144–1147. doi: 10.1126/science.1095210. [DOI] [PubMed] [Google Scholar]
- 38.Tremblay Y, et al. Analytical approaches to investigating seabird-environment interactions: A review. Mar Ecol Prog Ser. 2009;391:153–163. [Google Scholar]
- 39.Falla RA. Oceanic birds as dispersal agents. Proc R Soc Lond B Biol Sci. 1960;152:655–659. [Google Scholar]
- 40.Rogers AD. Evolution and biodiversity of Antarctic organisms: A molecular perspective. Philos Trans R Soc Lond B Biol Sci. 2007;362:2191–2214. doi: 10.1098/rstb.2006.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whinam J, Chilcott N, Bergstrom DM. Subantarctic hitchhikers: Expeditioners as vectors for the introduction of alien organisms. Biol Conserv. 2005;121:207–219. [Google Scholar]
- 42.Martin AC, Barkley WD. Seed Identification Manual. Berkeley, CA: University of California Press; 1961. [Google Scholar]
- 43.Webb CJ, Simpson MJA. Seeds of New Zealand Gymnosperms & Dicotyledons Vol. 1. New Zealand: Manuka, Cromwell; 2001. [Google Scholar]
- 44.Cappers RTJ, Bekker RM, Jans JEA. Digital Seed Atlas of the Netherlands. Groningen, The Netherlands: Barkhuis; 2006. [Google Scholar]
- 45.Sweedman L, Merritt D, editors. Australian Seeds. A Guide to Their Collection, Identification and Biology. Collingwood, Australia: CSIRO; 2006. [Google Scholar]
- 46.Hughes KA, Lee JE, Ware C, Kiefer K, Bergstrom DM. Impact of anthropogenic transportation to Antarctica on seed viability. Polar Biol. 2010;33:1125–1130. [Google Scholar]
- 47.Council of Managers of National Antarctic Programs . COMNAP Report to ATCM XXXI. ATCM IP 127. Antarctic Treaty Consultative Meeting, June 2–13, 2008, Kiev, Ukraine. Buenos Aires: Secretariat of the Antarctic Treaty; 2008. [Google Scholar]
- 48.Thomson JW, Cooper APR. The SCAR Antarctic digital topographic database. Antarct Sci. 1993;5:239–244. [Google Scholar]
- 49.Chown SL, Gremmen NJM, Gaston KJ. Ecological biogeography of southern ocean islands: Species-area relationships, human impacts, and conservation. Am Nat. 1998;152:562–575. doi: 10.1086/286190. [DOI] [PubMed] [Google Scholar]
- 50.Rienecker MM, et al. MERRA: NASA's Modern-Era Retrospective Analysis for Research and Applications. J. Climate. 2011;24:3624–3648. [Google Scholar]
- 51.Trudgill DL, Squire GR, Thompson K. A thermal time basis for comparing the germination requirements of some British herbaceous plants. New Phytol. 2000;145:107–114. [Google Scholar]
- 52.Bannister P. A touch of frost? Cold hardiness of plants in the southern hemisphere. NZ J Bot. 2007;45:1–33. [Google Scholar]
- 53.Hennion F, Walton DWH. Seed germination of endemic species from Kerguelen phytogeographic zone. Polar Biol. 1997;17:180–187. [Google Scholar]
- 54.Sierra-Almeida A, Cavieres LA, Bravo LA. Freezing resistance varies within the growing season and with elevation in high-Andean species of central Chile. New Phytol. 2009;182:461–469. doi: 10.1111/j.1469-8137.2008.02756.x. [DOI] [PubMed] [Google Scholar]
- 55.Gordon H, et al. Melbourne: Commonwealth Scientific and Industrial Research Organisation and Bureau of Meteorology; 2010. The CSIRO Mk3.5 Climate Model. Centre for Australian Weather and Climate Research Technical Report 21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.