Abstract
During insect metamorphosis, neuronal networks undergo extensive remodeling by restructuring their connectivity and recruiting newborn neurons from postembryonic lineages. The neuronal network that directs the essential behavior, ecdysis, generates a distinct behavioral sequence at each developmental transition. Larval ecdysis replaces the cuticle between larval stages, and pupal ecdysis externalizes and expands the head and appendages to their adult position. However, the network changes that support these differences are unknown. Crustacean cardioactive peptide (CCAP) neurons and the peptide hormones they secrete are critical for ecdysis; their targeted ablation alters larval ecdysis progression and results in a failure of pupal ecdysis. In this study, we demonstrate that the CCAP neuron network is remodeled immediately before pupal ecdysis by the emergence of 12 late CCAP neurons. All 12 are CCAP efferents that exit the central nervous system. Importantly, these late CCAP neurons were found to be entirely sufficient for wild-type pupal ecdysis, even after targeted ablation of all other 42 CCAP neurons. Our evidence indicates that late CCAP neurons are derived from early, likely embryonic, lineages. However, they do not differentiate to express their peptide hormone battery, nor do they project an axon via lateral nerve trunks until pupariation, both of which are believed to be critical for the function of CCAP efferent neurons in ecdysis. Further analysis implicated ecdysone signaling via ecdysone receptors A/B1 and the nuclear receptor ftz-f1 as the differentiation trigger. These results demonstrate the utility of temporally tuned neuronal differentiation as a hard-wired developmental mechanism to remodel a neuronal network to generate a scheduled change in behavior.
Keywords: neuronal identity, neuropeptide, neuronal plasticity
Neuronal remodeling is a pervasive mechanism that matches network output to the physiological and behavioral demands of the organism throughout development and in response to changing input or injury (1, 2) via morphological remodeling (3, 4), neuronal addition through neurogenesis (5), and functionally significant changes in gene expression (6–8). Drosophila metamorphosis has provided many striking and informative examples. Most larval neurons undergo morphological restructuring to rewire into adult networks. Also, postembryonic waves of neurogenesis and programmed cell death combine to remodel existing or adult-specific networks through the addition of newly born neurons or subtraction of larval-specific neurons (9–13).
The network of neurosecretory neurons that directs ecdysis must function at each major developmental transition in insects; however, the behavioral sequence generated by this network differs at each stage (14). Larval ecdysis sheds the old cuticle between larval stages, pupal ecdysis everts and extends the head and appendages to their adult position in 12-h pupae, and adult ecdysis ecloses the adult from the pupal case and inflates the wings (15). The crustacean cardioactive peptide (CCAP) neuron population plays a critical role; targeted ablation of Drosophila CCAP neurons disrupts the timing of larval ecdysis and results in a lethal failure of pupal ecdysis (16).
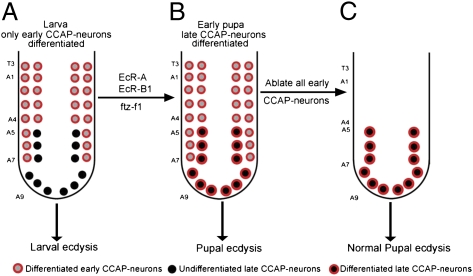
Previous work had shown that CCAP neurons undergo morphological remodeling after pupal ecdysis (17). Here, we find that the CCAP neuronal network is functionally remodeled between larval and pupal ecdysis by the delayed differentiation of a subset of CCAP neurons. We find that 12 late CCAP neurons differentiate to express the functionally critical peptide hormones CCAP, Bursα, and Bursβ immediately before pupal ecdysis and at the same time project axons out of the central nervous system. Notably, these late CCAP neurons are sufficient for pupal ecdysis; in genetic ablation studies wherein only the late-differentiating subset of CCAP neurons survived, pupal ecdysis was entirely wild type. Further analysis showed that late CCAP neurons become postmitotic in the embryo in segments A5–A9 (segment designations are shown in Fig. S1), but, unlike the other CCAP neurons, their terminal differentiation is triggered at pupariation by ecdysone pathway signaling. These data provide evidence for temporally tuned terminal differentiation as a mechanism to remodel a functioning neuronal network at a specific time point to enable a switch in behavioral output. Evidence of similar delayed differentiation within other insect neuronal subsets supports our proposal that this mechanism is used by a number of neuronal networks (18–23).
Results
CCAP neurons in Drosophila can be identified robustly using CCAP-GAL4 (Fig. S1) (16, 24). In the ventral nerve cord (VNC), there is a single CCAP interneuron (CCAP-IN) in each hemisegment T1–A7 as well as a single CCAP efferent (CCAP-EN), which exits the VNC along the lateral segmental nerve trunk, in each hemisegment T3–A4 (Fig. 1B and Fig. S1 A and A′) (25). Here, we examined CCAP neuron subsets in pupae (Fig. 1 and Fig. S1) and identified 12 late-emerging CCAP neurons restricted to segments A5–A9. These late CCAP neurons emerge during early pupariation before pupal ecdysis (APF). A single late CCAP neuron emerges adjacent to each CCAP-IN in hemisegments A5–A7, and six late CCAP neurons emerge in the A8/A9 segments (Fig. 1 C–E and Fig. S1 B and B′). Previously, we found that CCAP-ENs can be discriminated from CCAP-INs by their coexpression of Dachshund (Dac), OK6-GAL4, and nuclear phosphorylated Mad (pMad) (25). Nuclear pMad is indicative of active bone morphogenetic protein (BMP) signaling and is exclusive, in the Drosophila VNC, to efferent neurons (26, 27). Applying these markers to late CCAP neurons, we found that A5–A7 CCAP neurons expressed Dac, OK6-GAL4, and pMad (Fig. 2 A, ii and Fig. S2 A, C, and E). We refer to these neurons as “late CCAP-ENs.” Late A8/A9 CCAP neurons expressed OK6-GAL4 and pMad (see Fig. S7 A–C) but not Dac (see Fig. S7 A and B). We refer to these neurons as “posterior lateral CCAP neurons” (CCAP-PLs) to discriminate them from the A5–A7 late CCAP-ENs. The late CCAP-ENs and CCAP-PLs reported here correspond to CCAP neurons that had been observed in certain previous reports, but their origin and subtype identity had not been established. The late CCAP-ENs correspond to the large dorsal CCAP neurons in A5–A7 segments that express CCAP, Bursα, and Bursβ reported in pharate adults (immediately before eclosion of adults) (17, 24, 28). This correspondence is verified below (see Fig. 4). Additionally, a set of A8/A9 CCAP neurons observed at the pupal ecdysis stage (29) likely corresponds to the CCAP-PLs characterized here.
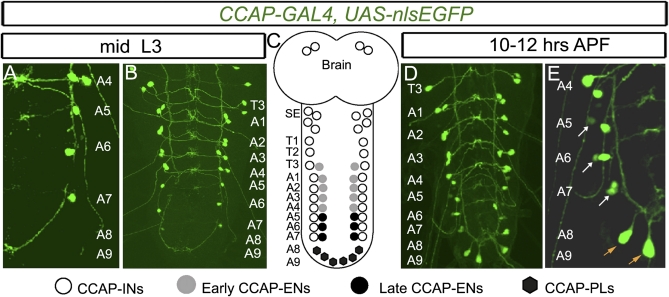
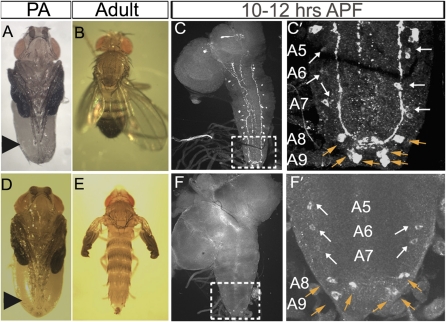
Fig. 1.
Emergence of late CCAP neurons in the A5–A9 abdominal VNC at pupariation. Expression of CCAP-GAL4,UAS-nlsEGFP (green) in the T3–A9 hemisegments in mid-L3 larvae (A and B) and in pupae 10–12 h APF (D and E) is shown with a summary depicting all CCAP neuronal subsets (C). Within each segment (T1–A7), CCAP neurons project across the midline forming a ladder-like structure that we used to confirm the segment identity of every CCAP neuron (see also Fig. S1). (A and B) At mid-L3, there is a CCAP neuron doublet in the T3–A4 hemisegments. In A5–A7 hemisegments, there is only a single CCAP neuron. (D and E) By 10–12 h APF, a second CCAP neuron has emerged in each A5–A7 hemisegment (white arrow). Also, six CCAP neurons emerge within hemisegments A8 and A9 (orange arrow). (C) Cartoon summary of CCAP neurons in the CNS. Based on previous work (25) and the identification of late CCAP-ENs and CCAP-PLs in Fig. 2, we summarize the identity of each CCAP neuron subtype here. Genotype: CCAP-GAL4,UAS-nlsEGFP/+.
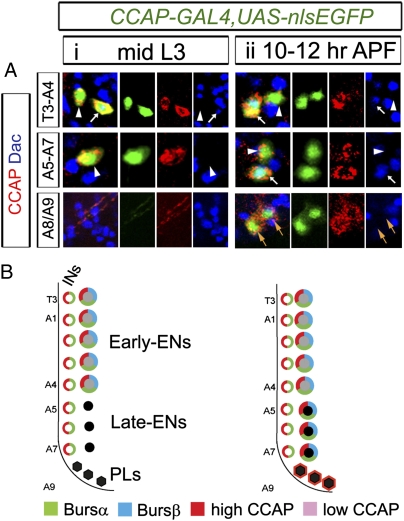
Fig. 2.
Late CCAP neurons differentiate at pupariation. Differentiation of peptide hormone-expressing late CCAP neurons occurs between late wandering L3 larvae and pupal ecdysis (at 12 h APF). (A) Representative images of CCAP neurons in hemisegments T3–A4, A5–A7, and A8 and A9 showing expression of CCAP-GAL4 (green) and immunoreactivity to CCAP (red) and Dac (blue) at mid-L3 (i) and at 10–12 h APF (ii). CCAP-ENs were identified by Dac expression. In segments T3–A4, CCAP is expressed in CCAP-INs (arrowheads) and early CCAP-ENs (arrows) from mid-L3 to pupal ecdysis. In segments A5–A7 and A8/A9, CCAP-GAL4 and CCAP immunoreactivity started after mid-L3 (arrows) in late CCAP-ENs (A5–A7) and in CCAP-PLs (A8/A9). CCAP-PLs did not express Dac. (B) Cartoon summaries of gene expression in VNC CCAP neurons at mid-L3 and 10–12 h APF. (See Fig. S2 for further details.) Genotypes: CCAP-GAL4,UAS-nlsEGFP/+.
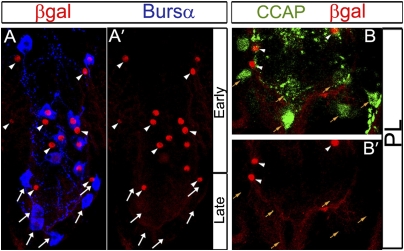
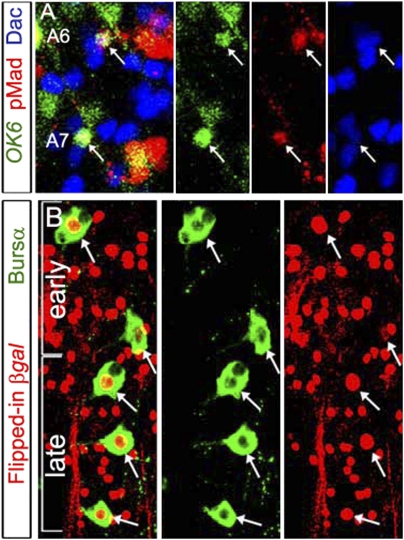
Fig. 4.
Late subsets of CCAP neurons emerge at late L3. Combining the TARGET and Flp/FRT systems, we permanently β-Gal–marked neurons that expressed CCAP-GAL4 in larvae. Animals were raised at 29 °C until mid-L3, and at 18 °C thereafter. Representative images of pharate adult VNC, showing anti-Bursα to identify CCAP-ENs (A) and anti-CCAP to identify CCAP-PLs (B). (A and A′) β-Gal immunoreactivity (red) was not observed in the six A5–A7 late CCAP-ENs (arrows) but was seen in CCAP-INs (arrowheads) and early CCAP-ENs (Bursα and β-Gal are colabeled). (B and B′) Posterior abdominal VNC showing that CCAP-PLs (orange arrows) expressed anti-CCAP (green) but did not express β-Gal (red). CCAP-INs (arrowheads) expressed anti-CCAP and β-Gal. Genotypes: CCAP-GAL4 /Act-FRT > STOP > FRT-nLacZ; tubP-GAL80TS, UAS-nlsEGFP/UAS-Flp.
We examined whether the late CCAP neurons activated CCAP-GAL4 expression late or actually underwent terminal differentiation at pupariation. No peptide hormone expression could be detected in late A5–A7 CCAP-ENs and A8/A9 CCAP-PLs by mid-L3 (Fig. 2 A, i and B and Figs. S1A and S2 B, i and C, i). However, by 10–12 h APF, late CCAP-ENs robustly expressed CCAP, Bursα, and Bursβ (Fig. 2 A, ii and B and Fig. S2 A, ii; B, ii; and C, ii), and CCAP-PLs expressed CCAP but not Bursα and Bursβ (Fig. 2 A, ii). Expression of these peptide hormones was retained in late CCAP neurons up to the pharate adult stage (Figs. S1 and S2 B, iii; C, iii; and D, iii). We also found that late CCAP neurons do not extend axons out of the VNC until pupariation (detailed below; see Fig. 6).
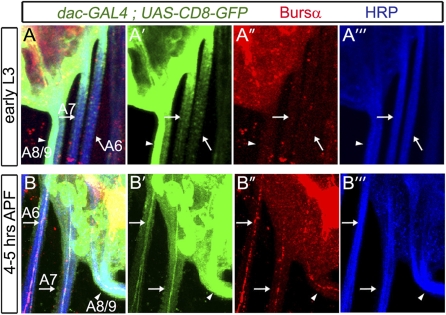
Fig. 6.
Late CCAP neurons exit the VNC during pupariation. We used dacGAL4,UAS-CD8-GFP expression in CCAP-ENs to show that late CCAP-ENs differentiate morphologically at pupariation. We show representative overlapping and split-fluorescence images of the posterior nerve trunks that exit the VNC in early L3 larvae and in pupae 4–5 h APF, triple-labeled for dacGAL4,UAS-CD8-GFP (green), Bursα (red), and anti-HRP (blue), which stains all neuronal membranes in Drosophila and outlines the nerve trunk. Arrows indicate nerve trunks in A6 and A7; the arrowhead indicates the fused A8/A9 nerve. (A–A′′′) In L3 larvae, dacGAL4,UAS-CD8-GFP–expressing and Bursα-expressing axons were not observed in A6 and A7 lateral nerve trunks (arrows). The A8/A9 nerve trunk (arrowhead) carries other dac-expressing efferents (see text for details) but did not carry Bursα-expressing axons. (B–B′′′) By 4–5 h APF, a single dacGAL4,UAS-CD8-GFP–expressing axon was detected in each of the A6 and A7 nerve trunks (arrows). This axon coexpressed Bursα. Bursα also was observed in the A8/A9 nerve trunk at this age. Genotypes: dacGAL4/UAS-CD8-GFP.
Late CCAP Neurons Are Sufficient for Pupal Ecdysis.
We postulated that late CCAP neurons function in pupal ecdysis. Previously, ablation of CCAP neurons (CCAP-GAL4 expressing the proapoptotic genes hid and reaper) resulted in a failure of head eversion and leg extension at pupal ecdysis (16). Here, we adapted this approach to examine pupal ecdysis after selective ablation of early CCAP neurons, leaving late CCAP neurons intact. To do so, we used a temperature-sensitive variant of GAL80 (GAL80TS), a potent GAL4 repressor (30). Larvae were raised at 29 °C up to mid-L3 and were switched to 18 °C thereafter. At 29 °C, GAL80TS is inactivated, and CCAP-GAL4 can drive UAS-hid/reaper expression, killing 100% of early CCAP neurons by mid-L3 (Fig. S3B). By subsequently shifting larvae to 18 °C, active GAL80TS then could block CCAP-GAL4 function upon its expression in late CCAP neurons at pupariation. This protocol selectively spared late CCAP neurons from cell death (n = 24 animals) (Fig. 3 F and F′ and Fig. S3D).
Fig. 3.
Late CCAP neurons are sufficient for pupal ecdysis. Early CCAP neurons were ablated selectively using temporally controlled expression of the cell death gene in larval CCAP neurons. (A and B) Control animals exhibit wild-type leg extension (arrowhead) and head eversion in pharate adults (PA) (A), and adults have wild-type wing inflation (B). (D and E) Selective ablation of early CCAP neurons results in wild-type leg extension (arrowhead) and head eversion in pharate adults (D), but adults have failed wing inflation (E). (C and F) CCAP immunoreactivity in whole CNS at 10–12 h APF. (C′ and F′) Magnified views of boxed areas in C and F. (C and C′) In controls, the full complement of CCAP neurons was seen, including A5–A7 late CCAP-ENs (white arrows in C′) and CCAP-PLs (orange arrows in C′). (F and F′) All early CCAP-ENs and CCAP-INs were ablated. Only late CCAP-ENs (white arrows in F′) and CCAP-PLs (orange arrows in F′) remained. Genotypes: (A–C′) CCAP-GAL4/+; tubP-GAL80TS, UAS-nlsEGFP/+; (D–F′) UAS-hid,UAS-reaper/w or Y; CCAP-GAL4; tubP-GAL80TS,UAS-nlsEGFP/+.
Remarkably, we found that the 12 late CCAP neurons were entirely sufficient to support wild-type pupal ecdysis. In 96% of these animals (22/23), head eversion and leg extension were as in wild-type animals, and 100% of the animals eclosed (n = 22) (Fig. 3 D and E). The only observable phenotype was a failure of wing inflation (Fig. 3E), which is known to require a subset of (ablated) early suboesophageal CCAP-INs (28, 31). As a control, we shifted animals from 29 °C to 18 °C at 14 h APF to repress UAS-hid/reaper expression after pupal ecdysis. This protocol resulted in a 100% failure of head eversion and leg extension (n = 15) (Fig. S4B). In a reciprocal test, we killed CCAP neurons only after pupal ecdysis by shifting animals from 18 °C to 29 °C at 14 h APF; pupal ecdysis and eclosion were as in wild-type animals, but wing inflation failed in 100% of animals (n = 12) (Fig. S4 C and C′).
We postulated that UAS-hid/reaper expression could be timed to kill different subsets of late CCAP neurons stochastically. We raised larvae at 29 °C until puparium formation, when CCAP neurons start to express CCAP-GAL4, and shifted the pupae to 18 °C thereafter. Intriguingly, we observed four primary pupal ecdysis phenotypic categories. Category 1 had a lethal failure of head eversion and leg extension (n = 36; 100% CCAP neuron lethality). Category 2 had wild-type pupal ecdysis and eclosion (n = 27; 5.7 ± 0.5 late CCAP-ENs; 5.6 ± 0.7 CCAP-PLs). Category 3 had wild-type leg extension but a failure of normal head eversion (n = 9; 6 ± 0 late CCAP-ENs; 1.8 ± 1.0 CCAP-PLs) (Fig. S4 D and D′). Category 4 had failure of leg extension but wild-type head eversion (n = 20; 2.7 ± 1.0 late CCAP-ENs; 5.9 ± 0.4 CCAP-PLs) (Fig. S4 E and E′). In each category, we examined the number and subset of remaining CCAP-immunoreactive late CCAP neurons (all early CCAP neurons were eliminated in all categories; Fig. S3 D′ and E′). The unexpected observation that head-eversion and leg-extension phenotypes could be uncoupled suggested their regulation by distinct CCAP neuronal subsets. In correspondence with this phenotypic data, ablation of most A5–A7 CCAP-ENs correlated only with failed leg extension. Conversely, ablation of most A8/A9 CCAP-PLs correlated only with head-eversion defects. These data were substantiated by previous observations that a group of A8/A9 CCAP neurons exhibit heightened Ca2+ activity during early fictive pupal ecdysis (corresponding to the time of head eversion) (29). These data led us to propose that late CCAP-ENs are required for leg extension, whereas late CCAP-PLs are required for head eversion.
Addition of Late CCAP Neurons.
Postembryonic neuroblast lineages generate newly born neurons in larvae that are recruited into existing or adult-specific circuits (11). We tested whether CCAP-neurons derived from post-embryonic lineages by feeding larvae BrdU from early L1 to late L3. However, despite very robust BrdU incorporation into neurons that reproduced the reported total BrdU larval incorporation pattern (32, 33), we never observed BrdU incorporation into late CCAP neurons (Fig. S5A). We also tracked early CCAP neurons with a permanent marker to test whether late CCAP neurons derived from early CCAP neurons. We raised animals of the genotype Act-FRT > STOP > FRT-nLacZ, UAS-Flp; CCAP-GAL4, UAS-nlsEGFP; tubP-GAL80TS. Here, Flp-mediated cis-FRT recombination brings lacZ under the actin promoter in neurons expressing CCAP-GAL4 but only at 29 °C when GAL80TS is inactive. Animals were raised at 29 °C to mid-L3 and then were switched to 18 °C. We verified that β-Gal was expressed in all early CCAP neurons by mid-L3 (Fig. S6 A and B′). However, by the pharate adult stage, β-Gal was not expressed in A5–A7 CCAP-ENs or in the CCAP-PLs but was expressed in CCAP-INs and in T3–A4 CCAP-ENs (Fig. 4 A and B). In control animals raised at 29 °C up to the pharate adult stage, we found that β-Gal expression could be induced in late CCAP neurons (Fig. S6 B and B′).
Late CCAP Neurons Derive from Early Lineages but Terminally Differentiate at Pupariation.
The lack of evidence supporting the emergence of late CCAP neurons from postembryonic lineages or early CCAP neuron transfating led us to test whether late CCAP neurons are embryonic but undergo delayed differentiation at pupariation. We previously reported that coexpression of Dac with either pMad or OK6-GAL4 could discriminate T3–A4 CCAP-ENs from all other cells in the dorsal VNC in embryos and larvae (25). Because late CCAP-ENs coexpressed these markers (Fig. 2 A, ii and Fig. S2 C, ii and E), we examined their coexpression in A5–A7 segments from stage 17 to mid-L3. At all ages, we identified a single neuron per A5–A7 hemisegment with the appropriate axial position to be an undifferentiated CCAP-EN (stage 17; Fig. 5A). By 10–12 h APF, this single neuron expressed CCAP, Bursα, and Bursβ (Fig. S2 C, ii and E). Strikingly, in stage 15–17 embryos we frequently observed precocious Bursα expression in an A5–A7 neuron that expressed Dac adjacent to a CCAP-IN (Fig. S5 B and C).
Fig. 5.
Late CCAP-ENs derive from an embryonic lineage. Postmitotic late CCAP-ENs can be observed at late embryonic stages. (A) Representative triple-labeled images and fluorophore splits of VNC hemisegments A6 and A7 at embryonic stage 17. In the dorsal-half VNC, only a single neuron (arrows) coexpressed pMad (red), OK6-GAL4 (green), and Dac (blue). In previous work (25), this overlap was unique to CCAP-ENs in dorsal hemisegments T3–A4. In Fig. 2 and Fig. S2, we show that this overlap is unique to A5–A7 late CCAP-ENs by 10–12 h APF. (B) Abdominal VNC of a pharate adult showing Bursα (green) and β-Gal (red) (with fluorophore splits). DacGAL4 was used to Flp-in constitutive lacZ expression only in embryos, using GAL80TS to control the time of UAS-Flp expression. By the pharate adult stage, β-Gal was observed in all CCAP-ENs including T3–A4 early CCAP-ENs and A5–7 late CCAP-ENs. Thus, all six late CCAP-ENs in A5–A7 expressed Dac before late stage 17. Genotypes: (A) OK6-GAL4, UAS-nlsEGFP/+; (B) dacGAL4/Act-FRT > STOP > FRT-nLacZ; tubP-GAL80TS, UAS-nlsEGFP /UAS-Flp.
To show more directly that late CCAP-ENs are born in the embryo, we used DacGAL4 to Flp-in a lacZ reporter conditionally only in embryos. Act-FRT > STOP > FRT-nLacZ, UAS-Flp; dac-GAL4,UAS-nlsEGFP; tubP-GAL80TS animals were raised at 29 °C up to the end of embryogenesis at late stage 17 and were switched to 18 °C thereafter. In pharates, we observed β-Gal immunoreactivity in all CCAP-ENs, including the six late CCAP-ENs in A5–A7 (Fig. 5B). Lack of BrdU incorporation into CCAP-PLs in larvae suggested that CCAP-PLs also are born in the embryo (Fig. S5A). However, the absence of Dac in CCAP-PLs precluded their direct identification by marker coexpression. However, we took advantage of their OK6-GAL4 expression to Flp-in lacZ selectively in the embryo. Animals of genotype Act-FRT > STOP > FRT-nLacZ, UAS-Flp; OK6-GAL4,UAS-nlsEGFP; tubP-GAL80TS were raised at 29 °C to late stage 17 and then were switched to 18 °C thereafter. In pharate adults, we observed β-Gal immunoreactivity in most CCAP-PLs (Fig. S7D), showing that CCAP-PLs are born and express OK6-GAL4 in the embryo. These results indicate that late CCAP neurons most likely are generated in the embryo in segments T3–A9 and that a mechanism exists to delay their peptide hormone differentiation in segments A5–A9.
We took advantage of early dac expression in CCAP-ENs to determine whether late CCAP-ENs morphologically differentiate at pupariation. Our previous work (25) demonstrated that T3–A4 CCAP-ENs exit the VNC via A1–A5 lateral segmental nerves, and that they are the only axons to express dacGAL4,UAS-CD8-GFP within those nerve trunks. Here we found that dacGAL4,UAS-CD8-GFP–expressing axons are not observed in A6 and A7 lateral segmental nerve trunks in larvae up to mid-L3 (Fig. 6A). The fused A8/A9 nerve trunk carries other dac-expressing efferents, such as dMP2 neurons (34); thus we could not discriminate CCAP efferents by dac expression. However, by 4–5 h APF, we observed a single dacGAL4,UAS-CD8-GFP–expressing axon in each of the A6 and A7 nerve trunks (Fig. 6B). Moreover, Bursα immunoreactivity was first observed in the A6, A7, and A8/A9 nerve trunks at pupariation and always within dacGAL4,UAS-CD8-GFP–expressing axons. These data indicate that late CCAP-EN axons do not extend an axon out of the VNC until pupariation. Thus, we conclude that late CCAP-EN axons do not morphologically differentiate until that time.
Ecdysone Cascade Drives Late CCAP Neuron Differentiation.
The ecdysone-induced nuclear hormone receptor cascade plays a critical role in the metamorphic changes that take place during pupariation. This cascade promotes postembryonic neurogenesis, remodeling of neuronal morphology, and, in certain cases, changes in neuronal gene expression (9, 35). We tested a cell-autonomous role for this cascade in temporally inducing late CCAP neuron differentiation. We used CCAP-GAL4 to drive isoform-specific blockers of the ecdysone receptor cell specifically and, as expected, found that dominant-negative transgenes to EcR-A (EcR-ADN) or EcR-B1 (EcR-B1DN) blocked the induction of late CCAP-EN peptide hormone (Fig. 7 B′ and C′). EcR-B2 expression had no effect (Fig. 7D′). In controls, CCAP immunoreactivity was observed in 14.7 ± 1.7 CCAP-ENs per VNC, Bursα in 14.7 ± 1.7 CCAP-ENs per VNC (n = 10 VNCs), and Bursβ in 14.1 ± 1.5 CCAP-ENs per VNC (n = 10 VNCs). EcR-ADN reduced CCAP expression to 6.4 ± 1.1 CCAP-ENs (n = 9, P < 0.0001), Bursα to 7.3 ± 1.2 CCAP-ENs (n = 6, P < 0.0001), and Bursβ to 7.7 ± 3.0 CCAP-ENs (n = 10, P < 0.0001). Similarly, EcR-B1DN reduced CCAP expression to 5.8 ± 2.4 CCAP-ENs (n = 8, P < 0.0001), Bursα to 6.7 ± 1.2 CCAP-ENs (n = 6, P < 0.0001), and Bursβ to 6.7 ± 1.8 CCAP-ENs (n = 9, P < 0.0001). Importantly, in all cases peptide hormone expression was eliminated in the A5–A7 late CCAP-EN subset. These data correspond well with pupal ecdysis phenotypes observed in these animals; expression of either UAS-EcR-ADN or UAS-EcR-B1DN (Fig. 7 B and C) resulted in a failure of leg extension, whereas UAS-EcR-B2DN had no effect (Fig. 7D).
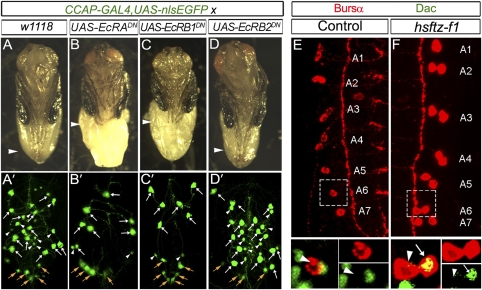
Fig. 7.
Ecdysone signaling is required for late CCAP-EN differentiation. CCAP-GAL4 was used to overexpress dominant-negative EcR-B1/A in CCAP neurons. This overexpression prevented further differentiation of late CCAP-ENs and resulted in a failure of leg extension. (A–D) Leg extension (arrowhead) in pharate adults for each dominant-negative EcR isoform. Control (A) and EcR-B2DN flies (D) had wild-type leg extension, but EcR-ADN (B) or EcR–B1DN (C) expression resulted in failed leg extension. (A′–D′) Pharate adult abdominal VNC showing distribution of all CCAP-GAL4,UAS-nlsEGFP–labeled neurons (green). In control (A′) and in EcR-B2DN (D′) flies, all CCAP neurons expressed CCAP-GAL4, including CCAP-ENs (white arrows), CCAP-INs (arrowheads), and CCAP-PLs (orange arrows). In EcR-ADN (B′) or EcR–B1DN (C′) flies, late CCAP-ENs lose CCAP-GAL4 expression. Early CCAP-ENs (white arrows) and CCAP-PLs (orange arrows) are unaffected. Segmental midline CCAP projections (Fig.1 and Fig. S1) were used as a reference to confirm that all remaining CCAP-ENs were within T3–A4 segments. (E and F) Early L2 VNCs double-labeled (with fluorophore splits of boxed area) for Bursα (red) and Dac (green), showing A1–A7 hemisegments. (E) (Upper) In hemisegments A5–A7 there was only a single CCAP neuron expressing Bursα. (Lower) Magnified view of boxed region in E shows that those neurons lack Dac expression (arrowhead), confirming them to be CCAP-IN. (F) (Upper) hs-ftz-f1 animals were heat shocked at late L1. CCAP neuron doublets expressing Bursα were observed in every hemisegment from T3–A7. (Lower) Magnified view of boxed region shows close-up of A6 hemisegment. The additional Bursα-expressing CCAP neuron (arrow) expressed Dac, indicative of a late CCAP-EN that had differentiated precociously. Genotypes: (A and A′) CCAP-GAL4, UAS-nlsEGFP/+; (B and B′) CCAP-GAL4, UAS-nlsEGFP/UAS-EcR-ADN ; (C and C′) CCAP-GAL4, UAS-nlsEGFP/UAS-EcR-B1DN; (D and D′) CCAP-GAL4, UAS-nlsEGFP/UAS-EcR-B2DN ; (E) CCAP-GAL4,UAS-nlsEGFP/+; (F) hs-ftz-f1/hs-ftz-f1.
We also examined the effect of expressing dsRNAi to the common isoform of EcR (UAS-EcR-CdsRNAi) under the control of dacGAL4 to knock down EcR expression in all CCAP-ENs. In correspondence with the EcRDN data above, we found that EcRdsRNAi significantly reduced CCAP and Bursα expression in late CCAP-ENs by 10 h APF, immediately before pupal ecdysis. The intensity of CCAP immunoreactivity per CCAP-EN neuron (normalized to the mean of the control) was reduced from 100.0 ± 61.7% (n = 55 late CCAP-ENs) to 50.0 ± 31.9% (n = 55 late CCAP-ENs) by EcRdsRNAi (P < 0.0001). Similarly, in late CCAP-ENs the intensity of Bursα immunoreactivity was reduced from 100.0 ± 59.3% (n = 50 late CCAP-ENs) to 30.0 ± 27.8% (n = 47 late CCAP-ENs) (P < 0.0001). These data confirm that peptide hormone expression in late CCAP-ENs requires EcR function. In contrast, EcR manipulation had little effect on CCAP expression in late CCAP-PLs. These data may explain the weak head-eversion phenotype upon EcR-B1DN/ADN expression using CCAP-GAL4 (Fig. 7 B–D). This lack of effect likely reflects EcR isoform and/or nuclear hormone receptor redundancy in CCAP-PLs or may reflect the significantly weaker expression of CCAP-GAL4 (and thus the lowered expression of the EcR-DN constructs) observed in those neurons compared with CCAP-ENs.
We examined whether late CCAP neurons are poised for ecdysone pathway-induced differentiation throughout larval stages. To test this hypothesis, we tested the effect of early induction of ftz-f1, a central player in the ecdysone-induced nuclear hormone receptor cascade (36, 37). Remarkably, we found that 1-h (37 °C) induction of a heat shock-inducible ftz-f1 transgene (36) in late L1 larvae resulted in precocious differentiation of late CCAP-ENs and CCAP-PLs within 4 h. We observed precocious Bursα immunoreactivity in late CCAP-ENs in 54% of animals (n = 13 animals), Bursβ immunoreactivity in 40% of animals (n = 20), and CCAP immunoreactivity in 38% of animals (n = 24) (results for Bursα are shown in Fig. 7F). Precocious CCAP expression also was observed in CCAP-PLs, but, appropriately for CCAP-PLs, neither Bursα nor Bursβ was observed. Although the results were not as robust, we also found that a 1-h heat shock in late stage 17 embryos also could induce some precocious peptide hormone expression in late CCAP neurons in L1 larvae. Thus, throughout larval development late CCAP neurons appear to be poised for an inductive ecdysone signal to undergo terminal differentiation into a peptide hormone-expressing CCAP neuron that can contribute to the execution of pupal ecdysis.
Discussion
We show that two additional subsets of CCAP neurons differentiate to express their mature peptide hormone battery and extend their axon into the periphery during the first 12 h of pupariation. Moreover, we show that these late CCAP neurons are sufficient for pupal ecdysis occurring 12 h after pupariation. We conclude that the CCAP neuronal network functionally recruits these late CCAP neurons to switch network output from a larval ecdysis mode to a pupal ecdysis mode. In other well-established models, postembryonic neurogenesis adds neurons to existing networks at metamorphosis to change network function. Here, we propose that temporally tuned terminal differentiation provides an alternate mechanism of functional recruitment to an existing neuronal network and show that this mechanism can support a change in network output. The extension of an axon into the periphery and the induction of peptide hormone expression in late CCAP neurons at pupariation are of critical relevance, because these peptide hormones are the primary output of CCAP neurons that direct ecdysis (14, 25, 38). Together, these data highlight an unanticipated functional heterogeneity in the CCAP neuronal network (24) and outline the regulatory mechanisms and functional relevance of temporally tuned neuronal differentiation in network remodeling.
A neuron typically is not considered to have terminally differentiated until it expresses the terminal differentiation genes and full morphology required for its function (39, 40). However, neuronal differentiation is not a singular event. In many neurons, the expression of genes critical for mature function (such as neuropeptides, neurotransmitter biosynthetic enzymes, or ion channels) or mature axon or dendritic branching patterns are induced only after many other aspects of neuronal identity are established. In many cases, these late-expressed genes also depend upon extrinsic input for their expression (26, 41, 42). Thus, here we refer to “terminal differentiation” as the completion of a protracted differentiation process required for mature function. In this context, we refer to terminal differentiation of late CCAP neurons as the completed induction of all genes critical for function, which requires the expression of their peptide hormone battery and the extension of their axon into the periphery (14, 25, 38). The development of tools to image late CCAP neuron morphology and other markers of CCAP neuron differentiation before pupariation would allow us to understand better how the entire differentiation program of late CCAP neurons proceeds from their birth in the embryo to pupariation. This understanding would be an important step toward resolving the extent of late CCAP neuron developmental stalling before pupariation. For example, it is intriguing that late CCAP neurons exhibit pMad signaling throughout larval development. Previously, only neurons that exit the VNC were found to exhibit nuclear pMad expression (26, 27). We were unable to resolve the morphology of late CCAP neurons at these early time points, but it would be interesting to determine whether these neurons abut the exit point for peripheral nerve trunks and can access BMP ligand there.
Most embryonic Drosophila neurons differentiate fully by early larval stages (43), but the literature does provide examples of delayed differentiation. Most motoneurons are born in the embryo and function in larvae and then are remodeled during metamorphosis and reused to innervate adult muscles (43). However, the MN5 mesothoracic motoneuron projects short, immature processes during embryogenesis but then arrests developmentally until metamorphosis, whereupon it elaborates mature arbors and innervates its muscle target (20). The functional relevance of this delayed-onset morphological differentiation is evident, but the underlying gene-regulatory mechanisms are unknown. Also, Tv2/3 neurons are born during embryogenesis but do not differentiate into FMRFamide-expressing neurons until metamorphosis (19, 21). Work in Manduca sexta has reported an increase in the number of CCAP-immunoreactive neurons in abdominal segments A2–A7 by day 1 of the fifth instar, before pupal ecdysis. Backfill experiments suggested that these neurons were present in the third and fourth instars but did not differentiate until the fifth instar (18, 22, 23). Our data suggest that those neurons are homologs of the late CCAP neurons that we identify in this work.
The role of ecdysone signaling in the metamorphic changes in the insect nervous system (e.g., in neuronal programmed cell death, postembryonic neurogenesis, and morphological remodeling) is well established (35, 44, 45). Ftz-f1 is an important downstream regulator of the ecdysone nuclear hormone cascade (46), but its role in neurons is less well studied. Previous studies demonstrated that ftz-f1 dictates the temporal specificity of ecdysone signaling by integrating messages between numerous nuclear receptors (E75, DHR3, DHR4, and potentially also E78 and DHR39) (36, 37). Despite its long-known expression in the nervous system, the only reported role for ftz-f1 in nervous system metamorphosis, to our knowledge, is in the remodeling of mushroom body neuron morphology (47–50). Here, we describe a role for ftz-f1 in triggering timed neuronal differentiation. It will be illuminating in the future to determine how EcR and Ftz-f1 coordinate peptide hormone differentiation in late CCAP neurons.
Why late CCAP neurons would undergo delayed terminal differentiation is unclear. Previously, we found that CCAP-ENs regulate both larval and pupal ecdysis (25). However, larval and pupal ecdyses are behaviorally distinct, and here we found that late CCAP-ENs had not differentiated in time for larval ecdysis. We postulate that the emergence of late CCAP-ENs immediately before pupal ecdysis offers an elegant means to meet the changing functional role of the CCAP neuron network from larval to pupal ecdysis. From this network perspective, we could regard late CCAP neuron terminal differentiation and the role of these neurons in pupal ecdysis in two ways. First, late CCAP neurons may be recruited functionally to the existing CCAP neuronal network to switch its ecdysis program from larval to pupal. Second, late CCAP neurons may constitute an independent subnetwork of CCAP neurons that is responsible for pupal ecdysis and supersedes a subnetwork of early CCAP neurons responsible larval ecdysis. Two lines of evidence led us to favor the first hypothesis. Adams and colleagues (29) imaged CCAP neuron activity during fictive pupal ecdysis using a CCAP::GcaMP transgene. They observed activity of CCAP neurons within segments T3–A4, which have only early CCAP neurons (29), and in segments A7–A9, which have late CCAP neurons. Thus, although we demonstrate here that T3–A4 early CCAP neurons are not essential for pupal ecdysis, this previous study shows that they are active and presumably perform some function. Also, CCAP neurons in the suboesophageal region are required in combination with abdominal CCAP-ENs for wing inflation in young adults. Thus, components of the CCAP network, in addition to the late CCAP neurons, continue to function beyond larval stages.
It is unclear why late CCAP-ENs do not differentiate into peptide hormone-expressing CCAP neurons until pupal ecdysis, but we propose three potential explanations. (i) Late CCAP-ENs may have distinct central connectivity or receptor expression that may mediate a difference in the sequence or activation of CCAP neuron activity that is specific to pupal ecdysis and perhaps detrimental to larval ecdysis. (ii) Early CCAP-ENs on their own may not secrete sufficient peptide hormone levels into the hemolymph for robust pupal ecdysis. Thus, late CCAP-ENs may be required to boost levels of secreted peptide hormones required for pupal ecdysis. It is possible that elevated peptide hormone expression may be detrimental to larval ecdysis. (iii) The temporally tuned differentiation mechanism may have evolved simply to put off the demands of secreting high levels of circulating peptide hormones until the time point at which such secretion is absolutely required.
The early neurogenesis of neurons that do not undergo terminal differentiation until pupariation was unexpected. However, we propose that such early neurogenesis offers a mechanism to generate late CCAP neurons that is simpler than postembryonic neurogenesis. The process of neuronal specification and differentiation is a highly orchestrated process through which complex cascades of spatially and temporally patterned transcription factors direct the emergence of specific neuronal subsets. Moreover, the Drosophila nervous system is segmented, with abdominal segments A1–A7 giving rise to essentially the same neuroblasts and mostly to the same neurons in each segment. Thus, generating a single CCAP-EN in each segment from the same neuroblast lineage would be an economical way to produce late CCAP-ENs with much the same terminal gene-expression profile as early CCAP-ENs. Additional segment-specific mechanisms then could be superimposed to delay late CCAP-EN differentiation until it received its ecdysone trigger. Recent work has described mechanisms to diversify the same postmitotic neuron in a segment-specific manner. The best described of these mechanisms is the segment-specific programmed cell death of dMP2 and Va neurons in the Drosophila VNC (51, 52). These studies indicate that segment-specific differences in Hox gene expression within these postmitotic neurons determine their survival versus apoptosis during embryogenesis. Here, we find that segment-specific differences in the timing of neuronal differentiation provide a mechanism for neuronal diversity. It will be intriguing in the future to investigate the potential role of segment-specific Hox gene expression in directing early versus late CCAP neuron differentiation.
Materials and Methods
Fly Stocks.
We used fly stocks dacGAL4 (53); CCAP-GAL4 (16); OK6-GAL4 (54); tubP-GAL80TS (55); UAS-nlsEGFP; UAS-CD8-GFP; UAS-EcR-ADN (BL9451) ; UAS-EcR-B1DN (BL6872); UAS-EcR-B2DN (BL9449); UAS-EcR-dsRNAi (BL9327), UAS-CD8-EGFPLL5 (Bloomington Drosophila Stock Center); Act-FRT > STOP > FRT.nlacZ, UAS-Flp (56); and hs-ftz-f1 (36). Lethal alleles were maintained over CyO,Act-EGFP or TM3,Ser,Act-EGFP balancer chromosomes. The control genotype was w1118. Flies were maintained on standard cornmeal food (25 °C, 70% humidity).
Immunohistochemistry.
Standard immunohistochemical protocols were used, as previously described (57). Primary antibodies were rabbit anti-CCAP (code 2TB; 1:2,000), a gift from H. Dircksen (Stockholm University, Stockholm, Sweden) (58); rabbit anti-Bursα (1:5,000), a gift from B. White (NIH, NIMH, Bethesda, MD) (24); mouse anti-Bursβ (1:2,000), a gift from C. Klein (Stanford University, Palo Alto, CA) (59); mouse anti-Dac (1:25; clone dac2-3) and mouse anti-BrdU (1:20; clone G3G4) (Developmental Studies Hybridoma Bank); rabbit anti-pMad (1:100) (41D10; Cell Signaling Technology); chicken anti–ß-Gal (1:1,000) (ab9361; Abcam); and goat anti-HRP-Cy5 (1:100) (Jackson ImmunoResearch). Secondary antibodies were anti-mouse, anti-chicken, anti-rabbit IgG (H+L) conjugated to either DyLight 488, Cy3, and Cy5 (1:200) (Jackson ImmunoResearch).
Image Analysis.
Images were acquired on an Olympus FV1000 confocal microscope and analyzed with Image J (National Institutes of Health). Images for comparison were processed identically, and image capture was set to avoid fluorescent saturation. Analysis was performed as described (13). Data are presented as the percentage intensity relative to the mean of the control.
Statistics.
Statistical analysis was performed using Graphpad Prism 4. Data for immunofluorescence and cell number underwent D'Agostino and Pearson Omnibus normality testing. Normally distributed data were compared using a two-tailed t test assuming equal variance. Non-normally distributed data were compared using a nonparametric Mann–Whitney test. Statistical data are presented to the exact P value to P < 0.0001.
Heat Shock Ftz-F1 Induction.
Larvae were heat shocked at 37 °C for 1 h and then were allowed to recover at 25 °C for 2–4 h before dissection and analysis.
Supplementary Material
Acknowledgments
We thank H. Dircksen, C. Klein, B. H. White, the Developmental Studies Hybridoma Bank, and the Bloomington Drosophila Stock Center for supplying key reagents used in this study. We thank Michael Gordon, Timothy O'Connor, and the D.W.A. laboratory (University of British Columbia) for support and valuable critiques before submission. This work was supported by the EJLB Foundation, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the British Columbia Knowledge Development Fund, and the Michael Smith Foundation for Health Research. D.W.A. is an EJLB Scholar, a Canadian Institutes of Health Research New Investigator, a Michael Smith Foundation for Health ResearchCareer Scholar, and a Tula Foundation Investigator. L.V. is a recipient of a College for Interdisciplinary Studies Graduate Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.W.T. is a guest editor invited by the Editorial Board.
See Author Summary on page 4725 (volume 109, number 13).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114710109/-/DCSupplemental.
References
- 1.Wilbrecht L, Kirn JR. Neuron addition and loss in the song system: Regulation and function. Ann N Y Acad Sci. 2004;1016:659–683. doi: 10.1196/annals.1298.024. [DOI] [PubMed] [Google Scholar]
- 2.Borodinsky LN, et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 3.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 4.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone SS, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31(38):13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu P, Hall AK. The role of activin in neuropeptide induction and pain sensation. Dev Biol. 2006;299:303–309. doi: 10.1016/j.ydbio.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demarque M, Spitzer NC. Neurotransmitter phenotype plasticity: An unexpected mechanism in the toolbox of network activity homeostasis. Dev Neurobiol. 2012;72:22–32. doi: 10.1002/dneu.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winbush A, Weeks JC. Steroid-triggered, cell-autonomous death of a Drosophila motoneuron during metamorphosis. Neural Dev. 2011;6:15. doi: 10.1186/1749-8104-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, et al. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: Sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 12.Levine RB, Morton DB, Restifo LL. Remodeling of the insect nervous system. Curr Opin Neurobiol. 1995;5:28–35. doi: 10.1016/0959-4388(95)80083-2. [DOI] [PubMed] [Google Scholar]
- 13.Brown HL, Truman JW. Fine-tuning of secondary arbor development: The effects of the ecdysone receptor on the adult neuronal lineages of the Drosophila thoracic CNS. Development. 2009;136:3247–3256. doi: 10.1242/dev.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewer J. Behavioral actions of neuropeptides in invertebrates: Insights from Drosophila. Horm Behav. 2005;48:418–429. doi: 10.1016/j.yhbeh.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Mesce KA, Fahrbach SE. Integration of endocrine signals that regulate insect ecdysis. Front Neuroendocrinol. 2002;23:179–199. doi: 10.1006/frne.2002.0228. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Schroeder AJ, Helfrich-Förster C, Jackson FR, Ewer J. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–2656. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- 17.Zhao T, et al. A Drosophila gain-of-function screen for candidate genes involved in steroid-dependent neuroendocrine cell remodeling. Genetics. 2008;178:883–901. doi: 10.1534/genetics.107.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai L, et al. Identification, developmental expression, and functions of bursicon in the tobacco hawkmoth, Manduca sexta. J Comp Neurol. 2008;506:759–774. doi: 10.1002/cne.21575. [DOI] [PubMed] [Google Scholar]
- 19.Schneider LE, Sun ET, Garland DJ, Taghert PH. An immunocytochemical study of the FMRFamide neuropeptide gene products in Drosophila. J Comp Neurol. 1993;337:446–460. doi: 10.1002/cne.903370308. [DOI] [PubMed] [Google Scholar]
- 20.Consoulas C, Restifo LL, Levine RB. Dendritic remodeling and growth of motoneurons during metamorphosis of Drosophila melanogaster. J Neurosci. 2002;22:4906–4917. doi: 10.1523/JNEUROSCI.22-12-04906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider LE, Roberts MS, Taghert PH. Cell type-specific transcriptional regulation of the Drosophila FMRFamide neuropeptide gene. Neuron. 1993;10:279–291. doi: 10.1016/0896-6273(93)90318-l. [DOI] [PubMed] [Google Scholar]
- 22.Loi PK, Emmal SA, Park Y, Tublitz NJ. Identification, sequence and expression of a crustacean cardioactive peptide (CCAP) gene in the moth Manduca sexta. J Exp Biol. 2001;204:2803–2816. doi: 10.1242/jeb.204.16.2803. [DOI] [PubMed] [Google Scholar]
- 23.Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. Crustacean cardioactive peptide-immunoreactive neurons in the hawkmoth Manduca sexta and changes in their immunoreactivity during postembryonic development. J Comp Neurol. 1993;338:612–627. doi: 10.1002/cne.903380410. [DOI] [PubMed] [Google Scholar]
- 24.Luan H, et al. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veverytsa L, Allan DW. Retrograde BMP signaling controls Drosophila behavior through regulation of a peptide hormone battery. Development. 2011;138:3147–3157. doi: 10.1242/dev.064105. [DOI] [PubMed] [Google Scholar]
- 26.Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. doi: 10.1016/s0092-8674(03)00204-6. [DOI] [PubMed] [Google Scholar]
- 27.Marqués G, et al. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- 28.Peabody NC, et al. Bursicon functions within the Drosophila CNS to modulate wing expansion behavior, hormone secretion, and cell death. J Neurosci. 2008;28:14379–14391. doi: 10.1523/JNEUROSCI.2842-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YJ, Zitnan D, Galizia CG, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Cur Biol. 2006;16(14):1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 30.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 31.Davis MM, O'Keefe SL, Primrose DA, Hodgetts RB. A neuropeptide hormone cascade controls the precise onset of post-eclosion cuticular tanning in Drosophila melanogaster. Development. 2007;134:4395–4404. doi: 10.1242/dev.009902. [DOI] [PubMed] [Google Scholar]
- 32.Prokop A, Bray S, Harrison E, Technau GM. Homeotic regulation of segment-specific differences in neuroblast numbers and proliferation in the Drosophila central nervous system. Mech Dev. 1998;74:99–110. doi: 10.1016/s0925-4773(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 33.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol. 1990;21:1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 34.Miguel-Aliaga I, Allan DW, Thor S. Independent roles of the dachshund and eyes absent genes in BMP signaling, axon pathfinding and neuronal specification. Development. 2004;131:5837–5848. doi: 10.1242/dev.01447. [DOI] [PubMed] [Google Scholar]
- 35.Schubiger M, Wade AA, Carney GE, Truman JW, Bender M. Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development. 1998;125:2053–2062. doi: 10.1242/dev.125.11.2053. [DOI] [PubMed] [Google Scholar]
- 36.Woodard CT, Baehrecke EH, Thummel CS. A molecular mechanism for the stage specificity of the Drosophila prepupal genetic response to ecdysone. Cell. 1994;79:607–615. doi: 10.1016/0092-8674(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 37.Broadus J, McCabe JR, Endrizzi B, Thummel CS, Woodard CT. The Drosophila beta FTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol Cell. 1999;3:143–149. doi: 10.1016/s1097-2765(00)80305-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim YJ, et al. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci USA. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobert O, Carrera I, Stefanakis N. The molecular and gene regulatory signature of a neuron. Trends Neurosci. 2010;33:435–445. doi: 10.1016/j.tins.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobert O. Regulatory logic of neuronal diversity: Terminal selector genes and selector motifs. Proc Natl Acad Sci USA. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Caraballo M, Dryer SE. Glial cell line-derived neurotrophic factor and target-dependent regulation of large-conductance KCa channels in developing chick lumbar motoneurons. J Neurosci. 2002;22:10201–10208. doi: 10.1523/JNEUROSCI.22-23-10201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tissot M, Stocker RF. Metamorphosis in drosophila and other insects: The fate of neurons throughout the stages. Prog Neurobiol. 2000;62:89–111. doi: 10.1016/s0301-0082(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 44.Weeks JC, Levine RB. Postembryonic neuronal plasticity and its hormonal control during insect metamorphosis. Annu Rev Neurosci. 1990;13:183–194. doi: 10.1146/annurev.ne.13.030190.001151. [DOI] [PubMed] [Google Scholar]
- 45.Robinow S, Talbot WS, Hogness DS, Truman JW. Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor isoform. Development. 1993;119:1251–1259. doi: 10.1242/dev.119.4.1251. [DOI] [PubMed] [Google Scholar]
- 46.King-Jones K, Thummel CS. Nuclear receptors—a perspective from Drosophila. Nat Rev Genet. 2005;6:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 47.Yamada M, et al. Temporally restricted expression of transcription factor betaFTZ-F1: Significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127:5083–5092. doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
- 48.Ruaud AF, Lam G, Thummel CS. The Drosophila nuclear receptors DHR3 and betaFTZ-F1 control overlapping developmental responses in late embryos. Development. 2010;137:123–131. doi: 10.1242/dev.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohno CK, Petkovich M. FTZ-F1 beta, a novel member of the Drosophila nuclear receptor family. Mech Dev. 1993;40:13–24. doi: 10.1016/0925-4773(93)90084-b. [DOI] [PubMed] [Google Scholar]
- 50.Boulanger A, et al. ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling. Nat Neurosci. 2011;14:37–44. doi: 10.1038/nn.2700. [DOI] [PubMed] [Google Scholar]
- 51.Suska A, Miguel-Aliaga I, Thor S. Segment-specific generation of Drosophila Capability neuropeptide neurons by multi-faceted Hox cues. Dev Biol. 2011;353:72–80. doi: 10.1016/j.ydbio.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miguel-Aliaga I, Thor S. Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development. 2004;131:6093–6105. doi: 10.1242/dev.01521. [DOI] [PubMed] [Google Scholar]
- 53.Heanue TA, et al. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aberle H, et al. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 55.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 56.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 57.Eade KT, Allan DW. Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling. J Neurosci. 2009;29:3852–3864. doi: 10.1523/JNEUROSCI.0213-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vömel M, Wegener C. Neurotransmitter-induced changes in the intracellular calcium concentration suggest a differential central modulation of CCAP neuron subsets in Drosophila. Dev Neurobiol. 2007;67:792–808. doi: 10.1002/dneu.20392. [DOI] [PubMed] [Google Scholar]
- 59.Luo CW, et al. Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc Natl Acad Sci USA. 2005;102:2820–2825. doi: 10.1073/pnas.0409916102. [DOI] [PMC free article] [PubMed] [Google Scholar]