Abstract
Islet transplantation is a feasible therapeutic alternative for metabolically labile patients with type 1 diabetes. The primary therapeutic target is stable glycemic control and prevention of complications associated with diabetes by reconstitution of endogenous insulin secretion. However, critical shortage of donor organs, gradual loss in graft function over time, and chronic need for immunosuppression limit the indication for islet transplantation to a small group of patients. Here we present a promising approach to address these limitations by utilization of a macrochamber specially engineered for islet transplantation. The s.c. implantable device allows for controlled and adequate oxygen supply and provides immunological protection of donor islets against the host immune system. The minimally invasive implantable chamber normalized blood glucose in streptozotocin-induced diabetic rodents for up to 3 mo. Sufficient graft function depended on oxygen supply. Pretreatment with the growth hormone-releasing hormone (GHRH) agonist, JI-36, significantly enhanced graft function by improving glucose tolerance and increasing β-cell insulin reserve in rats thereby allowing for a reduction of the islet mass required for metabolic control. As a result of hypervascularization of the tissue surrounding the device, no relevant delay in insulin response to glucose changes has been observed. Consequently, this system opens up a fundamental strategy for therapy of diabetes and may provide a promising avenue for future approaches to xenotransplantation.
Keywords: treatment of diabetes, immune isolation, beta cells
Transplantation of pancreatic islets has evolved into a feasible treatment option for a subset of patients with type 1 diabetes. However, several obstacles limit the widespread application of this approach. First, the overall shortage of donor organs remains a critical challenge in the transplantation field, especially in the context of islet transplantation, where often more than one donor per recipient is needed (1). Second, islet transplantation features an especially complex immunological situation. Patients transplanted with donor islets may develop allotype immunization (2), which could complicate future transplants. Furthermore, the life-long use of immunosuppressive therapy is essential for controlling alloimmunity and ideally also autoimmunity. However, immunosuppression is associated with potentially severe side-effects and may additionally impair islet revascularization and regeneration (3–5). Finally, although the procedure performed is minimally invasive, complications such as portal vein thrombosis or i.p. bleeding may occur.
The typical requirement of more than one donor per recipient to achieve insulin-independence for even a limited time implies substantial loss of viable islets during the isolation process and in the early posttransplant period. Massive loss of beta cells is evident even upon syngeneic transplantation in mice after only a few days (6). Multiple factors contribute to such a loss. An important determinant is the limited oxygen supply for pancreatic islets, which have a high oxygen demand (7–10). Initially, diffusion alone is adequate, but subsequently, loss is due to insufficient vascularization (11, 12). In a healthy pancreas, oxygen is supplied to the islets through a dense, glomerular-like network of capillaries that provides blood inflow of nearly 15% of the overall pancreatic supply and ensures that no oxygen gradient is formed between the surface and the core of the islet (13). Thus, when encapsulated islets are implanted and neovascularization is prevented from direct contact with the graft, graft loss is even more severe.
Immunoisolation of pancreatic islets has the potential to prevent/minimize the use of immunosuppressive agents; thus, encapsulation of islets in hydrogels has been pursued for many years. In this technology, donor islets are coated with a semipermeable membrane that blocks cells and large molecules of the host immune system but is permeable to essential nutrients, glucose, and insulin. Microencapsulation has been shown to be feasible in various experimental systems (14–19) and in pilot clinical trials (20). However, the limited lifetime of microencapsulated islets and the large islet tissue volume required are obstacles that must be overcome to make this approach clinically viable.
Inadequate oxygen supply causes a gradual loss of islet mass and functional potency (21, 22). This effect was even more pronounced when islets are encapsulated (21–24). Experimental approaches to overcome this problem include the generation of very small capsules (25), use of hypoxia-resistant islet cells (26), stimulation of blood vessel growth around implanted devices (27, 28), increased oxygen permeability of the encapsulating material (29, 30), use of small islet cell clusters to reduce the diffusion distance to the islet (30–32), and the use of islets engineered to contain an intracellular oxygen carrier such as myoglobin (33). Another approach focuses on in situ generation of oxygen, close to the islets, by an electrochemical process (34) or local photosynthesis (35). However, none of these methods are efficient enough to maintain viable physiological conditions.
Besides experimental efforts to create the most beneficial environment for the islet graft, it is essential to produce viable, robust, and uncompromised functional islets in the most efficient and economic manner (36). To this end, several growth factors that may have potential for enhancing mass and function of β-cells have been identified (37). A natural adaptation of islet cell mass, mediated by growth factors, occurs due to increased demand during pregnancy as well as with obesity (38). Hypothalamic neurohormone, growth hormone-releasing hormone (GHRH), stimulates the release of growth hormone from the pituitary and has been the focus of intense studies. GHRH and GHRH receptors of pituitary type and splice variants thereof are expressed in many human tissues (39). Recently, we have shown splice variant-1 expression of GHRH receptor in rat insulinoma INS-1 cells, as well as in rat and human pancreatic islets (40, 41). We have also analyzed the effect of a synthetic agonist of GHRH JI-38 on beta-cell survival and cell proliferation in vitro and in vivo (42). Islets treated with GHRH agonist, JI-36, were able to achieve normoglycemia earlier and more consistently than untreated islets. Furthermore, in contrast to diabetic animals transplanted with untreated islets, animals receiving islets pretreated with Jl-36 agonist displayed a better insulin response in glucose tolerance tests; this response was comparable to the response of normal healthy mice (40).
A more efficient use of donor islets could facilitate the widespread use of islet transplantation as a treatment approach for type 1 diabetes. Therefore, we investigated the combination of GHRH agonism with ideal oxygenation using a bioartificial macrochamber, which proved beneficial for transplanted islets. Encapsulated islets are maintained within an alginate slab configuration adjacent to an oxygen-permeable membrane, which creates a sufficient immune barrier and allows for adequate oxygen supply to the islet graft (Fig. 1). We achieved enhanced function of encapsulated islets by pretreating them with GHRH analogs. Together, a strategy capable of restoring and maintaining long-term euglycemia in diabetic models is thereby presented.
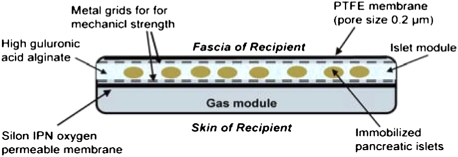
Fig. 1.
Schematic view of the bioartificial chamber system.
Results
Transplantation and Metabolic Follow-Up.
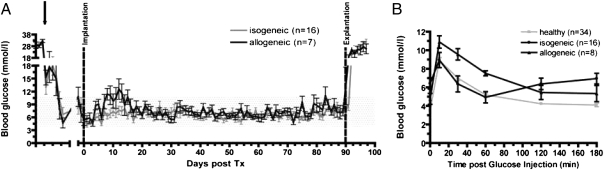
Streptozotocin (STZ)-induced diabetic rats were implanted with encapsulated rodent islets (2,400 islet equivalents; 3,600 IEQ/cm2) in isogeneic (n = 16) and allogeneic (n = 7) islet transplantation models. Monitoring of nonfasting blood glucose levels showed normoglycemia during the entire follow-up period of 90 d (Fig. 2A). Upon explantation of graft on day 90, the animals immediately reverted to a diabetic state.
Fig. 2.
Transplantation of encapsulated rodent islets into diabetic rats (isogeneic and allogeneic model) and metabolic follow-up. (A) Nonfasting blood glucose profile of animals transplanted with either isogeneic or allogeneic encapsulated islets. After STZ-injection and assuring diabetic state, animals were treated with slow-releasing insulin (arrow) until transplantation. After initial blood glucose variations, stable normoglycemia was maintained during the follow-up period of 90 d regardless of experimental group. After explantation of the islet containing chamber-system, blood glucose levels of all animals rapidly increased. (B) At week 3 posttransplantation, an i.v. glucose tolerance test was performed (0.5 g/kg body weight i.v.) in both experimental groups and compared with healthy animals. Regardless of experimental group, all animals reached normal blood glucose levels within the observation period.
To determine the insulin reserve and kinetic response of encapsulated islets to glucose challenge, an i.v. glucose tolerance test (ivGTT) was performed at 3 wk posttransplantation. Transplanted animals were compared with healthy animals. All animals showed a rapid increase in blood glucose levels upon glucose injection followed in comparison with healthy animals by a gradual and slightly delayed decrease to finally reach normal blood glucose levels. Regardless of experimental group, all animals reached target blood glucose levels at 120 min following glucose injection. (Fig. 2B).
Biocompatibility Measures.
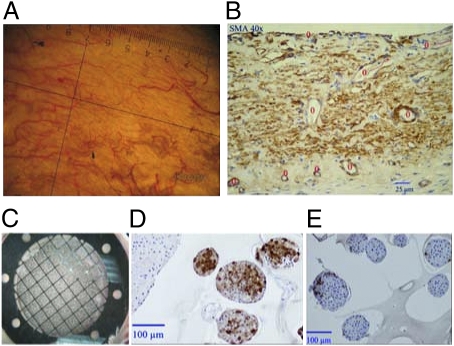
Upon explantation of the chamber system, surrounding tissue was examined macroscopically and histologically. The device itself was intact without any damage to the membranes and the housing; the tissue pocket displayed no signs of inflammation or fibrosis but had prominent vascularization at the site of the islet module.
Histological assessment of tissue surrounding the device confirmed the macroscopic appearance displaying no inflammatory reactions such as leukocyte infiltration. Quantification of capillary density showed a considerable difference between the site facing the islet-module site (18 ± 7.5 blood vessels per high-power field) and the gas-module site (12 ± 3.8 per high-power field; Fig. 3).
Fig. 3.
Morphological analysis of the pocket surrounding an explanted encapsulated graft. (A) Macroscopic view of islet facing tissue. (B) Smooth muscle actin (SMA) stained tissue, blood vessel are marked with red circles. (C) Macroscopic view of the islet-containing slab, integrated into the chamber system. (D and E) Immunohistochemical staining of immobilized islets for insulin (D) and glucagon (E).
When the islet containing alginate slab was retrieved from the device, macroscopic and unstained microscopic examination showed intact islet clusters with well preserved islet morphology. Immunohistochemical staining of alpha and beta cells using glucagon and insulin antibodies showed intense staining and a normal distribution typical for rat islets (Fig. 3).
Enhancement of Encapsulated Islet Function by Pre-Treatment with GHRH Agonist JI-36.
When GHRH agonist JI-36 was introduced to the experiment, the total number of transplanted islets was significantly reduced by one half, to 1,000 islet equivalents per animal.
Pre-Transplant Assessment.
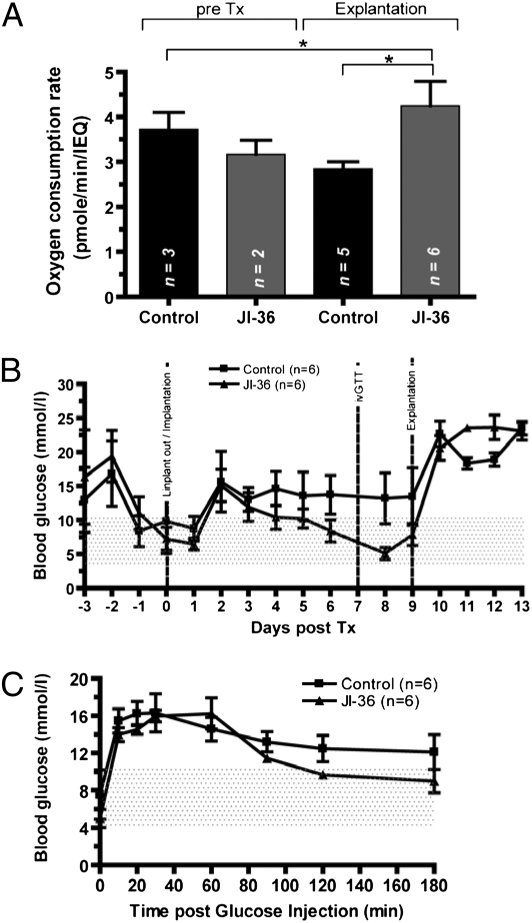
Following 48 h of culture either in complete CR medium supplemented with vehicle (0.1% dimethyl sulfoxide; DMSO) or JI-36, determination of oxygen consumption rate (OCR) was used as a quality control assay before transplantation. As shown in Fig. 4, no relevant difference was observed between the groups before transplantation.
Fig. 4.
Enhancement of encapsulated islet function by pretreatment with GHRH agonist JI-36. (A) Pretransplant and posttransplant assessment. By determination of OCR on representative samples of each group before transplantation, no relevant difference between the groups was observed. Upon retrieval of the islets after 9 d, a significant difference in OCR was shown in favor of JI-36 pretreated islets (*P < 0.05). Metabolic follow-up of transplanted animals with encapsulated islet grafts either untreated (n = 6) or pretreated with JI-36 (n = 6). (B) After initial increase, nonfasting blood glucose levels normalized within the JI-36 pretreated group, control animals remained hyperglycemic throughout the observation period. The area under the curve of blood glucose for days 1–9 showed a significant difference between the groups in favor of JI-36 treated animals (*P < 0.05). Retrieval of the graft caused an immediate recurrence of diabetic state in all animals. (C) During ivGTT, control animals did not reach target blood glucose levels, whereas the JI-36 group normalized at 120 min postglucose injection.
Metabolic Follow-Up.
Twelve diabetic animals were transplanted with a minimal mass of pretreated encapsulated islets with either vehicle (n = 6) or JI-36 (n = 6) and followed for 2 wk. After an initial rise in blood glucose levels in both groups, the treatment group reached normoglycemia, until explantation of the graft, whereas control animals remained slightly hyperglycemic throughout the observation period (Fig. 4B). The calculation of the area under the curve of blood glucose levels resulted in a significant difference for days 1–9 post implantation in favor of JI-36 treated animals (P < 0.05). Upon retrieval of the graft, all animals returned to a diabetic metabolic state. On day 7 posttransplantation, an ivGTT was performed. Control animals did not reach target blood glucose levels within the observation period of 180 min following glucose injection, whereas the JI-36 pretreated group displayed normalization of glucose at 120 min (Fig. 4C).
Quality Control Assessment Post Explantation.
Upon retrieval of the chamber after 9 d, morphological and functional characterization of the islets was performed by histology, measurement of OCR, and insulin release (GSIR) stimulated by glucose.
Slabs containing retrieved islets were morphologically intact; however, islets from the group treated with JI-36 appeared more compact and dense compared with control islets. Insulin staining was markedly more intense in the pretreated group (Fig. 5 A and B). At the time of explantation, the OCR was determined and compared with pretransplantation analysis. No significant change was observed during the in situ period. However, islets pretreated with JI-36 had significantly superior levels in OCR compared with control islets (P < 0.05; Fig. 4).
Fig. 5.
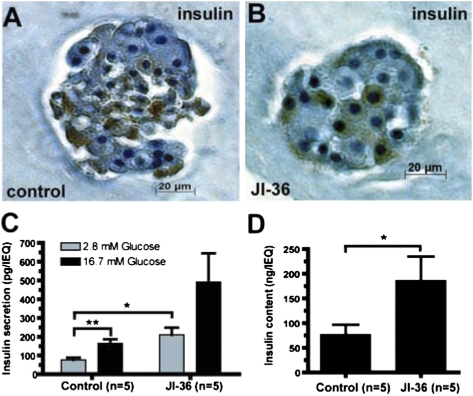
Postexplantation assessments. (A and B) Representative image of encapsulated islets stained for insulin. JI-36 pretreated islets appeared more compact and dense compared with control islets and staining for insulin was intense and distinct. (C) Glucose stimulated insulin release (GSIR). After equilibration at glucose concentration of 2.8 mM, islets were stimulated in vitro with high glucose of 16.7 mM. Basal insulin release was significantly increased in pretreated islets compared with controls, both groups showed a regular stimulation index of 2.2 (control) and 2.4 (JI-36) upon stimulation indicating intact insulin reserve. (D) Islets were analyzed for insulin content following glucose stimulation. The quantification showed significantly higher levels of insulin in the JI-36 treated islets compared with control (*P < 0.05). For the GSIR-experiment, one set of islets per group was excluded due to technical issues.
After explantation, islet slabs were also stimulated with glucose in vitro in a static model. At basal glucose concentration of 2.8 mM, the release of insulin into culture media was significantly higher in the JI-36 group (129 ± 59 pg/islet) compared with control (54 ± 15 pg/islet). This difference cannot be explained by the leakiness of the islets treated with JI-36, because upon stimulation with high levels of glucose (16.7 mM), insulin release from both groups was increased 2.2-fold in controls and 2.4-fold in the JI-36 group, compared with basal conditions, thereby demonstrating a positive insulin reserve (Fig. 5C). Quantification of insulin content following glucose stimulation showed significantly higher insulin levels in the islets treated with JI-36 compared with controls (P < 0.05; Fig. 5D).
Discussion
More widespread use of transplantation of pancreatic islets for diabetic patients is limited by (i) the general shortage of donor organs; (ii) the insufficient long-term function mainly due to inadequate oxygen supply and exposure to inflammatory, antiregenerative and antiproliferative factors in the intraportal microenvironment; and (iii) the chronic need for immunosuppressive therapy. In this study, we evaluated a macrochamber developed specifically for encapsulation of pancreatic islets that provides physiological oxygen levels to the islets, creates a sufficient immunobarrier to prevent allogeneic immune response, and allows for mechanical protection of the islet graft. In addition, the device is designed for s.c. implantation and is therefore minimally invasive and easily retrievable.
Transplantation of an optimized islet preparation with viable and functionally potent islets is a key prerequisite for successful transplantation regardless of other transplantation conditions. Besides donor selection and optimization of the pancreas preservation and isolation process, several approaches have been made to precondition islets before transplantation (43–47). Conditioning of pancreatic islets with GHRH agonist JI-36 improves beta-cell survival and metabolic function in vivo mainly due to a reduction in apoptosis and preservation of the proliferative capacity of the islet cells (40). Pronounced expression of GHRH-receptor on beta cells in rat and human islets was a strong indicator for a direct signal transduction within beta cells. Synthetic agonists of GHRH, such as JI-36, are more potent and longer acting than native GHRH or other growth factors. Even more potent GHRH agonists than JI-36 (42) have now been synthesized by the group (A.V.S.).
The administration of JI-36 to islet culture before transplantation not only improves the quality of the islets to a statistically significant extent, but moreover allows for a reduction in the islet mass needed to reverse diabetes. Therefore, the use of JI-36 provides a more efficient strategy for islet transplantation within the setting of encapsulation as well as in islet transplantation in general. Moreover, the encapsulation of pretreated islets within a device that protects the graft from the host tissue allows for safe application of the agonist in a clinical setting.
Another key prerequisite for artificial device use in humans is biocompatibility of all materials used as well as the composite design. For the chamber system used, all materials have been tested for biocompatibility and, as shown here in rodents and as described in minipigs (48), the implantation pocket showed no signs of inflammatory reactions. Moreover, the surrounding tissue displayed markedly increased vascularization at the site of the islet chamber. This observation is of major importance for proper function of the islet graft. The immobilization of the islets in alginate and their encapsulation within macroporous membranes allows for free diffusion of small molecules such as glucose and insulin. The crucial issue for any artificial device that is not directly connected to the blood stream is an adequate and rapid route of metabolic exchange. Interestingly, performance of an ivGTT in our system indeed demonstrated a minimally delayed response time. This important advantage of the system is most likely based on the very short diffusion distance and large diffusion area developed in close proximity to the islet module. The hypervascularization observed may be induced by proangiogenic factors released from the islets, possibly the JI-36, or the local oxygenation (49–51).
As demonstrated in several studies (52–54), the supply of oxygen in both the early posttransplant period and long-term is largely limited at the intraportal transplantation site, but is nevertheless crucial for islet survival and function. Islets exposed to chronic hypoxia are especially susceptible to hypoxia-mediated inflammatory reactions, oxidative stress, and apoptosis. The model proposed here provides a unique solution for adequate islet oxygenation. Gas phase oxygen is infused into a compartment that is an integral part of the device and the oxygen diffuses across a thin gas-permeable membrane and through the adjacent alginate hydrogel to supply the islets. During extensive series of tests, the structure of the device, islet density, oxygen concentration, and infusion intervals were adjusted to continuously provide the islets with oxygen at partial pressure over 100 mmHg and below toxic levels. Thus, a stable and prolonged normalization of diabetic conditions could be observed in animals treated by transplantation with this device. We attribute these favorable results mainly to adequate oxygen supply of the graft in the early implantation period (55, 56).
In the majority of centers, islet transplantation is done intraportally by either transhepatic cannulation of the portal vein or through minilaparotomy. Complications associated with this procedure are, overall, rare (57). However, bleeding complications or portal vein thrombosis have been reported in particular cases, and the number of possible transplants is generally restricted to three attempts per individual. The s.c. location of a graft containing device has the advantage of an easy and minimally invasive implantation procedure and the opportunity to easily replenish the device in case of graft failure.
For quality control of islets pretransplant and after retrieval, measurement of the oxygen consumption rate was used as a standard method. This assay is especially well suited to encapsulated islets and was proven to be directly correlated to islet performance in vivo. According to several other groups, this assay is well established and verified as an excellent predictor of transplantation outcomes (58, 59).
One of the major aims of macroencapsulation of islets is the immunoisolation of the graft from the host's immune function, thus allowing reduction or avoidance of the immunosuppression necessary to prevent rejection of the graft. In an allogeneic transplantation setting, we could show that the system provided sufficient immunoisolation of the graft throughout the experimental period. As indicated by normalized blood glucose levels, no rejection episodes were observed within the 90 d of follow-up period. During extensive experimental series, we determined that the combination of alginate encapsulation and the multilayered membrane barrier is necessary to reach complete immunoisolation.
The approach presented here for islet macroencapsulation addresses the current critical issues of islet transplantation. By preconditioning of encapsulated islets with a potent antiapoptotic agent, such as GHRH agonist JI-36, the efficacy of islet transplantation can be substantially improved by reduction of the islet mass necessary for metabolic control. A robust immune barrier, adequate oxygen supply, and minimally invasive surgical procedure are the hallmarks of this approach. These features provide promising avenues to design therapeutic cell therapies for the rising numbers of patients with diabetes mellitus.
Materials and Methods
All animal experiments were conducted in Israel and approved by the Israeli National Animal Ethical Committee. Animal models, islet isolation and culture, determination of islet equivalent, glucose tolerance test, histology and immunohistochemistry, measurement of insulin secretion by static challenge with glucose, and statistical analysis are described in detail in SI Materials and Methods.
OCR.
OCR was used as a quality control for the viability and function in isolated and immobilized islets, pre- and post- implantation. Equal amounts of 250 islet equivalents were analyzed inside a 620-μL chamber within a temperature control unit (Eurotherm 808; Eurotherm) at 37 °C. Data were analyzed using Labview 7.2 software (National Instruments). OCR was calculated as described (60).
Implantable Macrochamber.
The macrochamber is a disk shaped, s.c. implantable chamber consisting of two major components: the islet module containing islets, encapsulated in alginate hydrogel, and the gas module (Fig. 1). The device is made of clinical grade polyether ether ketone (PEEK Optima LT1R40). The exterior diameter and height of the device are 31.3 mm and 7 mm, respectively. The gas compartment holds a total volume of 3 mL and is connected to 2 s.c. access ports (Soloport; Instech Solomon). The internal islet module (diameter: 11.5 mm; surface area: 1.05 cm2) contains > 2000 islet equivalents/cm2, embedded in ultrapure, high guluronic acid (68%) alginate (UP-MVG, Novamatrix). A custom-fabricated 25-μm-thick Silon IPN (interpenetrating network of polydimethylsiloxane and polyetherfluoroethylene) oxygen-permeable membrane (Bio Med Sciences) separates the gas module from the islet module and a 25-μm hydrophylized PTFE membrane (Biopore; Millipore) with a pore size of 0.2 μm creates a barrier between the islet module and the recipient's tissue. A metal grid on each side of the islet module provides mechanical strength. Oxygen permeability of the Silon IPN membrane as measured in a diffusion apparatus at 37 °C is 4.36 × 10–14 moles/(s × cm2 × mmHg).
Assembly of the Macrochamber.
Samples of 2,500 islet equivalents were collected and gently mixed with 1.8% (wt/vol) of high guluronic acid alginate (G = 0.68) and transferred to a sterile mold. Alginate was cross-linked by slightly hypotonic strontium solution (70 mM SrCl2/25 mM Hepes). The islet containing slab was then washed twice in complete CR medium and installed into the islet module. The Biopore membrane was fixed onto the device using a Viton75/27 mm O-ring (McMaster Carr) and sealed to the plastic housing with Med 1000 silicone glue (Nusil). The fully assembled chamber system was washed in complete CR medium at 37 °C for 2 h before implantation.
Implantation of the Macrochamber.
Rats were anesthetized by Ketamine/Xylazine followed by isoflurane inhalation. Through a dorsal skin incision, a s.c. pocket was dissected for implantation of the macrochamber. A second incision was made between scapulas for the gas access ports and connected to the device through s.c. tunnelled silicone tubes. The system was implanted with the islet module facing the fascia. An oxygen enriched gas mixture (40% oxygen, 55% nitrogen, 5% CO2) was used for daily “refuelling” of the gas module.
Posttransplant Follow-Up.
Animals were followed for up to 90 d after transplantation. The nonfasting blood glucose levels were measured daily. At week 3, rats were subjected to an ivGTT; for GHRH experiments, ivGTT was performed on day 7. After explantation of the device, animals were monitored for several days to confirm recurrence of diabetes.
Supplementary Material
Acknowledgments
We thank Silke Zeugner for her assistance with immunohistochemistry and Martina Talke for help in preparation of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft Grants SFB 655 “From Cells to Tissues” (to S.R.B.), KFO 252 (to B.L., T.C., and S.R.B.), and BR 1179 (to M.D.B. and B.L.); Dresden Tumor Centre of Excellence, Center for Regenerative Therapies Dresden (CRTD); Paul Langerhans Institute Dresden; and the German Center for Diabetes Research (DZD). Studies in Miami were supported by the Medical Research Service of the Veterans Affairs Department and University of Miami Miller School of Medicine, Division of Hematology/Oncology, Departments of Pathology and Medicine (all to A.V.S.) and the Austin Weeks Family Endowment for Urologic Research (to N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201868109/-/DCSupplemental.
References
- 1.Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Cardani R, et al. Allosensitization of islet allograft recipients. Transplantation. 2007;84:1413–1427. doi: 10.1097/01.tp.0000290388.70019.6e. [DOI] [PubMed] [Google Scholar]
- 3.Hyder A, Laue C, Schrezenmeir J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005;19:541–546. doi: 10.1016/j.tiv.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann T, et al. Diabetes mellitus and islet cell specific autoimmunity as adverse effects of immunsuppressive therapy by FK506/tacrolimus. Exp Clin Endocrinol Diabetes. 2000;108:347–352. doi: 10.1055/s-2000-8127. [DOI] [PubMed] [Google Scholar]
- 5.Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- 6.Biarnés M, et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson PO, et al. Glucose-induced islet blood flow increase in rats: Interaction between nervous and metabolic mediators. Am J Physiol Endocrinol Metab. 2002;283:E457–E464. doi: 10.1152/ajpendo.00044.2002. [DOI] [PubMed] [Google Scholar]
- 8.Jansson L. The regulation of pancreatic islet blood flow. Diabetes Metab Rev. 1994;10:407–416. doi: 10.1002/dmr.5610100405. [DOI] [PubMed] [Google Scholar]
- 9.Jansson L, Bodin B, Källskog O, Andersson A. Duct ligation and pancreatic islet blood flow in rats: Physiological growth of islets does not affect islet blood perfusion. Eur J Endocrinol. 2005;153:345–351. doi: 10.1530/eje.1.01966. [DOI] [PubMed] [Google Scholar]
- 10.Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466–473. [PubMed] [Google Scholar]
- 11.Davalli AM, et al. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59:817–820. [PubMed] [Google Scholar]
- 12.Lau J, Carlsson PO. Low revascularization of human islets when experimentally transplanted into the liver. Transplantation. 2009;87:322–325. doi: 10.1097/TP.0b013e3181943b3d. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 14.Antosiak-Iwańska M, et al. Isolation, banking, encapsulation and transplantation of different types of Langerhans islets. Pol Arch Med Wewn. 2009;119:311–317. [PubMed] [Google Scholar]
- 15.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: The untouchable islets. Trends Mol Med. 2002;8:363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 16.Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: Proof of concept. Transplantation. 2006;81:1345–1353. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 17.Duvivier-Kali VF, Omer A, Parent RJ, O'Neil JJ, Weir GC. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:1698–1705. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 18.Elliott RB, et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005;37:466–469. doi: 10.1016/j.transproceed.2004.12.198. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, et al. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: Implications for diabetes cell therapies. Transplantation. 2009;87:983–991. doi: 10.1097/TP.0b013e31819c86ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott RB, et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 21.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 22.Kühtreiber WM, Lanza RP, Beyer AM, Kirkland KS, Chick WL. Relationship between insulin secretion and oxygen tension in hybrid diffusion chambers. ASAIO J. 1993;39:M247–M251. [PubMed] [Google Scholar]
- 23.Lewis ASC, Colton CK. In: Engineering Challenges in Immunobarrier Device Development. Lanza R-P, Langer R, Vacanti J, editors. Boston, MA: Elsevier; 2006. pp. 405–418. [Google Scholar]
- 24.Lewis ASC, Colton CK. In: Tissue Engineering for Insulin Replacement in Diabetes. Ma P-X, Elisseeff J, editors. New York: Taylor & Francis; 2006. pp. 585–608. [Google Scholar]
- 25.Hallé JP, et al. Studies on small (< 300 microns) microcapsules: II—Parameters governing the production of alginate beads by high voltage electrostatic pulses. Cell Transplant. 1994;3:365–372. doi: 10.1177/096368979400300503. [DOI] [PubMed] [Google Scholar]
- 26.Wright JR, Jr, Yang H, Dooley KC. Tilapia—a source of hypoxia-resistant islet cells for encapsulation. Cell Transplant. 1998;7:299–307. doi: 10.1177/096368979800700308. [DOI] [PubMed] [Google Scholar]
- 27.Josephs SF, Loudovaris T, Dixit A, Young SK, Johnson RC. In vivo delivery of recombinant human growth hormone from genetically engineered human fibroblasts implanted within Baxter immunoisolation devices. J Mol Med (Berl) 1999;77:211–214. doi: 10.1007/s001090050338. [DOI] [PubMed] [Google Scholar]
- 28.Lembert N, et al. Encapsulation of islets in rough surface, hydroxymethylated polysulfone capillaries stimulates VEGF release and promotes vascularization after transplantation. Cell Transplant. 2005;14:97–108. [PubMed] [Google Scholar]
- 29.Chin K, Khattak SF, Bhatia SR, Roberts SC. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol Prog. 2008;24:358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AS, Fisher RJ, Weir GC, Colton CK. Oxygen consumption and diffusion in assemlages of reprising spheres: Performance enhancement of a bioartificial pancreas. Chem Engineer Science. 2009;64:4470–4487. [Google Scholar]
- 31.Lehmann R, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 32.O'Sullivan ES, et al. Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia. 2010;53:937–945. doi: 10.1007/s00125-009-1653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilakaratne HK, Hunter SK, Rodgers VG. Mathematical modeling of myoglobin facilitated transport of oxygen in devices containing myoglobin-expressing cells. Math Biosci. 2002;176:253–267. doi: 10.1016/s0025-5564(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, et al. In situ electrochemical oxygen generation with an immunoisolation device. Ann N Y Acad Sci. 1999;875:105–125. doi: 10.1111/j.1749-6632.1999.tb08497.x. [DOI] [PubMed] [Google Scholar]
- 35.Bloch K, et al. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 2006;12:337–344. doi: 10.1089/ten.2006.12.337. [DOI] [PubMed] [Google Scholar]
- 36.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7:38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen JH, Svensson C, Galsgaard ED, Møldrup A, Billestrup N. Beta cell proliferation and growth factors. J Mol Med (Berl) 1999;77:62–66. doi: 10.1007/s001090050302. [DOI] [PubMed] [Google Scholar]
- 38.Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- 39.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler CG, et al. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: Antiproliferative effects of peptide analogues. Proc Natl Acad Sci USA. 2009;106:15879–15884. doi: 10.1073/pnas.0907843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izdebski J, et al. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92:4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berney T, Johnson PR. Donor pancreata: Evolving approaches to organ allocation for whole pancreas versus islet transplantation. Transplantation. 2010;90:238–243. doi: 10.1097/TP.0b013e3181e25a40. [DOI] [PubMed] [Google Scholar]
- 44.Caballero-Corbalán J, et al. Using HTK for prolonged pancreas preservation prior to human islet isolation. J Surg Res. 2011;31:1–6. doi: 10.1016/j.jss.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi H. Pancreas procurement and preservation for islet transplantation: Personal considerations. J Transplant. 2011;2011:783168. doi: 10.1155/2011/783168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott WE, 3rd, et al. Persufflation improves pancreas preservation when compared with the two-layer method. Transplant Proc. 2010;42:2016–2019. doi: 10.1016/j.transproceed.2010.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro AM. Strategies toward single-donor islets of Langerhans transplantation. Curr Opin Organ Transplant. 2011;16:627–631. doi: 10.1097/MOT.0b013e32834cfb84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ludwig B, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42:918–922. doi: 10.1055/s-0030-1267916. [DOI] [PubMed] [Google Scholar]
- 49.Brissova M, et al. Pancreatic islet production of vascular endothelial growth factor-a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 50.Vériter S, et al. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials. 2011;32:5945–5956. doi: 10.1016/j.biomaterials.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 51.Watada H. Role of VEGF-A in pancreatic beta cells. Endocr J. 2010;57:185–191. doi: 10.1507/endocrj.k09e-035. [DOI] [PubMed] [Google Scholar]
- 52.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 53.Mandrup-Poulsen T. Beta-cell apoptosis: Stimuli and signaling. Diabetes. 2001;50(Suppl 1):S58–S63. doi: 10.2337/diabetes.50.2007.s58. [DOI] [PubMed] [Google Scholar]
- 54.Yin D, et al. Liver ischemia contributes to early islet failure following intraportal transplantation: Benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6:60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 55.Hughes SJ, Davies SE, Powis SH, Press M. Hyperoxia improves the survival of intraportally transplanted syngeneic pancreatic islets. Transplantation. 2003;75:1954–1959. doi: 10.1097/01.TP.0000066805.39716.23. [DOI] [PubMed] [Google Scholar]
- 56.Lacy PE, Finke EH, Janney CG, Davie JM. Prolongation of islet xenograft survival by in vitro culture of rat megaislets in 95% O2. Transplantation. 1982;33:588–592. doi: 10.1097/00007890-198206000-00004. [DOI] [PubMed] [Google Scholar]
- 57.CITR Research Group 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR) Cell Transplant. 2009;18:753–767. doi: 10.3727/096368909X470874. [DOI] [PubMed] [Google Scholar]
- 58.Papas KK, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papas KK, et al. Prediction of marginal mass required for successful islet transplantation. J Invest Surg. 2010;23:28–34. doi: 10.3109/08941930903410825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98:1071–1082. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.