Abstract
Recent studies suggest that Cu/Zn superoxide dismutase (SOD1) could be pathogenic in both familial and sporadic amyotrophic lateral sclerosis (ALS) through either inheritable or nonheritable modifications. The presence of a misfolded WT SOD1 in patients with sporadic ALS, along with the recently reported evidence that reducing SOD1 levels in astrocytes derived from sporadic patients inhibits astrocyte-mediated toxicity on motor neurons, suggest that WT SOD1 may acquire toxic properties similar to familial ALS-linked mutant SOD1, perhaps through posttranslational modifications. Using patients’ lymphoblasts, we show here that indeed WT SOD1 is modified posttranslationally in sporadic ALS and is iper-oxidized (i.e., above baseline oxidation levels) in a subset of patients with bulbar onset. Derivatization analysis of oxidized carbonyl compounds performed on immunoprecipitated SOD1 identified an iper-oxidized SOD1 that recapitulates mutant SOD1-like properties and damages mitochondria by forming a toxic complex with mitochondrial Bcl-2. This study conclusively demonstrates the existence of an iper-oxidized SOD1 with toxic properties in patient-derived cells and identifies a common SOD1-dependent toxicity between mutant SOD1-linked familial ALS and a subset of sporadic ALS, providing an opportunity to develop biomarkers to subclassify ALS and devise SOD1-based therapies that go beyond the small group of patients with mutant SOD1.
Amyotrophic lateral sclerosis (ALS) is characterized by degeneration and loss of upper and lower motor neurons, leading to paralysis and death by 3–5 y after diagnosis (1). The majority of ALS cases are sporadic (sALS), with no apparent hereditary contribution; only 5% of cases are familial (fALS), with genetic mutations dominantly inherited (2, 3). Disease-causing mutations in various genes have been identified (4–6); abundant among these are mutations in the gene encoding for Cu/Zn superoxide dismutase (SOD1) (7). It is now established that mutated SOD1 (mutSOD1) acquires new toxic functions, most likely through mutation-driven conformational changes (8, 9). Despite multiple epidemiologic studies investigating possible correlations between environmental and/or genetic triggers and sALS, the etiology of sALS remains unknown (10). Thus, it is imperative that we at least find markers of disease for patients with sALS.
The two forms, fALS and sALS, are clinically indistinguishable and share many pathological abnormalities (11), suggesting common disease mechanisms and possibly common triggers. SOD1 has been proposed as such a common trigger (12). It has been suggested that either age- or environmental-dependent posttranslational modifications lead to conformational rearrangements in the structure of WT SOD1 that are similar to those imparted by disease-causative SOD1 mutations in fALS, resulting in a similar gain of toxic pathogenic functions (13). For instance, in cultured cells, oxidized WT SOD1 forms proteinaceous aggregates that trigger cell death (14). Moreover, Zn-depleted WT SOD1 induces death of cultured motor neuron and metal-deficient WT SOD1 conforms, similar to mutSOD1, in an unstable and aggregation-prone structure (15). The use of conformation-specific antibodies raised against fALS-linked mutSOD1 has led to the detection of atypical WT SOD1 species in a subset of sALS (16, 17). In vitro, these mutSOD1-specific conformational antibodies also recognize the oxidized form of recombinant WT SOD1, suggesting that the WT SOD1 detected in sALS patients may be oxidized as well (17). Bosco et al. (17) recently reported that, similar to fALS-linked mutSOD1, recombinant oxidized WT SOD1 inhibits kinesin-based axonal transport in isolated squid axoplasm, suggesting a common mechanism of toxicity in mutant and oxidatively modified WT SOD1 in vitro. Although the foregoing studies suggest that oxidized SOD1, formed perhaps as a consequence of environmental- or age-related factors, could trigger or affect the course of sALS, evidence of the presence of oxidized SOD1 in sALS patients remains circumstantial. Similarly, whether WT SOD1 inclusion is a pathological occurrence or gains pathogenic relevance perhaps by targeting the same intracellular pathways as the disease-causative mutSOD1 is unclear.
Here we decisively report the presence of an over-oxidized WT SOD1 in a subset of sALS patients. Using Epstein–Barr virus (EVB)-immortalized lymphocytes (lymphoblasts) (18), we found that WT SOD1 is significantly more oxidized above baseline oxidation in a group of sALS patients with bulbar onset compared with non-neurologic controls, mutSOD1-derived patients, and patients with non-bulbar sALS. We term this over-oxidized SOD1 iper-oxidized SOD1 (iperOxSOD1). We found that induction of oxidative stress by treating lymphoblasts with hydrogen peroxide (H2O2) did not further increase the iperOxSOD1 oxidative state, although it did trigger iperOxSOD1 aggregation and formation of a toxic complex with mitochondrial Bcl-2, similar to the complex that we recently reported for fALS-linked mutSOD1 (19). Like mutSOD1 (20) and other toxic proteins (21, 22), iperOxSOD1 induces a conformational change in Bcl-2 that exposes the toxic BH3 domain, damaging the lymphoblast mitochondria.
Our data demonstrate the presence of an iper-oxidized form of SOD1 in a subset of sALS patients with bulbar onset and conclusively confirm, in patient-derived cells, the potential of WT SOD1 to become pathogenic through a disease-specific posttranslational modification. IperOxSOD1 and the toxic complex with Bcl-2 were not detected in all sALS samples, suggesting that modified SOD1 (and toxicity of the iperOxSOD1/Bcl-2 complex) may be involved in a subset of sALS cases by analogy with mutSOD1, which accounts for only ∼25% of fALS cases. Thus, our data highlight the existence of a converging pathogenic pathway between a portion of fALS and sALS cases, leading to the possibility of subclassifying ALS based on the identification of specific biomarkers. Moreover, taken together with recent data showing that SOD1 can be targeted therapeutically in sporadic ALS (23), our finding that Bcl-2 becomes a toxic target of iperOxSOD1 through the same mechanism as mutSOD1 (20) can permit the design of target-based therapies against the SOD1/Bcl-2 complex with efficacy possibly exceeding that of therapies for the small percentage of patients with mutSOD1.
Results
WT SOD1 Is iper-Oxidized in a Subset of sALS Patients.
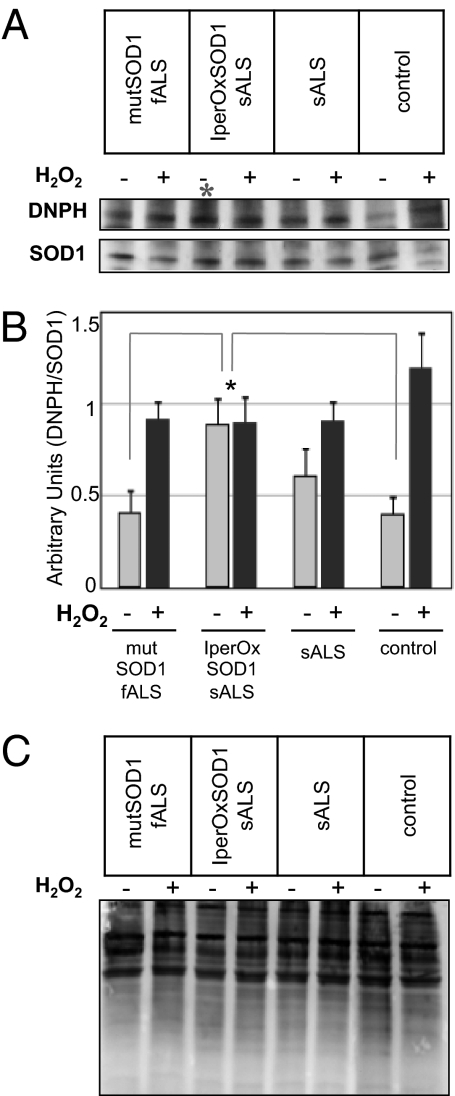
Correlative evidence suggests the potential for oxidization of WT SOD1 in sALS (17). The correlation is based on the observation that antibodies positive for a misfolded WT SOD1 in sALS spinal cord sections also recognize recombinant oxidized SOD1 (17) in vitro. However, thus far no hard evidence of the existence of such oxidized SOD1 in sALS patients has been published. To determine whether WT SOD1 is indeed oxidized in sALS, we performed derivatization analyses of carbonyl compounds with 2,4-dinitrophenylhydrazine (DNPH) on SOD1 immunoprecipitated from lymphoblasts derived from sALS patients, mutSOD1-fALS patients, and healthy control subjects. DNPH Western blot analysis is a sensitive method for detecting oxidized carbonyl groups in the protein of interest (22–24). We found that at baseline (without oxidative stress), SOD1 oxidization was greater in a subset of sALS patients with bulbar onset compared with mutSOD1-fALS patients and controls (Fig. 1A). On densitometric analysis, this group of sALS patients (termed iperOxSOD1-sALS) exhibited a twofold increase in baseline SOD1 oxidation (P < 0.05) compared with mutSOD1-fALS patients, non-neurologic controls (Fig. 1B), and other sALS patients. This increased WT SOD1 oxidation is a specific effect and not a generalized phenomenon affecting several proteins; the total carbonyl content showed no differences in global protein oxidative levels (Fig. 1C). To determine whether iperOxSOD1 could respond to further oxidation, we treated lymphoblasts from these patients with H2O2. In contrast to normally oxidized or mutSOD1 lymphoblasts, the iperOxSOD1 lymphoblasts did not respond to treatment (Fig. 1 A and B), suggesting that maximum oxidation had already occurred. Seven of the 20 sALS cases analyzed in the present study displayed iperOxSOD1 (Table 1). Initial analysis showed a correlation between iperOxSOD1 and site of disease onset, with all seven iperOxSOD1-sALS patients diagnosed with bulbar ALS. We found no correlations between the appearance of iperOxSOD1 and patient sex, age of onset, or duration of disease progression.
Fig. 1.
Identification of an iper-oxidized SOD1 in a subset of sALS patients. (A) Representative immunoblot of SOD1 oxidation detected by a derivatization assay of carbonyl compounds. Proteins extracted from sALS, fALS, and healthy control lymphoblasts, before and after H2O2 treatment, were precipitated with rabbit anti-SOD1 antibody and analyzed by Western blot using anti-DNPH (Upper) and sheep anti-SOD1 (Lower) antibodies. (B) Ratio of DNPH and precipitated SOD1. For each patient's line, samples were analyzed in triplicate in three independent blots (with and without H2O2), and densitometric analysis was performed on each blot. The graph represents the mean ± SD SOD1 oxidation for each experimental group. ANOVA revealed significantly higher oxidized SOD1 in a group of sALS lymphoblasts compared with lymphoblasts from mutSOD1-fALS and healthy controls (*P < 0.05). (C) Representative blot of derivatization assay of carbonyl compounds on total protein content from fALS, sALS, and healthy control lymphoblasts before and after H2O2 treatment, showing no differences in total oxidation levels.
Table 1.
iperOxSOD1-sALS appears to correlate with bulbar onset
| Total | Men | Women | |
| No. of ALS patients | 24 (4 fALS) | 12 (2 fALS) | 8 (2 fALS) |
| Age of onset (mean ± SE) | 55.0 ± 2.5 | 55.9 ± 2.2 | 53 ± 2.8 |
| Illness duration (mo ± SE) | 40.5 ± 4.9 | 42.7 ± 4.7 | 38.3 ± 5.0 |
| Site of onset | 9 LL; 4 UL; 7 B | 6 LL; 3 UL; 3 B | 2 LL; 2 UL; 4 B |
| Patients with iperOxSOD1 | 0 LL; 1 UL; 7 B | 0 LL; 0 UL; 3 B | 0 LL; 1 UL; 4 B |
| Response to H2O2 | 9 LL; 3 UL; 0 B | 6 LL; 3 UL; 0 B | 2 LL; 2 UL; 0 B |
B, bulbar; LL, lower limb; UL, upper limb.
IperOxSOD1 Aggregates in Lymphoblasts of a Subset of sALS Patients.
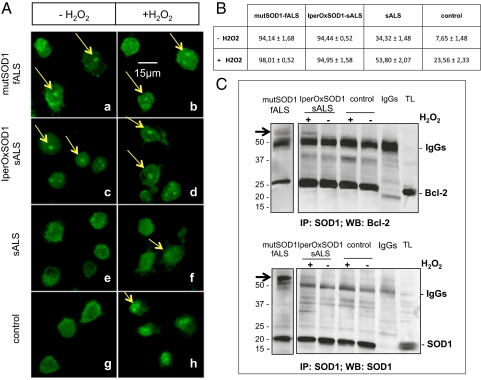
It was previously reported that in vitro, a posttranslationally modified WT SOD1 becomes unstable and undergoes misfolding and aggregation in a mutSOD1-like fashion (13). Accordingly, we performed immunofluorescence analysis of iperOxSOD1-sALS lymphoblasts and compared the patterns of aggregation in iperOxSOD1 and mutSOD1. We found SOD1-positive inclusions in lymphoblasts of iperOxSOD1-sALS and mutSOD1-fALS patients (Fig. 2A, a–c), but no SOD1 inclusions in lymphoblasts of control subjects (Fig. 2A, g) or the other sALS patients (Fig. 2A, e). Treatment with H2O2 did not change the aggregation pattern of iperOxSOD1 (Fig. 2A, d) or of mutSOD1 (Fig. 2A, b); however, it did induce SOD1 aggregation in lymphoblasts of control subjects and the other sALS patients (Fig. 2A, f–h), although these aggregates were more diffuse and not as well defined as those seen at baseline in iperOxSOD1-sALS and mutSOD1-fALS patients. Thus, given that oxidative stress induced aggregation of WT SOD1, our findings suggest that the aggregation of SOD1 that we observed in the seven sALS patients with bulbar onset at baseline is a consequence of disease-induced stress.
Fig. 2.
Like mutSOD1, iperOxSOD1 aggregates in patient cells. (A) SOD1 appearance in lymphoblasts of sALS, mutSOD1-fALS, and controls analyzed by immunofluorescence using a rabbit antiSOD1 antibody. Confocal experiments revealed SOD1-positive aggregates (arrows) in both mutSOD1-fALS and iperOxSOD1-sALS patients before (a and c) and after (b and d) H2O2 treatment (100 μM, 2 h). Control and sALS lymphoblasts exhibited SOD1 aggregation only after H2O2 treatment (f–h). (B) Percentage (± SD) of cells containing SOD1-positive aggregates before and after H2O2 treatment. Counting was done selecting seven different fields for each group. MutSOD1-fALS and iperOxSOD1-sALS patients showed a significantly higher percentage of cells containing aggregates compared with both remaining sALS and control patients (P < 0.0001). (C) Representative blots showing the aberrant bond of SOD1 and Bcl-2 in mutSOD1-fALS and iperOxSOD1-sALS patients (arrows). Proteins extracted from fALS, iperOxSOD1-sALS, and control lymphoblasts treated with 100 μM H2O2 were precipitated as above, and membranes were stained with mouse anti–Bcl-2 antibody (Upper). SOD1 precipitation was confirmed by restaining the blot with a sheep anti-SOD1 antibody (Lower). After H2O2 treatment, high molecular weight aggregates positive for both Bcl-2 (Upper) and SOD1 (Lower) were detected in mutSOD1-fALS and iperOxSOD1-sALS patients, but not in healthy controls (or the remaining sALS patients). The SOD1/Bcl-2 complex was recovered in the presence of the precipitating antibody, but not with the corresponding IgGs, indicating specificity of the binding. TL, total lysate.
IperOxSOD1 Acquires mutSOD1-Like Toxic Properties and Aggregates with Mitochondrial Bcl-2.
Numerous toxic properties have been attributed to mutSOD1. If mutSOD1 and iperOxSOD1 share toxic properties, then this toxicity must converge to common intracellular molecular targets, possibly damaging the same organelles in cells. We examined mitochondria as a converging point of toxicity for two reasons: (i) Mitochondrial defects are a common feature of sALS and fALS, with mitochondrial degeneration, vacuolization, and swelling documented in both sALS and fALS patients as well as in mutSOD1 mice models of ALS (25), and (ii) we recently reported an aberrant toxic interaction between mutSOD1 and mitochondrial Bcl-2 and found that mutSOD1 relies on this interaction to damage the mitochondria (20). We also showed that formation of the toxic mutSOD1/Bcl-2 complex is mitochondria-specific and driven by conformational changes of the unstable mutSOD1 (20). Consequently, we conducted co-immunoprecipitation experiments to determine whether iperOxSOD1 aberrantly binds with Bcl-2 in mitochondria from iperOxSOD1-sALS patients. We first confirmed the interaction between SOD1 (WT and mutant) and Bcl-2 in lymphoblasts of ALS patients and controls. We found that monomeric SOD1 co-immunoprecipitated with Bcl-2 from mitochondria isolated from mutSOD1-fALS, sALS (with or without iperOxSOD1), and control lymphoblasts (Fig. 2B). Based on our previous report that misfolded mutSOD1 aberrantly binds Bcl-2 forming a high molecular weight SDS-resistant complex with Bcl-2 (19), we expected misfolded iperOxSOD1 (but not regular SOD1) to similarly form insoluble high molecular weight complexes with Bcl-2. Although we confirmed the presence of a high molecular weight SDS-resistant SOD1/Bcl-2 complex in lymphoblasts of mutSOD1 patients, we did not detect these aggregates in iperOxSOD1-positive lymphoblasts (Fig. 2B), suggesting that iperOxSOD1 can act only partly like mutSOD1, perhaps by initially becoming unstable and only later maturing into a mutSOD1-like toxic molecule through exposure to additional stressors. To test this hypothesis, we treated lymphoblasts with H2O2 and found that on exposure to further oxidative stress, iperOxSOD1 formed high molecular weight SDS-resistant aggregates with Bcl-2 (Fig. 2B, arrow), whereas no aberrant aggregates of SOD1 and Bcl-2 were detected in control cells (Fig. 2B). This finding indicates that although oxidative stress does not change the oxidative state of the already iper-oxidized SOD1 (Fig. 1), it does trigger features similar to those seen in ALS-causative SOD1 mutations. Confocal microscopy analysis of H2O2-treated lymphoblasts showed colocalization of SOD1 and Bcl-2 within cellular aggregates (Fig. 3, g and h). We found inclusions immunopositive for both SOD1 and Bcl-2 in mutSOD1 and iperOxSOD1-sALS lymphoblasts (Fig. 3, a, b, d, and e), but not in lymphoblasts from sALS patients without iperOxSOD1 (Fig. 3, c–f). Moreover, visual inspection showed lower overall expression of SOD1 and less evident colocalization of SOD1 and Bcl-2 in these non-iperoxSOD1 sALS groups (Fig. 3, i).
Fig. 3.
Like mutSOD1, iperOxSOD1 colocalizes and aggregates with Bcl-2 in patient cells. Colocalization of SOD1 and Bcl-2 was evaluated by immunofluorescence with rabbit anti-SOD1 (A–C) and mouse anti-Bcl-2 (D–F) antibodies. The merging signals show colocalization of the two proteins within the aggregates in iperOxSOD1-sALS and mutSOD1-fALS patients after H2O2 treatment (G and H, arrows). sALS lymphoblasts with no iperOxSOD1 and no SOD1/Bcl-2 high molecular weight aggregates did not demonstrate colocalization of the two proteins (C, F, and I).
OxSOD1 Requires Bcl-2 to Damage in Vitro Isolated Mitochondria.
MutSOD1 relies on mitochondrial Bcl-2 to damage in vitro isolated mitochondria (20). To determine whether over-oxidized SOD1 has similar damaging properties to mitochondria through the aberrant complex with Bcl-2, we incubated recombinant oxSOD1 with mitochondria isolated from HEK293T cells, which do not express endogenous detectable levels of Bcl-2 (20, 21), and mitochondria from HEK293T cells transfected with Bcl-2. Mitochondrial damage was examined by ELISA, with the amount of cytochrome C released from mitochondria measured. Similar to what we reported previously for mutSOD1 (20), oxSOD1 induced cytochrome C release only from Bcl-2–positive mitochondria (Fig. 4A). In contrast to oxSOD1, WT SOD1 did not cause the release of cytochrome C (26) from either Bcl-2–negative or Bcl-2–positive mitochondria (Fig. 4A). Thus, iperOxSOD1 and mutSOD1 share a common mechanism of mitochondrial toxicity that requires the formation of a toxic complex with mitochondrial Bcl-2.
Fig. 4.
oxSOD1-mediated toxicity requires binding with Bcl-2 and induces a toxic rearrangement of Bcl-2 structure. (A) In isolated mitochondria, recombinant oxSOD1 induces cytochrome C release only in the presence of Bcl-2. Bcl-2–negative or Bcl-2–positive mitochondria isolated from HEK293T cells were incubated with 5 μL of recombinant oxSOD1 for 30 min. Cytochrome C immunoreactivity was measured in supernatants by ELISA. OxSOD1, but not WT SOD1, caused an increase in cytochrome C release from damaged mitochondria. Data are mean ± SD of three independent experiments performed in duplicate (P < 0.05) with CaCl2 as a positive control for maximal cytochrome C release. (B) Representative blot showing that Bcl-2 is conformationally modified in lymphoblasts of iperOxSOD1-sALS and mutSOD1-fALS patients. In mutSOD1-fALS and iperOxSOD1-sALS lymphoblasts, there is increased exposure of the toxic BH3 domain paralleled by decreased exposure of the pocket region, indicating a conformational change in the Bcl-2 protein. (C) Densitometric analysis of the BH3/pocket ratio. Each patient's line was analyzed in three independent experiments. The graph represents BH3 and pocket exposure normalized on the total amount of Bcl-2 in all samples. The BH3/pocket ratio was significantly higher in mutSOD1-fALS and iperOxSOD1s-ALS lymphoblasts compared with control and other sALS lymphoblasts. *P < 0.05; **P < 0.0001. (D) Exposure of BH3 domain also was assessed by flow cytometry using the conformation-specific anti-BH3 antibody. The shift in fluorescence of mutSOD1-fALS and iperOxSOD1-sALS lymphoblasts compared with controls indicates increased exposure of the BH3 domain.
Given our finding that mutSOD1 induces a toxic conformational change in Bcl-2 exposing the otherwise hidden BH3 toxic domain, we wished to determine whether iperOxSOD1 behaves similarly, transforming Bcl-2 into a toxic protein. We immunoprecipitated H2O2-treated lymphoblasts using two anti-Bcl-2 (αBcl-2) conformation-specific antibodies: (i) the αBcl-2/pocket antibody, which recognizes Bcl-2 in its normal, prosurvival conformation, and (ii) the αBcl-2/BH3 antibody, which does not recognize Bcl-2 unless it undergoes a toxic conformational change to expose the BH3 domain (20). The amount of Bcl-2 immunoprecipitated by αBcl-2/pocket antibody was significantly lower in lymphoblasts from iperOxSOD1-sALS patients compared with controls, which included lymphoblasts from both healthy individuals and sALS patients without iperOxSOD1 (Fig. 4B). Accordingly, αBcl-2/BH3 precipitates Bcl-2 to a greater extent in iperOxSOD1-sALS lymphoblasts than in control lymphoblasts (Fig. 4B). We also used mutSOD1-fALS lymphoblasts as a positive control (Fig. 4B, Left) to set the BH3/pocket ratio. Densitometric analysis found a higher BH3/pocket ratio in iperOxSOD1-sALS lymphoblasts than in lymphoblasts from controls and sALS patients without iperOxSOD1, with the highest ratio seen in mutSOD1-fALS cells (Fig. 4C). We confirmed this finding by flow cytometry using αBcl-2/BH3 antibody to sort cells based on exposure of the BH3 domain (Fig. 4D). Expression of the toxic BH3 domain was higher in iperOxSOD1-sALS lymphoblasts than in controls (Fig. 4D). As revealed by immunoprecipitation analyses (Fig. 4 B and C), the BH3/pocket ratio in iperOxSOD1-sALS lymphoblasts was between that in mutSOD1-fALS (highest ratio) and control (lowest ratio) lymphoblasts.
We next determined whether the iperOxSOD1/Bcl-2 complex acquired toxic properties that could compromise the integrity of mitochondria in iperOxSOD1-sALS patients. We examined mitochondrial morphology by transmission electron microscopy in H2O2-treated iperOxSOD1-sALS lymphoblasts and found that, in contrast to control lymphoblasts, where H2O2 treatment triggered WT SOD1 oxidation but not formation of the SOD1/Bcl-2 complex, iperOxSOD1-sALS cells showed considerable mitochondrial damage (Fig. 5, Upper). Control lymphoblasts exhibited normally shaped mitochondria with elongated structure and organized cristae (Fig. 5, Lower). In iperOxSOD1-sALS lymphoblasts, few intact mitochondria were detected. In the few structures reminiscent of mitochondria, vacuoles and a completely disorganized outer and inner membrane structure were seen (Fig. 5, Upper).
Fig. 5.
H2O2-induced formation of the toxic iperOxSOD1/Bcl-2 complex triggers mitochondrial damage in patient cells. Control and iperOxSOD1-sALS cells treated for 2 h with 100 μM H2O2 underwent morphological evaluation by transmission electron microscopy. IperOxSOD1-sALS cells show derangement of mitochondria matrix and disappearance of morphological structure.
Discussion
The lack of reliable biomarkers precludes a complete understanding of the etiology of sALS. As for sALS, the etiology of fALS is heterogeneous, with different subgroups of fALS caused by mutations in different genes (27). Although studies in animal and cellular models of mutSOD1-fALS have advanced our understanding of the disease, critical questions remain: How well do these models represent the totality of ALS? Are the toxic mechanisms identified in a fraction of fALS relevant to sALS? Are all sALS cases equal or, like fALS, can sALS be subclassified based on different primary causes? Do all forms of ALS share the same pathogenic mechanisms despite being set off by different triggers? A common assumption is that pathogenic mechanisms must converge because the different forms of ALS are clinically indistinguishable. Supporting this idea, glutamate excitotoxicity and mitochondria dysfunction are shared by fALS and sALS (25, 28, 29). In addition, mutations and proteinaceous inclusions of TDP-43 and FUS, and more recently of ubiquilin 2, have been identified in both fALS and sALS (30, 31). No genetic mutations of SOD1 have been found in sALS patients, despite the occurrence of SOD1 proteinaceous inclusions (32). In the absence of SOD1 mutations in sALS, we cannot determine whether WT SOD1-positive inclusions are pathogenic or simply a pathological finding. The fact that these inclusions resemble those formed by fALS-linked mutSOD1 has been recently proposed as an indication of their pathogenicity, and triggering by aging or other ALS disease-associated posttranslational modifications, most likely oxidation, has been suggested (17). However, whether WT SOD1 is indeed oxidized in patients and eventually acquires toxic properties that make it pathogenic remains unknown.
Here we conclusively show that WT SOD1 is posttranslationally modified and iper-oxidized in a subset of sALS patients, and that through this oxidation, modified SOD1 acquires toxic properties similar to those induced by disease-causing genetic mutations in patient-derived cells. First, the iper-oxidation of SOD1 is specific, not generalized to all ALS patients but occurring only in a subset of patients with bulbar onset (iperOxSOD1-sALS). Thus, oxidation is not a secondary phenomenon triggered by global metabolic changes associated with the disease. Second, iper-oxidation is not a phenomenon triggered by different culturing and cloning conditions in immortalized lymphoblasts. Indeed, realizing that these cells may have varying biochemical phenotypes independent of disease but induced by different culturing conditions, we standardized our EVB immortalization protocol, experimental procedures, and culturing conditions among the different patient lines. Third, in iperOxSOD1-sALS patients, oxidation is not an overall age-associated phenomenon that targets other proteins; rather, it is directed toward the one protein that, if modified genetically, is sufficient to cause ALS, arguing in favor of a potential pathogenic rather than pathological role of iperOxSOD1. The finding that only 30% of sALS involves iperOxSOD1 underscores the concept that, analogous to fALS (triggered by different genetic mutations), sALS comprises multiple subgroups with differing etiologies (27). Taken together, our results indicate the potential to identify biomarkers to subclassify various forms of ALS.
In iperOxSOD1-sALS, highly oxidized SOD1 is present at baseline and, in contrast to normally oxidized SOD1 (mutant or WT), is not sensitive to further oxidation. However, this iperOxSOD1 requires further oxidative stress to acquire fully toxic characteristics leading to aberrant binding with Bcl-2 and mitochondrial damage. This finding is in agreement with previous studies showing that aberrant misfolding and aggregation of SOD1 is enhanced by oxidative stress (16). In patient-derived cells, the extent to which iper-oxidation promotes conformational modifications in SOD1 compared with those triggered by genetic mutations is unclear. From our experiments, it appears that the structural modifications induced by genetic mutations and oxidation differ in nature, with oxidation causing more subtle changes in SOD1 structure that seem to predispose to a full modification induced by environmental stressors. Indeed, whereas mutSOD1-containing lymphoblasts form the aberrant and toxic mutSOD1/Bcl-2 complex at baseline, in the iperOxSOD1 lymphoblasts, the appearance of the iperOxSOD1/Bcl-2 complex and the toxic conformational change in Bcl-2 requires an additional oxidative stress. Based on our previous studies, we know that, at least for mutSOD1, the aberrant binding with Bcl-2 is conformation-specific and requires structural rearrangement of both proteins (20). By analogy, we believe that the binding between iperOxSOD1 and Bcl-2 also requires a full structural rearrangement of SOD1 that for iperOxSOD1-sALS lymphoblasts is a “two-step” process caused by intrinsic disease-driven oxidation and extrinsic additional stress.
Both mutSOD1/Bcl-2 and iperOxSOD1/Bcl-2 complexes, but not H2O2 treatment alone, significantly altered mitochondrial morphology in the patients’ lymphoblasts. There was no apparent cell death, however. Similarly, despite the presence of mutSOD1 or iperOxSOD1, fALS-SOD1 and iperOxSOD1-sALS lymphoblasts could be easily cultured as control lymphoblasts, probably because we used immortalized lymphoblasts, which might have selected for cells inherently more resistant to disease-induced changes. A careful comparison of lymphocytes and lymphoblasts of these patients may provide insight into the mechanisms and/or factors that confer resistance to immortalized cells. Resistance to SOD1 (mutant or iper-oxidized) toxicity and/or changes induced by damaged mitochondria also may reflect the fact that we used peripheral and renewable cells that are not affected by ALS. A comparative analysis of these peripheral cells with induced pluripotent stem cell-derived motor neurons from the same patient(s) may reveal why motor neurons are more susceptible to mitochondrial changes induced by SOD1 toxicity. Despite the lack of an evident phenotype (e.g., cell loss), the use of patient lymphoblasts has allowed us to identify disease-driven pathogenic characteristics of an aberrant SOD1 in sALS. We emphasize that we are not claiming that these—or any other peripheral cells—represent a viable model for mimicking human disease in culture-like induced pluripotent stem cell-derived disease-relevant cells (neurons and glia). Rather, these cells provide a useful “first-pass” screening tool to identify specific pathogenic signatures of subgroups of ALS. Of the 20 sALS patients analyzed in the present study, all 7 with iperOxSOD1 were diagnosed with bulbar ALS. We found no correlation between iperOxSOD1 and sex, age of onset, or disease duration. Although the potential correlation between iperOxSOD1 and site of onset requires further validation using larger cohorts of patients and eventually postmortem tissues from subgroups of patients, our data support the idea of subclassifying ALS based on disease-specific biochemical markers. They also support the use of lymphoblasts (or other readily obtainable peripheral cells) to subclassify complex diseases like ALS by identifying specific aberrant pathways or targets, enabling the design of better-tailored therapeutic strategies with potentially higher efficacy. Interestingly, a recent study found that reducing SOD1 levels in astrocytes derived from patients with sALS (with no apparent aberrant SOD1) eliminates the toxicity of these sporadic astrocytes on motor neurons (23). Although this study confirms the potential to target SOD1 therapeutically beyond fALS, it does not identify which population of sporadic astrocytes has the toxic SOD1 (and thus may benefit from an SOD1-based therapy), or which form of SOD1 confers toxicity to these sporadic astrocytes. In the present study, by showing that aberrant WT SOD1 is iper-oxidized and that iperOxSOD1 in sALS seems to correlate with bulbar onset, we provide these two key elements of the puzzle that may aid the design of specific SOD1-based therapies in sALS. Whether the iperOxSOD1/Bcl-2 complex is also toxic to astrocyte mitochondria, and whether it triggers astrocyte-derived toxicity on motor neurons, remain to be determined. However, the notion that Bcl-2 is a common toxic target of both mutSOD1 and iperOxSOD1 allows the design of target-based therapies against the SOD1/Bcl-2 complex, whose efficacy may extend beyond application to the small number of mutSOD1 patients.
Methods
Data were collected from 20 sALS patients with a diagnosis of ALS based on the revised El Escorial criteria and 4 fALS patients carrying three different SOD1 mutations (A4V, L106V, and G37R) (Table 1). Ten healthy age-matched controls were included in the analysis. Ethics Committee approval was obtained from the Foundation “C. Mondino” Institute of Neurology, University of Pavia (for the sALS patients and controls) and Massachusetts General Hospital (for the fALS patients). All patients and controls provided written informed consent to participate in the study. EBV-immortalized lymphoblasts were derived and analyzed as described in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Robert H. Brown, Jr and Diane Mckenna-Yasek for providing some of the lymphoblast lines used in these studies. This work was supported by National Institutes of Health Grants NINDS R01-NS051488 (to P.P.) and NINDS R01-NS064488 (to D.T.), and ALS Association Grant 1066 (to P.P.), and the Farber Family Foundation (P.P. and D.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115402109/-/DCSupplemental.
References
- 1.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 3.Bosco DA, Landers JE. Genetic determinants of amyotrophic lateral sclerosis as therapeutic targets. CNS Neurol Disord Drug Targets. 2010;9:779–790. doi: 10.2174/187152710793237494. [DOI] [PubMed] [Google Scholar]
- 4.Ticozzi N, et al. Genetics of familial amyotrophic lateral sclerosis. Arch Ital Biol. 2011;149:65–82. doi: 10.4449/aib.v149i1.1262. [DOI] [PubMed] [Google Scholar]
- 5.Renton AE, et al. ITALSGEN Consortium A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson PA, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay M, Valentine JS. Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS. Antioxid Redox Signal. 2009;11:1603–1614. doi: 10.1089/ars.2009.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw PJ. Motor neurone disease. BMJ. 1999;318:1118–1121. doi: 10.1136/bmj.318.7191.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: Common pathways in neurodegenerative disease. Hum Mol Genet. 2006;15(Special Issue 2):R182–R187. doi: 10.1093/hmg/ddl202. [DOI] [PubMed] [Google Scholar]
- 12.Proctor EA, Ding F, Dokholyan NV. Structural and thermodynamic effects of post-translational modifications in mutant and wild-type Cu, Zn superoxide dismutase. J Mol Biol. 2011;408:555–567. doi: 10.1016/j.jmb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 14.Kabashi E, Valdmanis PN, Dion P, Rouleau GA. Oxidized/misfolded superoxide dismutase-1: The cause of all amyotrophic lateral sclerosis? Ann Neurol. 2007;62:553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- 15.Banci L, et al. SOD1 and amyotrophic lateral sclerosis: Mutations and oligomerization. PLoS One. 2008;3:e1677. doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakhit R, et al. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat Med. 2007;13:754–759. doi: 10.1038/nm1559. [DOI] [PubMed] [Google Scholar]
- 17.Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Middleton T, Gahn TA, Martin JM, Sugden B. Immortalizing genes of Epstein-Barr virus. Adv Virus Res. 1991;40:19–55. doi: 10.1016/s0065-3527(08)60276-6. [DOI] [PubMed] [Google Scholar]
- 19.Pasinelli P, et al. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Pedrini S, et al. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19:2974–2986. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin B, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 22.Niebrój-Dobosz I, Dziewulska D, Kwieciński H. Oxidative damage to proteins in the spinal cord in amyotrophic lateral sclerosis (ALS) Folia Neuropathol. 2004;42:151–156. [PubMed] [Google Scholar]
- 23.Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantle D, Siddique S, Eddeb F, Mendelow AD. Comparison of protein carbonyl and antioxidant levels in brain tissue from intracerebral haemorrhage and control cases. Clin Chim Acta. 2001;312:185–190. doi: 10.1016/s0009-8981(01)00623-4. [DOI] [PubMed] [Google Scholar]
- 25.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Lei X, et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB J. 2006;20:2147–2149. doi: 10.1096/fj.05-5665fje. [DOI] [PubMed] [Google Scholar]
- 27.Chiò A, Calvo A, Moglia C, Mazzini L, Mora G. PARALS study group Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population-based study. J Neurol Neurosurg Psychiatry. 2011;82:740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- 28.Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 30.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: The FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng HX, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.