Abstract
In plants, plasmodesmata (PD) serve as channels for micromolecular and macromolecular cell-to-cell transport. Based on structure, PD in immature tissues are classified into two types, simple and branched (X- and Y-shaped) or twinned. The maximum size of molecules capable of PD transport defines PD aperture, known as the PD size exclusion limit. Here we report an Arabidopsis mutation, decreased size exclusion limit1 (dse1), that exhibits reduced cell-to-cell transport of the small (524 Da) fluorescent tracer 8-hydroxypyrene-1,3,6-trisulfonic acid at the midtorpedo stage of embryogenesis. Correspondingly, the fraction of X- and Y-shaped and twinned PD was reduced in dse1 embryos compared with WT embryos at this stage, suggesting that the frequency of PD is related to transport capability. dse1 is caused by a point mutation in At4g29860 (previously termed TANMEI) at the last donor splice site of its transcript, resulting in alternative splicing in both the first intron and the last intron. AtDSE1 is a conserved eukaryotic 386-aa WD-repeat protein critical for Arabidopsis morphogenesis and reproduction. Similar to its homologs in mouse, null mutants are embryo-lethal. The weak loss-of-function mutant dse1 exhibits pleiotropic phenotypes, including retarded vegetative growth, delayed flowering time, dysfunctional male and female organs, and delayed senescence. Finally, silencing of DSE1 in Nicotiana benthamiana leaves leads to reduced movement of GFP fused to tobacco mosaic virus movement protein. Thus, DSE1 is important for regulating PD transport between plant cells.
In plants, micromolecules and macromolecules, such as water, ions, metabolites, proteins, RNAs, and plant viruses, spread from cell to cell through plasmodesmata (PD) (1–3). PD are plasma membrane-lined channels spanning plant cell walls that directly connect the cytoplasm between adjacent cells. The axial center of PD contains a cylinder of compressed endoplasmic reticulum (ER) called the desmotubule (DT). Cell-to-cell transport occurs through the cytoplasmic space between the plasma membrane and the DT (1, 2, 4) and also along the ER membrane of the DT (5). Actin and myosin may regulate PD aperture by forming helically arranged particles and spoke-like structures that connect the DT to the plasma membrane (6–11).
PD are classified as primary and secondary according to their origin. Primary PD form during cytokinesis when ER becomes trapped in the newly formed cell wall. Secondary PD form after cytokinesis during cell wall extension and during the development of different cell layers (1–3). Morphologically, PD can be classified into simple and branched or twinned forms. Simple PD are single channels connecting two neighboring cells that are predominant in young tissues, such as sink leaves (6, 12), root and shoot apical meristems (13, 14), and embryos (15). Such young tissues also exhibit branched (X- and Y-shaped) PD and twinned PD, the latter consisting of two closely paired simple PD (15, 16). Twinned PD may represent the formation of new secondary PD from existing simple PD via X- and Y-shaped intermediates (3, 17). In contrast, mature tissues, such as source leaves, contain mostly branched PD comprising multiple channels that interconnect to form branches through the cytoplasmic sleeve (6, 12).
PD have different transport capacities, usually measured by their size exclusion limit (SEL), defined as the size of the largest molecules that can diffuse through PD. PD SEL is an important regulator of plant development and has been studied extensively during leaf maturation. Simple PD in sink tissues have larger SELs than highly branched PD in mature source tissue in leaves (12). PD SEL in leaves is not static (18) and is affected by such biotic and abiotic factors as turgor, wounding, long-term osmotic stress, hypoxia, and pathogenic infection (19).
Embryogenesis is an emerging system for studying PD function and SEL. The Arabidopsis embryo undergoes temporal and spatial changes in PD transport resulting in the establishment of symplastic fields corresponding to the morphogenetic regions that will give rise to the major plant organs: cotyledon, shoot apical meristem, hypocotyl, and root (20). Down-regulation of PD aperture in embryo development occurs at the midtorpedo stage (21). This transition in embryonic PD aperture provided the basis for a genetic screen that identified two mutants, increased size exclusion limit1 (ise1) and ise2, that fail to down-regulate PD permeability (22, 23). These mutants also have increased proportions of branched (X- and Y-shaped) PD and twinned PD (15). Here we report a mutation with the opposite phenotype—decreased PD aperture—termed decreased size exclusion limit 1 (dse1). Reduced PD transport was observed in Arabidopsis dse1 embryos and also after virus-induced gene silencing of DSE1 in Nicotiana benthamiana leaves. Consistent with results from ise1 and ise2 mutants, dse1 mutants contain fewer X- and Y-shaped PD and twinned PD than their WT counterparts. Thus, increased or decreased movement via PD correlates with the extent of X- and Y-shaped and twinned PD.
Results
Identification of a Mutant with Decreased PD SEL in Arabidopsis Embryos.
We previously used hydrophilic fluorescent (F) symplastic tracers to measure the transport capacity of cells during different stages of embryogenesis. The low molecular weight (524 Da) tracer 8-hydroxypyrene-1, 3, 6-trisulfonic acid (HPTS) moved through all cells in embryos from globular to late torpedo stages; in contrast, 10-kDa F-dextrans moved from cell-to-cell only during early embryogenesis up to the midtopedo stage (21). We originally used this down-regulation of F-dextran movement to screen torpedo embryos from embryo defective (emb) lines to identify ise-type mutants with the increased capacity to transport F-dextrans (21). In the present study, we again performed a large-scale screening of ethyl methane sulfonate mutagenized emb lines, but instead we scored for the opposite phenotype, reduced PD movement of the low molecular weight tracer HPTS. HPTS movement was severely restricted in the defective embryos of one mutant line, dse1 (Fig. 1 B and C), but HPTS moved freely from cell-to-cell in WT embryos segregating in the same siliques, as expected (Fig. 1A).
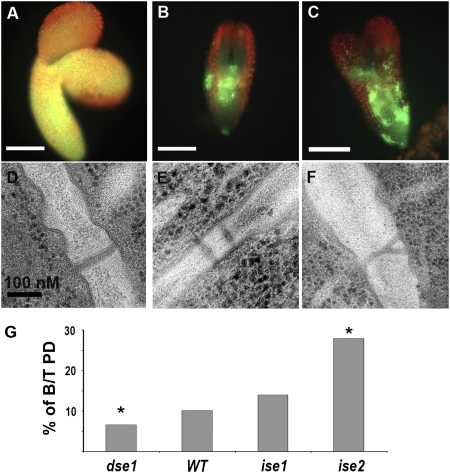
Fig. 1.
Characterization of dse1. (A–C) HPTS tracer movement assay in torpedo-staged embryos. HPTS moved freely in WT embryos (A), but was restricted in dse1 embryos (B and C). (D–F) Classification of PD in dse1 embryos: simple (D), twinned (E), and branched Y-shaped (F). (G) Fractions of branched (X- and Y-shaped) and twinned PD (B/T) in dse1, WT, and ise1 and ise2. Data for branched and twinned PD in ise1 and ise2 were published previously (15). *P < 0.05 compared with WT.
Frequency of X- and Y-Shaped and Twinned PD Is Decreased in dse1 Embryos.
In young developing tissues with predominantly simple PD, do novo PD formation likely occurs via the formation of intermediate structures, such as branched PD without central cavities, herein defined as X- and Y-shaped PD, resulting in twinned PD (3, 15–17). We predicted that PD transport is related to the frequency of PD formation, that is, the frequency of X- and Y-shaped and twinned PD. Indeed, ise1 and ise2 mutants exhibit increased intercellular movement and increased frequencies of X- and Y-shaped and twinned PD (15). Thus, we tested whether the frequency of X- and Y-shaped and twinned PD is altered in dse1 embryos with reduced PD transport. We examined at least 350 PD in the hypocotyls of three separate torpedo-staged embryos from both dse1 and WT. PD were classified as simple single channels or as X- and Y-shaped and twinned PD (Fig. 1 D–F). Twinned PD are pairs of simple PD within <100 nm (15, 16). We found that dse1 embryos contained only 6.6% X- and Y-shaped and twinned PD, significantly less than the 10.2% in WT embryos observed here (Fig. 1G) and in a previous study (∼10%) (15). In contrast, ise1 and ise2 embryos with increased PD transport contain ∼14% and ∼28% X- and Y-shaped and twinned PD, respectively (Fig. 1G) (15). Taken together, these data indicate that that the frequency of X- and Y-shaped and twinned PD structure is strongly correlated with PD transport function.
Molecular Cloning and Characterization of dse1.
dse1 mutants are grown as dse1 heterozygous plants, in which the defective embryos segregate in siliques at the expected ratio for a single-recessive mutant. Map-based cloning localized dse1 to chromosome 4 close to marker PRHA (Fig. S1). Deep sequencing of this region revealed a single nucleotide change in At4g29860, which consists of 13 exons and 12 introns (Fig. 2A). In dse1, the splice donor site of the last intron of At4g29860 is mutated from guanine (GT) to adenine (AT) (Fig. 2A). At4g29860 was previously known as TANMEI/EMB2757 (TAN) (24). Similar to tan, dse1 embryos are developmentally retarded and accumulate anthocyanin at the junction between the cotyledons and hypocotyls, corresponding to the shoot apical meristem (Fig. S2). These data suggest that reduced PD transport in dse1 at this critical source of meristematic cells likely causes stress, resulting in accumation of anthocyanin. Crossing heterozygous dse1 to heterozygous tan-2 (Salk 097510), a T-DNA insertion mutant in At4g29860, demonstrated that dse1 and tan-2 are allelic. HPTS movement in tan-2 embryos was restricted as well (Fig. S3). Constructs carrying either the genomic fragment of At4g29860 or the At4g29860 cDNA under control of the native promoter fully rescued the dse1 phenotype. Thus, the dse1 mutant identified here results from G-to-A mutation at the splice junction of exon 12 in At4g29860.
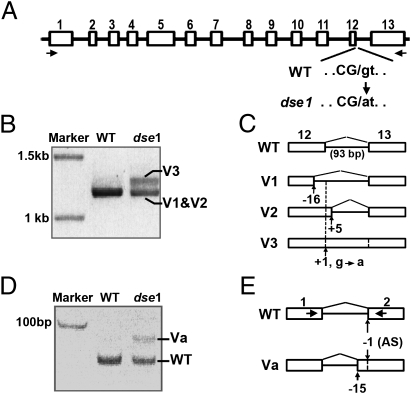
Fig. 2.
Splice donor site in At4g29860 is mutated in dse1. (A) Diagram of At4g29860. Exons are in white boxes and labeled numerically. The 5′ splicing donor site of the last intron (intron 12) is mutated from G to A in dse1. Exon and intron nucleotides at the junction in WT versus dse1 are in capital letters and lowercase letters, respectively, and separated with a slash. (B) Full-length At4g29860 cDNAs from Ler (WT) and dse1 embryos. cDNAs were separated on a 0.8% agarose gel. Primer positions are arrows in A underneath the exons. Splice variants of intron 12 (V1–3) in dse1 are labeled. (C) Diagram of splice variants of intron 12 in dse1. Because of the G-to-A mutation at the donor splice site (1 bp downstream of the exon 12; position +1), dse1 intron 12 is spliced using cryptic donor sites either 16 bp upstream (position −16) or 4 bp downstream (position +5), resulting in variants 1 and 2 (V1 and V2). Note that full-length V1 and V2 do not resolve from each other on the 0.8% agarose gel in B. Variant 3 (V3) retains intron 12. Splicing of intron 12 in WT is shown at the top. (D) Alternative splicing of intron 1 in dse1 embryos. cDNA fragments were amplified with primers (arrows in E) from WT and dse1 embryos and separated on 8% polyacrylamide gel. dse1 intron 1 is spliced normally (WT) or as variant “a” (Va). (E) Diagram of alternative splicing of intron 1 in dse1 embryos with either WT acceptor sites (AS) 1 bp upstream of exon 2 (position −1) or a cryptic site 14 bp upstream (position −15).
We predicted the dse1 mutation would lead to defective splicing of dse1 mRNA, because the mutation occurs at a splice donor site (Fig. 2A). Indeed, dse1 cDNA products were detected as two bands (Fig. 2B); sequencing of dse1 cDNAs across exon 12-intron 12-exon 13 revealed that the lower band contains two variants (V1 and V2), whereas the upper band (V3) is due to the retention of intron 12 (93 bp) (Fig. 2C). V1 and V2 are due to alternative splice donor sites either 16 bp upstream or 4 bp downstream of the mutated donor splice site in dse1 (Fig. 2C). Interestingly, we also observed an intron 1 splice variant, Va, in dse1 mRNA with an alternative splicing acceptor site 14 bp upstream of the WT site (Fig. 2 D and E); the resulting mRNA contains several premature stop codons. Sequencing dse1 mRNA in heterozygous embryos also revealed alternative splicing of the first and last introns; however, no alternative splicing was detected in DSE1 transcripts from homozygous WT embryos (Fig. 2 B and C). This indicates that the alternative splicing observed in embryos is specific to dse1 transcripts. Previous studies reported alternative splicing in the first intron only in WT tissues after embryogenesis (24).
Reduced DSE1 Expression in N. benthamiana Reduces Movement of P30-GFP.
To examine whether a lack of DSE1 has similar effects on cell-to-cell movement in other plant species and in nonembryonic tissues, we cloned and silenced DSE1 homologs NbDSE1 (GenBank accession no. HQ873433) in N. benthamiana leaves via virus-induced gene silencing with tobacco rattle virus (TRV)-based vectors. Agrobacteria containing TRV-NbDSE1 were introduced into 3-wk-old N. benthamiana leaves via infiltration. NbDSE1 mRNA was significantly reduced in the leaves above the initial inoculated leaf at 14 d postinfiltration in TRV-NbDSE1 plants compared with control plants infiltrated with TRV-GUS (Fig. 3G).
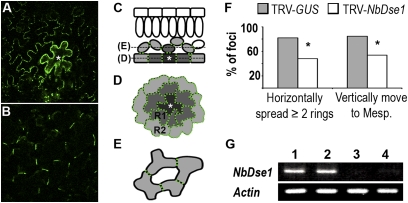
Fig. 3.
Intercellular movement of P30-1xGFP in DSE1 silenced N. benthamiana leaves. NbDSE1 was silenced by infiltration with TRV-NbDSE1. Plants infiltrated with TRV-GUS were used as controls. (A–E) P30-GFP movement from a primary transformed epidermal cell in N. benthamiana leaves. (A) Leaf epidermis showing horizontal movement from a primary transformed epidermal cell (marked with white asterisk). (B) P30-GFP also moves vertically from the same primary transformed cell into the mesophyll layer of cells. (C–E) Illustration of horizontal and vertical movement of P30-GFP in leaf tissue. (C) Side view of leaf tissue. (D) Top-down view of epidermal cells. (E) Top-down view of spongy mesophyll cells. P30-GFP moves from primary transformed epidermal cells (dark gray with star) to neighboring cells (light gray) and forms puncta (green dots) at PD in the cell wall of the cells to which it moves. In D, R1 and R2 represent the first and second rings of epidermal cells surrounding primary transformed foci. (F) Both horizontal and vertical movements of P30-GFP were reduced in NbDSE1-silenced leaves. Horizontal movement was recorded as the number of rings of epidermal cells into which P30-GFP spread from primary foci. Vertical movement was recorded when P30-GFP moved to mesophyll cells. *P < 0.05 compared with controls. (G) Semiquantitative RT-PCR. NbDSE1 and Actin were amplified in 32 and 25 cycles, respectively. Lanes 1 and 2 are from control TRV-GUS–infected leaves; lanes 3 and 4 are from NbDSE1-silenced leaves.
PD function in DSE1 silenced leaves was examined by monitoring the cell-to-cell movement of tobacco mosaic virus (TMV) movement protein P30 fused to GFP (P30-GFP). TMV P30 targets to PD and facilitates intercellular transport of viral RNA. We used P30-GFP rather than soluble GFP (sGFP) to monitor cell-to-cell movement, because P30-GFP forms puncta that mark PD in all of the cells to which it has moved and is easier to track by fluorescence microscopy compared with sGFP (15). In leaves, P30-GFP moves from an originally transfected epidermal cell both horizontally to adjacent epidermal cells (Fig. 3 A, C, and D) and vertically to adjacent mesophyll cells below (Fig. 3 B, C, and E). We used dilute concentrations of Agrobacterium carrying the 35S::P30-GFP expression construct to ensure that individual transformed foci were well separated from one another, to allow accurate monitoring of P30-GFP movement. We monitored movement in more than 40 foci in five experiments at 48 h postinfiltration (hpi) of Agrobacterium. At 48 hpi, P30-GFP moved horizontally to at least one ring of epidermal cells away from all primary infected cells. However, movement to two or more rings of cells was greater in control leaves than in DSE1-silenced leaves, in ∼80% versus ∼50% of the foci, respectively (Fig. 3F). Vertical movement into the mesophyll layer was similarly reduced in DSE1-silenced leaves (Fig. 3F). Thus, reducing DSE1 expression in N. benthamiana reduces PD-mediated horizontal and vertical transport in leaves, consistent with the reduced movement of fluorescent tracers in Arabidopsis dse1 embryos.
Subcellular Localization of AtDSE1.
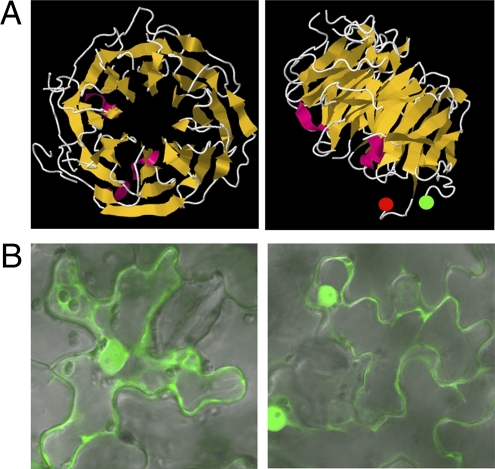
AtDSE1 is a putative 386-aa WD40 protein (24) that has homologs in all eukaryotes and only in eukaryotes (Fig. S4). AtDSE1 forms an eight-bladed propeller-like structure (Fig. 4A), according to the I-TASSER prediction program (25). N- and C- termini of native AtDSE1 are predicted to be exposed and to not be part of the propeller structure (Fig. 4A), so fusing GFP to either terminus of AtDSE1 is unlikely to perturb the native structure. Indeed, both fusion proteins GFP-AtDSE1 and AtDSE1-GFP expressed under the DSE1 native promoter are fully functional and rescue dse1 mutants and the null embryo lethal tan-2 mutation. We could not observe the GFP signal or detect fusion proteins using GFP antibody in any rescued plants, however. Thus, we examined the subcellular localization of AtDSE1 in N. benthamiana leaves after 35S promoter-mediated transient expression of N- and C-terminal GFP fusions to AtDSE1. Western blot analysis revealed both fusions of the expected size of 70 kDa, with no breakdown products. GFP-AtDSE1 or AtDSE1-GFP was consistently detected in both cytoplasm and nuclei (Fig. 4B); thus, AtDSE1 likely functions in both the nucleus and the cytoplasm.
Fig. 4.
Characterization of AtDSE1. (A) Predicted AtDSE1 structure with eight blades. (Left) Top view. (Right) Side view. N and C termini are labeled with green and red circles, respectively. (B) Subcellular localization of AtDSE1. AtDSE1 fused to GFP at either the N-terminus (Left) or the C-terminus (Right) localize to cytoplasm and nuclei after transient expression in epidermal cells of N. benthamiana leaves.
DSE1 Is Expressed Ubiquitously and Is Essential for Arabidopsis Reproduction.
To further investigate the role of DSE1 during development, we characterized its expression pattern and the growth pattern of dse1 mutant plants. Northern blot analyses and quantitative PCR revealed At4g29860 (DSE1/TAN) expression at different developmental stages in all tissues examined, including roots, rosette leaves, shoots, flowers, and siliques (24). Here we expressed GUS under the DSE1 promoter to provide more details about the cell and tissue types in which DSE1 is expressed; for example, the DSE1 promoter is very active in the shoot apex, and its activity in roots is confined to the vascular system (Fig. S5). Overall, our data indicate that the DSE1 promoter is active throughout the plant life cycle, from embryogenesis to seed set (Fig. S5). Not surprisingly, dse1 plants exhibit growth defects at all developmental stages, as described below.
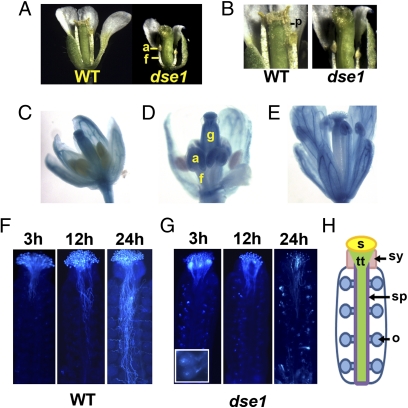
Previous studies with tan-1 monitored seedlings only at the cotyledon stage (24). We found that germination on agar medium supplemented with sucrose allowed dse1 seedlings to develop true leaves (Fig. S6 A and B). Such plants survive in soil and can initiate flowering, albeit after a delay of 1 or 2 wk compared with WT grown under the same conditions (Fig. S6C). dse1 plants lose apical dominance and produce smaller abnormal flowers, often with altered organ numbers. dse1 gynoecia do not set seeds (Fig. 5A and Fig. S6D). This infertility is due at least in part to the abnormal development of dse1 stamen; anther filaments do not extend, and no pollen is released from anthers (Fig. 5 A and B). The requirement for DSE1 in stamen development is consistent with the DSE1 expression pattern revealed by the pDSE1::GUS reporter line. DSE1 expression in anthers begins just when the filaments start to elongate (stage 12) (Fig. 5D), and continues to later stages (Fig. 5E).
Fig. 5.
dse1 plants exhibit multiple pleiotropic phenotypes. (A) Dissected WT and dse1 mature flowers. (B) Gynoecia of of WT and dse1 mature flowers. No pollen was released from dse1 anthers. (C–E) GUS staining in flowers of transgenic Arabidopsis carrying the pDSE1::GUS gene at different stages: stage 10 (C), stage 12 (D), and stage 13 (E). (F–G) WT pollen tube growth in dse1 and WT gynoecia. Gynoecia of dse1 (F) and WT (G) were collected at 3, 12, and 24 hpp and stained with analine blue. (F, Inset) dse1 ovules showing callose deposition. (H) Diagram of a gynoecium in Arabidopsis. a, anther; f, anther filament; g, gynoecium; p, pollen; s, stigma; sy, style; sp, septum; tt, transmitting track; o, ovule.
Because DSE1 is constitutively expressed in gynoecia (Fig. 5 C–E), we suspected that dse1 also affects female organ development. Indeed, no seeds were produced after hand pollination with WT pollen. To further investigate this dysfunction, we examined WT pollen tube growth on dse1 gynoecia. We found that WT pollen germinates normally on dse1 stigma and produces tubes throughout the style by 3 h postpollination (hpp), as on WT stigma (Fig. 5 F and G). Afterward, pollen tubes grow very slowly on dse1; at 24 hpp, pollen tubes barely reach the middle region of the gynoecium, remain in the transmitting tract, and do not migrate toward ovules (Fig. 5G). No further growth is observed at 48 hpp. In contrast, on WT gynoecia, pollen tubes grow basipetally to the bottom of the transmitting tract, and at 24 hpp, pollen tubes emerge from the septum and grow toward and start to penetrate ovules (Fig. 5F). Mature dse1 ovules are also abnormal, accumulating internal aggregates of aniline blue staining callose (Fig. 5G, Inset). dse1-defective ovules likely fail to signal pollen tube elongation. Thus, DSE1 is essential for signal transduction and development of both male and female organs.
Discussion
Here we analyze a mutant with reduced PD-mediated intercellular transport, dse1. Movement of the low molecular weight tracer HPTS is severely restricted in Arabidopsis dse1 embryos and tan-2 (an allele of dse1) embryos. Similarly, P30-GFP movement is reduced in N. benthamiana leaves after gene silencing of NbDSE1. Significantly, the proportion of X- and Y-shaped and twinned PD is reduced in Arabidopsis dse1 torpedo-staged embryos compared with WT. The correlation between reduced transport in dse1 embryos and reduced frequencies of X- and Y-shaped and twinned PD is consistent with our findings in ise1 and ise2 mutations with the opposite phenotype (15, 22). In Arabidopsis ise1 and ise2 mutant embryos and ISE1- or ISE2-silenced N. benthamiana young leaves, increased cell-to-cell transport is associated with increased X- and Y-shaped and twinned PD (15). Taken together, these data suggest a simple strategy for regulating intercellular transport: more PD orifices result in more transport.
Another mutant with reduced intercellular movement, GFP arrested trafficking 1 (gat1) contains 9% branched PD in the root apical meristem (26), similar to the 10% observed in WT embryos in the present study and in a previous intensive quantitative study of PD in immature WT tissues (15). However, 5% of gat1 PD were occluded, but none were occluded in dse1 embryos. Thus, dse1 and gat1 regulate PD structure and intercellular movement via PD in a different manner. This hypothesis is further supported by two major differences in the manner in which the mutants were isolated. gat1 was identified by reduced movement of 27-kDa GFP in vascular tissues, whereas dse1 was identified by reduced movement of the small 524-Da tracer HPTS in cells throughout developing embryos.
PD structure and function are important to plant development. Here and in our previous studies, we used mutants that are embryo-defective and can develop to a stage at which their tissues can be assayed for PD function. We specifically chose the midtorpedo stage for investigation because we determined that cell-to-cell transport was specifically altered (reduced) at this stage compared with earlier stages of embryogenesis (21). However, it is critical to note that null mutants of DSE1, ISE1, and ISE2 are embryo-lethal (22–24), attesting to their essential roles. The dse1 point mutation has less severe phenotypes but still exhibits developmental defects. During the first 6 wk of seedling development, dse1 plants are smaller than WT, and flower initiation is delayed. dse1 flowers are abnormal; both male and female organs are infertile. AtDSE1 encoded by DSE1/EMB2757/TANMEI belongs to the WD-repeat (WDR) protein superfamily. It forms a β-propeller structure with eight blades (25), six of which are WDRs. AtDSE1 is critical to plant development but is undetectable by Western blot analysis, suggesting that it functions at low protein levels or experiences rapid turnover. The integrity of the propeller structure is critical to the function of AtDSE1, as demonstrated by the finding that the absence of only the C-terminal 34 amino acids of the last WDR in dse1 leads to notable pleiotropic phenotypes. DSE1 transcription is regulated in a complex manner. The amount of DSE1 mRNA and the activity of DSE1 promoter are distinct in different tissues (24) (Fig. S5). Moreover, DSE1-specific mRNAs are distinct in different tissues; alternative splicing at the first intron of DSE1 that disrupts the coding frame occurs in WT seedlings (24), but not in WT embryos, as shown here.
WDR proteins are thought to function as structural platforms for mediating the formation of stable or reversible protein complexes that play important roles in various pathways, including signal transduction, cytoskeleton assembly, protein trafficking, nuclear export, and pre-RNA processing (27). Phylogenic analyses have revealed numerous AtDSE1 homologs in all eukaryotes, from protozoa to mammals. Mutation of the DSE1 homolog in mouse (Gnbl1) also leads to embryo lethality (28), but no DSE1 homologs have known exact functions. The only clue is that the DSE1 homolog in yeast, YPR085C /Asa1, interacts with telomere-binding protein interacting protein 1 (29), suggesting a possible role in telomeric chromatin regulation and chromatin remodeling. (Note that the yeast asa1 mutant is lethal as well.)
Five mutants with altered PD function in Arabidopsis have been described to date: ise1, ise2, gat1, dse1, and glucan synthase like 8 (gsl8). All five of these mutants produce severe phenotypes during embryo or seedling development; however, except GSL8 none encodes proteins that localize to PD. ISE1 encodes a mitochondrial DEAD-box RNA helicase (22), ISE2 encodes a chloroplast DEVH-type RNA helicase (23, 30), GAT1 encodes a chloroplast thioredoxin (26), and DSE1 encodes a WDR protein that localizes to the nucleus and cytoplasm. Only, GSL8 encodes a putative glucan synthase, that may localize close to PD, as it is involved in callose deposition in cell walls at the cell plate (31). These data strongly support that in addition to PD localized proteins, proteins regulating cellular homeostasis are critical for PD function and concomitant developmental pathways. The cellular processes influenced by ISE1, ISE2, GAT1, and DSE1 likely affect common factors that regulate the modification of PD structure and function. Plastid homeostasis is very important to PD structure and function. GAT1 localizes to plastids (26), and ise1 and ise2 both dramatically affect the expression of more than 300 nuclear genes essential for chloroplast function (30). Finally, a mutant of corn, sucrose export defective 1, with decreased PD mediated transport from the phloem encodes a protein that localizes to chloroplasts (32).
In summary, dse1 profoundly affects PD transport, reducing the transport of even small molecules (<500 Da). It will be interesting to examine whether PD themselves are modified or occluded as a result of altered PD protein composition or whether external factors, such as callose deposition at cell walls surrounding PD, result in the dse1 PD phenotype. Finally, we are excited to investigate whether DSE1 also plays a role in chloroplast homeostasis (like the mutants mentioned above) or whether it acts to regulate PD formation and function through another pathway.
Materials and Methods
Plant Materials and Growth Conditions.
Here 5,000 ethyl methane sulfonate-mutagenized lines of Arabidopsis thaliana Landsberg erecta (Ler) previously identified as carrying embryo-defective mutants capable of developing to the torpedo stage of embryogenesis (21) were screened individually by fluorescence microscopy for reduced transport of HTPS. Lines were propagated as heterozygotes, so that homozygous defective embryos were observed segregating in developing siliques. The dse1 heterozygous line was backcrossed four times to Ler WT before analysis. tan-2 [SALK_097510, ecotype Columbia-0 (Col-0)] was obtained from the Arabidopsis Biological Resource Center. Unless noted otherwise, plants were grown in soil under long-day conditions (16-h light/8-h dark) at 22 °C with a light intensity of 120 μmol photons m−2 s−1. To obtain homozygous dse1 plants, seeds from heterozygous dse1 plants were surface-sterilized, plated on 1/2 Murashige and Skoog medium supplemented with 0.5% sucrose, stored at 4 °C for 3 d, and placed under continuous light for germination and growing. Two-wk-old dse1 seedlings were transferred to soil and grown as above.
dse1 Complementation.
For complementation, dse1 heterozygous plants were transformed with binary vectors pCBM3 or pCBM5. The genomic DSE1 fragment (∼5.9 kb) starting from 2.5 kb upstream of the ATG of DSE1 to 1.0 kb downstream from the stop codon was PCR-amplified from Ler and cloned into the PstI site of pCAMBIA3300 (Cambia), generating pCBM3. For pCBM5, the PCR-amplified 2.5-kb DSE1 promoter was first cloned into pRTL2-sGFP (33) between EcoRV and NcoI sites, generating pRTL2- pDSE1:: sGFP. Next, sGFP in pRTL2- pDSE1:: sGFP was removed by NcoI-NotI digestion and replaced with RT-PCR–amplified DSE1 coding sequences (CDS) generating pRTL2- pDSE1::DSE1CDS. Finally, the pDSE1::DSE1CDS::nos&35s terminator cassette was released from the latter plasmid using SbfI, and this fragment was cloned into the PstI site of pCAMBIA3300, generating pCBM5.
Constructing GUS Reporter Lines.
The GUS gene was amplified and used to replace sGFP in pRTL2- pDSE1:: sGFP. pDSE1::GUS cloned into pCAMBIA3300, and then transformed into Col-0.
Dye Loading Assay, GUS Staining, and in Vivo Pollen Tube Guidance Assay.
Torpedo-staged embryos were loaded with HPTS (Molecular Probes) as described previously (21). GUS staining was done following published protocols (34). For in vivo pollen guidance assays, gynoecia were stained with analine blue as described previously (35).
GFP Constructs for AtDSE1 Subcellular Localization.
DSE1CDS without the stop codon was PCR-amplified from pCBM5 and inserted into the NcoI site upstream of sGFP in pRTL2-sGFP, generating pRTL2-DSE1-sGFP. DSE1CDS with the stop codon was PCR-amplified from PCBM5 and cloned to NcoI-NotI–digested pRTL2-3xsGFP (18) between the first sGFP and the nos terminator, generating pRTL2-sGFP-DSE1. 35S:: DSE1-sGFP::nos&35S terminator and 35S::sGFP-DSE1::nos&35s terminator were released by Sbf1 and cloned into pCAMBIA3300 to generate C- and N-terminal GFP fusions to DSE1, respectively. Both constructs were introduced into GV3101 and infiltrated into leaves of N. benthamiana (15). GFP fluorescence was observed and imaged after 48 h by confocal laser scanning microscopy.
RNA Extraction and RT-PCR.
Total RNA from Arabidopsis embryos was isolated with the Absolutely RNA Nanoprep Kit (Stratagene). Total RNA from N. benthamiana leaves was extracted with the Qiagen RNeasy Plant Mini Kit. DNA was removed with the DNA-Free Kit (Ambion), and total RNAs were reverse-transcribed using oligo (dT)18 and SuperScript II Reverse Transcriptase (Invitrogen). First-strand cDNA was subjected to PCR with iProof polymerase (Bio-Rad). The products were separated by electrophoresis, purified, and cloned to pCR 2.1-TOPO (Invitrogen) for sequencing.
Protein Structure Prediction.
The amino acid sequence of AtDSE1 was deduced by ExPASy (http://ca.expasy.org/tools/dna.html). The AtDSE1 3D structure was predicted by I-TASSEL (25).
Transmission Electronic Microscopy.
Thin-sections of torpedo-staged embryos from dse1 and Ler were prepared for transmission electron microscopy as described previously (15). PD structures in longitudinal sections of hypocotyls were examined. Three embryos from each genotype were evaluated. Data were analyzed with the Student t test.
Molecular Cloning of NbDSE1 from N. benthamiana.
A BLAST search of the Arabidopsis DSE1 CDS against the Solanaceace database from the Solanaceae Genomics Resource Web site (http://solanaceae.plantbiology.msu.edu/) revealed several hits with high similarity. Based on this information, forward (5′-CCCAGTCTCAGTGCTACGAG) and reverse (5′ TTCCCATAGAGCCACTGTCGTG) primers were designed to amplify a partial NbDSE1 cDNA fragment via RT-PCR. This partial NbDSE1 cDNA fragment was cloned into the pCR 2.1-TOPO vector (Invitrogen) for sequencing. According to the sequence of the partial NbDSE1 cDNA fragment, primers 5′- CAGCAGCTCCAGAGAGACCACCTTTGC and 5′-GCAGGGGAACAAGCTTCTGTGGTTGAG were designed for 5′ and 3′ rapid amplification of cDNA ends (RACE), respectively, using the Smart RACE cDNA Amplification Kit (Clontech). Products were cloned into the pCR 2.1-TOPO vector for sequencing. Finally, sequences from the 5′ and 3′ RACE products were aligned to generate full-length NbDSE1 cDNA (GenBank accession no. HQ873433).
Virus-Induced Gene Silencing and Light Microscopy.
A 443-bp fragment of NbDSE1 cDNA (corresponding to the nucleotide sequences encoding amino acids 68–213 of NbDSE1) was amplified by RT-PCR and cloned into the XbaI and XhoI sites of pYL156 (36) to generate pTRV2-NbDSE1 for silencing of NbDSE1 in N. benthamiana. pYC1, pYL156 carrying a GUS fragment (22), was used as a control. Virus-induced gene silencing was performed by infiltration as described previously (15). At 14 d postinfiltration, total RNA was extracted from newly emerged leaves to test the expression level of NbDSE1. P30-1xGFP was transiently expressed in silenced plants and examined at 48 hpi by confocal laser scanning microscopy as described previously (15). The experiment was repeated five times. More than 40 P30-1xGFP foci were imaged for both NbDSE1-silenced and control tissues, and the data were analyzed using the Student t test.
NOTE ADDED IN PROOF
A recent Plant Cell Advance Online Publication describes a mutant, aluminum tolerant 2 (alt2) with reduced uptake of toxic aluminum (37). alt2 is allelic to tanmei and therefore is also allelic to dse1 described here. Thus, reduced uptake of aluminum in alt2 may be in part be explained by reduced PD mediated cell-to-cell transport.
Supplementary Material
Acknowledgments
We thank Steve Ruzin and Denise Schichnes of the College of Natural Resources Biological Imaging Center for advice and support. This research was supported by a University of California (Berkeley) Miller postdoctoral fellowship (to T.M.B.-S.) and National Institutes of Health Grant GM45244 (to P.C.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202919109/-/DCSupplemental.
References
- 1.Zambryski PC, Crawford K. Plasmodesmata: Gatekeepers for cell-to-cell transport of developmental signals in plants. Annu Rev Cell Dev Biol. 2000;16:393–421. doi: 10.1146/annurev.cellbio.16.1.393. [DOI] [PubMed] [Google Scholar]
- 2.Maule AJ. Plasmodesmata: Structure, function and biogenesis. Curr Opin Plant Biol. 2008;11:680–686. doi: 10.1016/j.pbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Burch-Smith TM, Stonebloom S, Xu M, Zambryski PC. Plasmodesmata during development: Re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma. 2011;248:61–74. doi: 10.1007/s00709-010-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overall RL, Blackman LM. A model of the macromolecular structure of plasmodesmata. Trends Plant Sci. 1996;1:307–311. [Google Scholar]
- 5.Guenoune-Gelbart D, Elbaum M, Sagi G, Levy A, Epel BL. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol Plant Microbe Interact. 2008;21:335–345. doi: 10.1094/MPMI-21-3-0335. [DOI] [PubMed] [Google Scholar]
- 6.Lucas WJ, Ding B, Van der Schoot C. Plasmodesmata and the supracellular nature of plants. New Phytol. 1993;125:435–476. doi: 10.1111/j.1469-8137.1993.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 7.Badelt K, White RG, Overall RL, Vesk M. Ultrastructural specializations of the cell wall sleeve around plasmodesmata. Am J Bot. 1994;81:1422–1427. [Google Scholar]
- 8.Radford JE, White RG. Localization of a myosin-like protein to plasmodesmata. Plant J. 1998;14:743–750. doi: 10.1046/j.1365-313x.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 9.Blackman LM, Overall RL. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 1998;14:733–741. [Google Scholar]
- 10.Reichelt S, et al. Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J. 1999;19:555–567. doi: 10.1046/j.1365-313x.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 11.White RG, Badelt K, Overall RL, Vesk M. Actin associated with plasmodesmata. Protoplasma. 1994;180:169–184. [Google Scholar]
- 12.Oparka KJ, et al. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 1999;97:743–754. doi: 10.1016/s0092-8674(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhu T, Lucas WJ, Rost TL. Directional cell-to-cell communication in the Arabidopsis root apical meristem, I: An ultrastructural and functional analysis. Protoplasma. 1998;203:35–47. [Google Scholar]
- 14.Ormenese S, Havelange A, Deltour R, Bernier G. The frequency of plasmodesmata increases early in the whole shoot apical meristem of Sinapis alba L. during floral transition. Planta. 2000;211:370–375. doi: 10.1007/s004250000294. [DOI] [PubMed] [Google Scholar]
- 15.Burch-Smith TM, Zambryski PC. Loss of INCREASED SIZE EXCLUSION LIMIT (ISE)1 or ISE2 increases the formation of secondary plasmodesmata. Curr Biol. 2010;20:989–993. doi: 10.1016/j.cub.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faulkner C, Akman OE, Bell K, Jeffree C, Oparka KJ. Peeking into pit fields: a multiple twinning model of secondary plasmodesmata formation in tobacco. Plant Cell. 2008;20:1504–1518. doi: 10.1105/tpc.107.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlers K, van Bel AJ. Dynamics of plasmodesmal connectivity in successive interfaces of the cambial zone. Planta. 2010;231:371–385. doi: 10.1007/s00425-009-1046-8. [DOI] [PubMed] [Google Scholar]
- 18.Crawford KM, Zambryski PC. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 2001;125:1802–1812. doi: 10.1104/pp.125.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillespie T, Oparka KJ. In: Intercellular Communication in Plants. Flemming AJ, editor. Oxford: Blackwell; 2005. pp. 109–146. [Google Scholar]
- 20.Kim I, Kobayashi K, Cho E, Zambryski PC. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA. 2005;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim I, Hempel FD, Sha K, Pfluger J, Zambryski PC. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development. 2002;129:1261–1272. doi: 10.1242/dev.129.5.1261. [DOI] [PubMed] [Google Scholar]
- 22.Stonebloom S, et al. Loss of the plant DEAD-box protein ISE1 leads to defective mitochondria and increased cell-to-cell transport via plasmodesmata. Proc Natl Acad Sci USA. 2009;106:17229–17234. doi: 10.1073/pnas.0909229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski PC. INCREASED SIZE EXCLUSION LIMIT 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. Plant Cell. 2007;19:1885–1897. doi: 10.1105/tpc.106.045666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamagishi K, et al. TANMEI/EMB2757 encodes a WD repeat protein required for embryo development in Arabidopsis. Plant Physiol. 2005;139:163–173. doi: 10.1104/pp.105.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benitez-Alfonso Y, et al. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA. 2009;106:3615–3620. doi: 10.1073/pnas.0808717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: A common architecture for diverse functions. Trends Biochem Sci. 1999;24:181–185. doi: 10.1016/s0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 28.Paylor R, et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: Implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burch-Smith TM, Brunkard JO, Choi YG, Zambryski PC. Organelle-nucleus cross-talk regulates plant intercellular communication via plasmodesmata. Proc Natl Acad Sci USA. 2011;108:E1451–E1460. doi: 10.1073/pnas.1117226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen XY, et al. The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol. 2009;150:105–113. doi: 10.1104/pp.108.133918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provencher LM, Miao L, Sinha N, Lucas WJ. Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell. 2001;13:1127–1141. doi: 10.1105/tpc.13.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford KM, Zambryski PC. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol. 2000;10:1032–1040. doi: 10.1016/s0960-9822(00)00657-6. [DOI] [PubMed] [Google Scholar]
- 34.Menand B, et al. Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proc Natl Acad Sci USA. 2002;99:6422–6427. doi: 10.1073/pnas.092141899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002;30:415–429. doi: 10.1046/j.1365-313x.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 37.Nezames CD, Sjogren CA, Barajas JF, Larsen PB. The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum-dependent root growth inhibition. Plant Cell. 2012 doi: 10.1105/tpc.112.095596. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.