Abstract
Rationale
The source of Ca2+ to activate pathological cardiac hypertrophy is not clearly defined. Ca2+ influx through the L-type Ca2+ channels (LTCCs) determines “contractile” Ca2+, which is not thought to be the source of “hypertrophic” Ca2+. However, some LTCCs are housed in caveolin-3 (Cav-3) enriched signaling microdomains and are not directly involved in contraction. The function of these LTCCs is unknown.
Objective
To test the idea that LTCCs in Cav-3 containing signaling domains are a source of Ca2+ to activate the calcineurin-nuclear factor of activated T cells (Cn-NFAT) signaling cascade that promotes pathological hypertrophy.
Methods and Results
We developed reagents that targeted Ca2+ channel blocking Rem proteins to Cav-3 containing membranes, which house a small fraction of cardiac LTCCs. Blocking LTCCs within this Cav-3 membrane domain eliminated a small fraction of the LTCC current, almost all of the Ca2+ influx induced NFAT nuclear translocation, but did not reduce myocyte contractility.
Conclusions
We provide proof of concept that Ca2+ influx through LTCCs within caveolae signaling domains can activate “hypertrophic” signaling, and this Ca2+ influx can be selectively blocked without reducing cardiac contractility.
Keywords: Caveolae, L-Type Calcium Channel, Hypertrophy, Contractility, NFAT
Introduction
Cardiovascular diseases increase cardiac systolic stress and cause myocyte hypertrophy. Increases in Ca2+ influx during pathological stress maintain pump function and activate signaling pathways that produce pathological hypertrophy and heart failure1,2, but the source of this “hypertrophic” Ca2+ is still not clearly defined.
Increases in Ca2+ influx are essential for the activation of pathological hypertrophic signaling, including the calcineurin-nuclear factor of activated T-cells (Cn-NFAT) pathway2, 3, the calmodulin-dependent protein kinase (CaMKII) pathway , and the protein kinase C (PKC) pathway5. The source of the Ca2+ that activates each of4 these signaling cascades is still not well known. Potential sources include L-6, 7 and T-8, 9 type Ca2+ channels and transient receptor potential (TRP) channels10.
In cardiac myocytes, voltage dependent L-type Ca2+ channels (LTCC, Cav1.2) are the predominant Ca2+ entry pathway and are essential for excitation-contraction coupling (ECC) and regulation of gene expression1. Myocyte LTCCs are concentrated in T-tubular membranes where they are organized in junctional complexes with ryanodine receptors (RyR2) on the SR1 to form a signaling microdomain involved in Ca2+-induced-SR Ca2+-release (CICR). This microdomain is the source of “contractile” [Ca2+].
Not all LTCCs are involved in CICR. A small fraction appears to be harbored in caveolae11, which are highly specialized membrane regions that are stabilized by the scaffolding protein caveolin. Caveolin-3 is the major caveolin expressed in the heart and it functions to help organize local signaling microdomains11. The function of caveolae based LTCCs are unknown and are the topic of this study.
Our studies test the hypothesis that “hypertrophic” Ca2+ enters the myocyte through LTCCs localized in Cav-3 based signaling microdomains and that this “signaling” Ca2+ is distinct from “contractile” Ca2+. This idea was examined with a novel LTCC blocker that selectively traffics to caveolae where it inhibits LTCCs within this signaling microdomain. Our results show that this Cav-3 localized LTCC antagonist can block “hypertrophic” Ca2+ without altering contractility. The caveolae targeted LTCC blocker was generated by molecular modification of Rem, a member of the RGK GTPase family that is known to potently inhibit LTCCs12. Expressing wild type Rem in cardiac myocytes blocked ICa-L and this inhibition required a c-terminal membrane-docking domain13. Truncation of this membrane-docking domain (Rem1-265) resulted in the inability of Rem to traffic to the membrane and to inhibit ICa-L. To specifically block caveolae-based LTCCs, the membrane-binding motif of native Rem was deleted and replaced with a canonical caveolin targeting domain sequence14, to create Rem1-265-Cav. Rem1-265-Cav targeted selectively to Cav-3 membranes, did not alter myocyte contractility, but blocked Ca2+-influx mediated activation of Cn-NFAT signaling. These data strongly support the hypothesis that LTCCs housed in Cav-3-containing microdomains is a source of “hypertrophic” Ca2+.
Methods
All methods have been described in detail in previously7,15-17 and an in depth account is available in the Online Data Supplement.
Adult feline left ventricular myocytes (AFLVMs) were isolated15 and adenoviral gene transfer was used to introduce WT-Rem, Rem1-265, Rem1-265-Cav, and/or NFATc3-GFP17, all at a multiplicity of infection (MOI) of 100. Myocytes were cultured for a period of up to 4 days. Localization of AdRem constructs was confirmed using standard sucrose gradient fractionation and caveolin-3 immunoisolation techniques16.
Results
Rem1-265-Cav Localizes to Caveolae
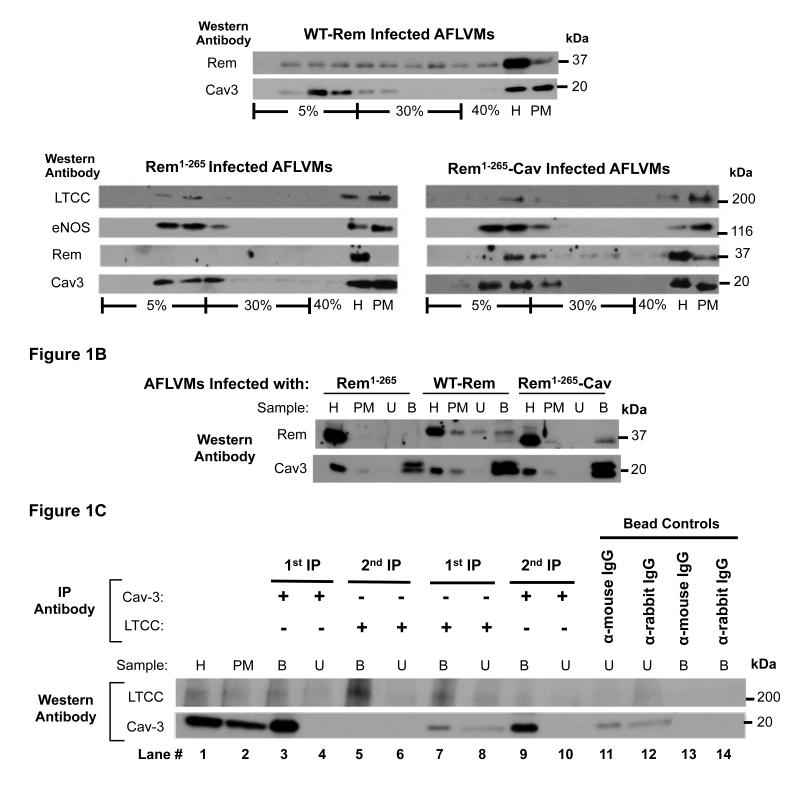
Native Rem was not detected in plasma membrane (PM) from AFLVMs. After adenoviral infection, WT-Rem was found in all AFLVM membrane fractions, consistent with the idea that it is in all PM domains. The Rem1-265 peptide lacking the membrane association motif remained in the general cell homogenate (H). Rem1-265-Cav localized to PM specifically within caveolin-containing lipid rafts (Figure 1A), verifying target specificity of the peptide containing the Cav binding motif. Rem1-265-Cav did not alter the normal raft/caveolae targeting of LTCCs, eNOS and caveolin-3 which demonstrates that Rem1-265-Cav does not displace molecules normally found in caveolae (Figure 1A). To prove that Rem1-265-Cav specifically localizes to caveolae, rather than lipid rafts in general, caveolae organelles were immunoaffinity purified16. Similar to our observation in raft membranes, only Rem1-265-Cav was seen in association with caveolin-3 (Figure 1B). These data show that Rem1-265-Cav specifically traffics to Cav-3 membrane microdomains.
Figure 1. Rem1-265-Cav and Cav1.2 distribution in plasma membranes.
(A) WT-Rem was in all plasma membrane (PM) populations. Rem1-265-Cav localized specifically within caveolin-containing lipid rafts. Rem1-265 was not in PMs and remained in the general cell homogenate (H). Rem1-265-Cav did not alter the normal raft/caveolae targeting of Cav1.2, eNOS and caveolin-3. (B) Caveolae organelles were immunoaffinity purified with anti-Cav-3. Rem1-265-Cav targeted specifically to caveolae whereas WT-Rem was both in caveolae (bound fraction) and in membrane samples depleted of caveolae (unbound fraction). Truncated Rem1-265 was not found in PMs. (C) To estimate the fraction of Cav1.2 localized to caveolae versus non-caveolae PMs, a Cav-3 IP was performed (lane 3). The unbound fraction (lane 4) from the Cav-3 IP was re-immunoprecipitated for Cav1.2 (lane 5). To estimate the fraction of caveolae that contain Cav1.2, PM preparations were immunoprecipitated for Cav1.2 (lane 7). The resulting unbound fraction (lane 8) was then re-immunoprecipitated for Cav-3 (lane 9) and the intensity of the bands in both fractions was compared. (U=unbound fraction, B=bound fraction, “bead controls” test for non-specific binding to the solid support used for immunoisolation of caveolae vesicles)
To further assess what fraction of LTCCs localizes to caveolae membrane domains, PM samples were purified from AFLVMs and subjected to immunoaffinity isolation with anti-Cav-3 (Figure 1C). The unbound fraction from the Cav-3 IP was then re-immunoprecipitated for Cav1.2 (Figure 1C). Densitometric analysis (n=3) showed that 26.2+/-12.7% of LTCCs resides within caveolae. To estimate the fraction of caveolae that contain LTCCs, AFLVM PM preparations were immunoprecipitated with an antibody for Cav1.2 (LTCC). The resulting unbound fraction was then re-immunoprecipitated for Cav-3 and the intensity of the bands in both fractions were compared. Only 13.4 +/− 10.1% (n=3) of caveolae PM contained LTCCs.
Rem1-265-Cav Blocks a Small Fraction of ICa,L and has no effect on Contractility
WT-Rem almost fully eliminated the L-type Ca2+ channel current (ICa,L) in AFLVMs at an MOI of 100 (Figure 2A). Rem1-265 had no significant effect on ICa,L. Rem1-265-Cav caused a small inhibition (about 15%) of ICa,L (Figure 2A). The smaller ICa,L block with Rem1-265-Cav versus WT-Rem suggests that only a small fraction of LTCCs are localized to Cav-3 signaling microdomains.
Figure 2. Rem1-265-Cav blocks a small fraction of ICa,L without reducing Contractility.
(A) WT-Rem (MOI of 100) almost completely abolished ICa,L while at equal MOI, Rem1-265-Cav inhibited only a small fraction of the current. Rem1-265 had no effect on ICa,L. Rem1-265-Cav infected myocytes responded normally to Iso. Myocyte contractions (B) and Ca2+ transients (C) were measured 36-48 hours after infection. Rem1-265-Cav had no significant effect on fractional shortening while WT-Rem almost completely abolished contraction. Rem1-265 had no effect. Rem1-265-Cav had no significant effect on Ca2+ transients (n=12-16). *** p<0.001
Inhibition of ICa,L with WT Rem eliminates LTCC regulation by catecholamines12. AFLVMs infected with Rem1-265-Cav responded normally to Isoproterenol (Figure 2A) while those infected with WT-Rem failed to respond (not shown). Myocytes infected with WT-Rem had markedly reduced fractional shortening (1.1+/-0.1% resting cell length) while those infected with truncated Rem1-265 (5.0+/-0.5%) and Rem1-265-Cav (4.4+/-0.4%) had contractions that were not significantly smaller than controls (4.7+/-0.5%) (Figure 2B). [Ca2+]i transients were also unaffected by Rem1-265-Cav (figure 2C). Collectively, these data support the idea that Rem1-265-Cav blocks a small fraction of Cav-3 associated LTCCs without significantly effecting myocyte excitation contraction coupling (ECC).
Rem1-265-Cav inhibits the β2-Adrenergic Regulation of LTCCs
Myocyte Cav-3 membrane domains are thought to contain both LTCCs and β2-adrenergic receptors11. The β2-adrenergic receptor specific agonist Zinterol in the presence of the β1-adrenergic receptor specific antagonist CGP increased ICa,L and fractional shortening in AFLVMs. This effect was inhibited by Rem1-265-Cav (Figure 3), suggesting that Rem1-265-Cav can selectively block Ca2+ entry through β2-adrenergic receptor regulated LTCCs within Cav-3 signaling microdomains.
Figure 3. β2AR Signaling is Inhibited by Rem1-265-Cav.
Zinterol in the presence of the β1AR specific antagonist CGP increased ICa-L (A; n=7) and fractional shortening (B; n=8). Rem1-265-Cav inhibited these effects. * p<0.05
Rem1-265-Cav Inhibits NFAT Translocation to the Nucleus
AFLVMs infected with AdNFATc3-GFP or AdNFATc3-GFP and AdRem1-265-Cav were electrically quiescent in culture. AFLVMs maintain low levels of cytosolic [Ca2+] with little or no spontaneous SR Ca2+ release9 and NFATc3-GFP is localized to the cytoplasm17. NFAT localization (cytoplasmic versus nuclear) was measured before (Figure 4A and C) and after (Figure 4B and D) 1 Hz pacing for 1 hour. Pacing caused NFAT to translocate from the cytoplasm to the nucleus in control AFLVMs (Figure 4B). AFLVMs infected with Rem1-265-Cav had normal contractions and Ca2+ transients (shown above) but more than 90% of NFAT nuclear translocation was inhibited (Figure 4E and F). These results were confirmed in experiments in which bath Ca2+ was raised to 4 mM, which also induced NFAT to translocate to the nucleus in control cells. Rem1-265-Cav inhibited NFAT translocation under these conditions (data not shown).
Figure 4. Rem1-265-Cav Inhibits Pathological Hypertrophic Signaling.
AFLVMs were infected with AdNFAT-GFP (A, B, E) or AdNFAT-GFP and AdRem1-265-Cav (C, D, F). NFAT-GFP translocation to the nucleus was measured before (A,C) and after (B,D) pacing at 1 Hz for 1 hour. Average data from 5 experiments is shown in E and F.
Discussion
Ca2+-Calmodulin (CaM) dependent activation of Cn-NFAT2, 3 produces pathological hypertrophy. Activation of this signaling cascade requires an increase in myocyte [Ca2+]. “Contractile” [Ca2+] does not appear to activate this hypertrophic process6.
The sources of “hypertrophic” [Ca2+] to activate Cn-NFAT signaling are not clearly defined. There is some evidence for a role for the LTCC6, 7, however, there is also evidence for Ca2+ entry through T-type Ca2+ channels (TTCC)8, 9 and TRP channels10. The role of TTCCs is controversial since some studies suggest that this pathway is anti-hypertrophic8 rather than pro-hypertrophic9. The observation that excess Ca2+ influx through TTCCs channels is anti-hypertrophic8 suggests that Ca2+ influx per se is not pro- or anti-hypertrophic. Rather the pathway for Ca2+ influx and possibly the localization of this pathway appear to be critical.
The present experiments suggest that a small number of LTCCs are localized in a fraction of Cav-3 containing membranes to form a signaling microdomain that can activate Cn-NFAT signaling. Our results are consistent with a recent study suggesting that a subpopulation of LTCCs in Cav-3 containing membranes are housed with AKAP150, which binds Cn18. These Cav-3 localized LTCCs do not participate in ECC or the regulation of “contractile” Ca2+. Our Cav-3 targeting strategy was able to selectively traffic an LTCC-blocking Rem protein (Rem1-265-Cav) to this signaling domain to block hypertrophic signaling without significantly reducing contraction or β1AR regulation of contractility. Our results suggest that any LTCCs associated with the small amount of Cav-3 others have found within T-tubules do not participate in ECC19.
Our results also show that not all Cav-3 containing membrane domains contain LTCCs, suggesting that hypertrophic signaling may take place in highly specialized signaling complexes. The signaling partners within these domains and their regulation in health and disease needs to be determined in future studies.
LTCCs are the major Ca2+ influx pathway in the adult mammalian heart and these channels are regulated biosensors that determine myocyte contractility and cardiac pump function1. Our results suggest that nature uses a subpopulation of these channels, housed away from ECC signaling domains, as a source of Ca2+ to modulate myocyte size when the heart is subjected to stress. Selective block of this Ca2+ influx pathway by the novel reagents developed in this study might provide an approach to reduce pathological hypertrophy without reducing cardiac contractility.
Supplementary Material
Novelty and Significance.
What is Known?
Pathological cardiac stressors such as hypertension and myocardial infarction activate signaling cascades that lead to cardiac myocyte hypertrophy.
Increases in myocyte [Ca2+]i are essential to initiate hypertrophic signaling through the cytoplasmic protein phosphatase Calcineurin (Cn).
The cytoplasmic [Ca2+]i transient is initiated by Ca2+ influx through L-type Ca2+ channels (LTCC) and drives cardiac contraction. This “contractile” [Ca2+]idoes not appear to be the source of [Ca2+] to initiate pathological hypertrophy.
What New Information Does This Article Contribute?
A small population of LTCCs is housed in Caveolin (Cav)-3 containing membranes (Caveolae) and is not involved in the regulation of contractile [Ca2+]i.
An LTCC-blocking protein can be trafficked to Cav-3 containing membranes to selectively block LTCCs in this signaling microdomain.
Block of Ca2+ entry through LTCCs within caveolae abolished activation of the Cn signaling pathway that is known to cause pathological hypertrophy.
There is strong epidemiological evidence to suggest that pathological cardiac hypertrophy predisposes patients to adverse cardiac events and to heart failure. Increases in [Ca2+]i are necessary to activate pathological hypertrophy, but the source of this Ca2+ appears to be distinct from the [Ca2+]i that is required for cardiac contraction. This study shows that a small population of LTCCs in a specialized signaling microdomain can be a source of Ca2+ to activate hypertrophic signaling. We developed a novel reagent that selectively blocks “hypertrophic” Ca2+ entry through Cav-3-based channels without blocking the LTCCs that induce and regulate “contractile” Ca2+. These experiments suggest that selective block of LTCCs in Cav-3 based signaling microdomains could reduce pathological hypertrophy without causing adverse negative inotropic effects.
Acknowledgments
Sources of funding: SRH-NIH Grants R01HL089312, T32HL091804, P01HL091799, R37HL033921, JDM-NIH Grants R01HL089312, R01HL62927, P01HL080101 CAM-AHA Pre-Doctoral Fellowship, RNC-NIH Post-Doctoral Fellowship
Non-Standard Abbreviations
- LTCC
L-Type Calcium Channel
- Cav-3
Caveolin-3
- Cn
Calcineurin
- NFAT
Nuclear Factor of Activated T cells
- CaMKII
Calmodulin-Dependent Protein Kinase
- PKC
Protein Kinase C
- TRP
Transient Receptor Potential
- ECC
Excitation Contraction Coupling
- RyR2
Ryanodine Receptor
- CICR
Ca2+-Induced-SR Ca2+-Release
- AFLVM
Adult Feline Left Ventricular Myocyte
- MOI
Multiplicity of Infection
- CaM
Ca2+-Calmodulin
- TTCC
T-Type Calcium Channel
Footnotes
Disclosures: The Authors have no disclosures
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual Review of Physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochemical and Biophysical Research Communications. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 3.Molkentin JD, Lu J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. Cam kinase signaling induces cardiac hypertrophy and activates the mef2 transcription factor in vivo. Journal of Clinical Investigation. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local insp3-dependent perinuclear ca2+ signaling in cardiac myocyte excitation-transcription coupling. The Journal of Clinical Investigation. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. The Journal of clinical investigation. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. Journal of molecular and cellular cardiology. 2011;50:460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakayama H, Bodi I, Correll RN, Chen X, Lorenz J, Houser SR, Robbins J, Schwartz A, Molkentin JD. Alpha1g-dependent t-type ca2+ current antagonizes cardiac hypertrophy through a nos3-dependent mechanism in mice. The Journal of clinical investigation. 2009;119:3787–3796. doi: 10.1172/JCI39724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx induced sarcoplasmic reticulum ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circulation Research. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 10.Eder P, Molkentin JD. Trpc channels as effectors of cardiac hypertrophy. Circulation research. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 11.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac ltype ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Marx SO, Colecraft HM. Molecular mechanisms, and selective pharmacological rescue, of rem-inhibited cav1.2 channels in heart. Circulation research. 2010;107:620–630. doi: 10.1161/CIRCRESAHA.110.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correll RN, Pang C, Finlin BS, Dailey AM, Satin J, Andres DA. Plasma membrane targeting is essential for rem-mediated ca2+ channel inhibition. The Journal of biological chemistry. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Journal of Biological Chemistry. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 15.Silver LH, Hemwall EL, Marino TA, Houser SR. Isolation and morphology of calcium-tolerant feline ventricular myocytes. American Journal of Physiology. 1983;245:H891–896. doi: 10.1152/ajpheart.1983.245.5.H891. [DOI] [PubMed] [Google Scholar]
- 16.Carlile-Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization in response to par1 stimulation is mediated by membrane rafts but not caveolae. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H366–H375. doi: 10.1152/ajpheart.01044.2006. [DOI] [PubMed] [Google Scholar]
- 17.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. Camkii negatively regulates calcineurin-nfat signaling in cardiac myocytes. Circulation research. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of akap5 with a subpopulation of l-type calcium channels / novelty and significance. Circulation Research. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scriven DRL, Klimek A, Asghari P, Bellve K, Moore EDW. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophysical Journal. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.