Abstract
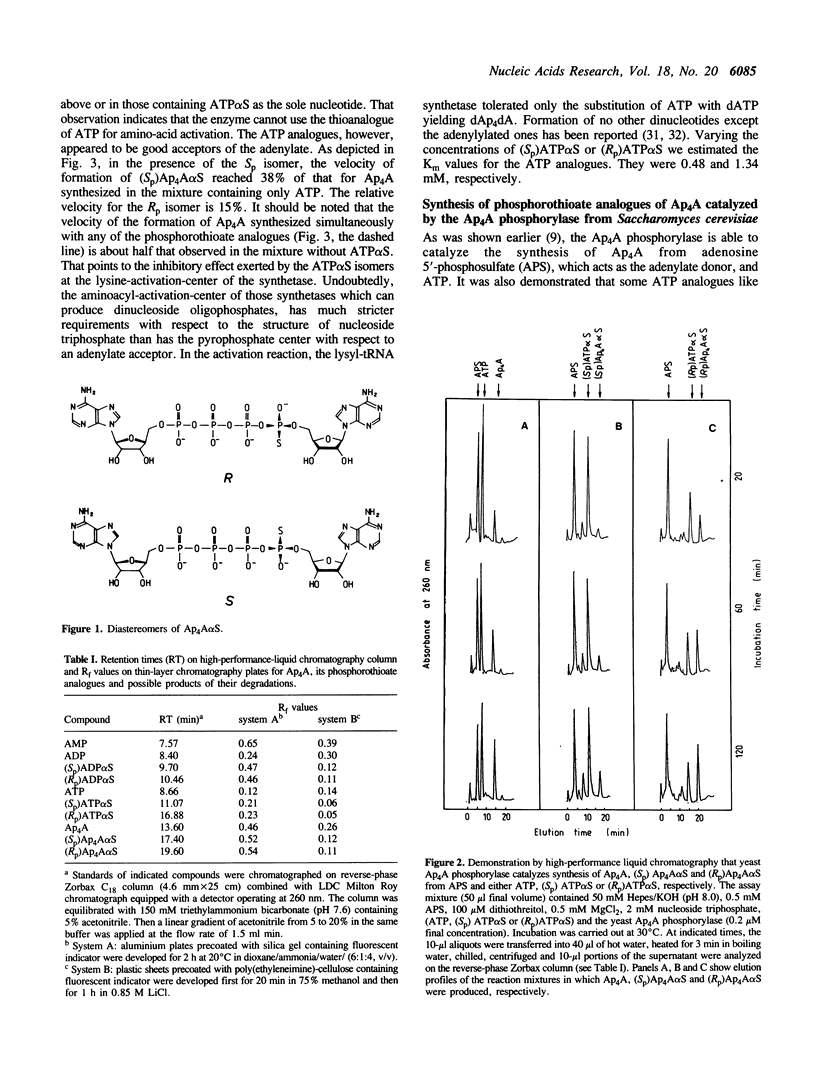
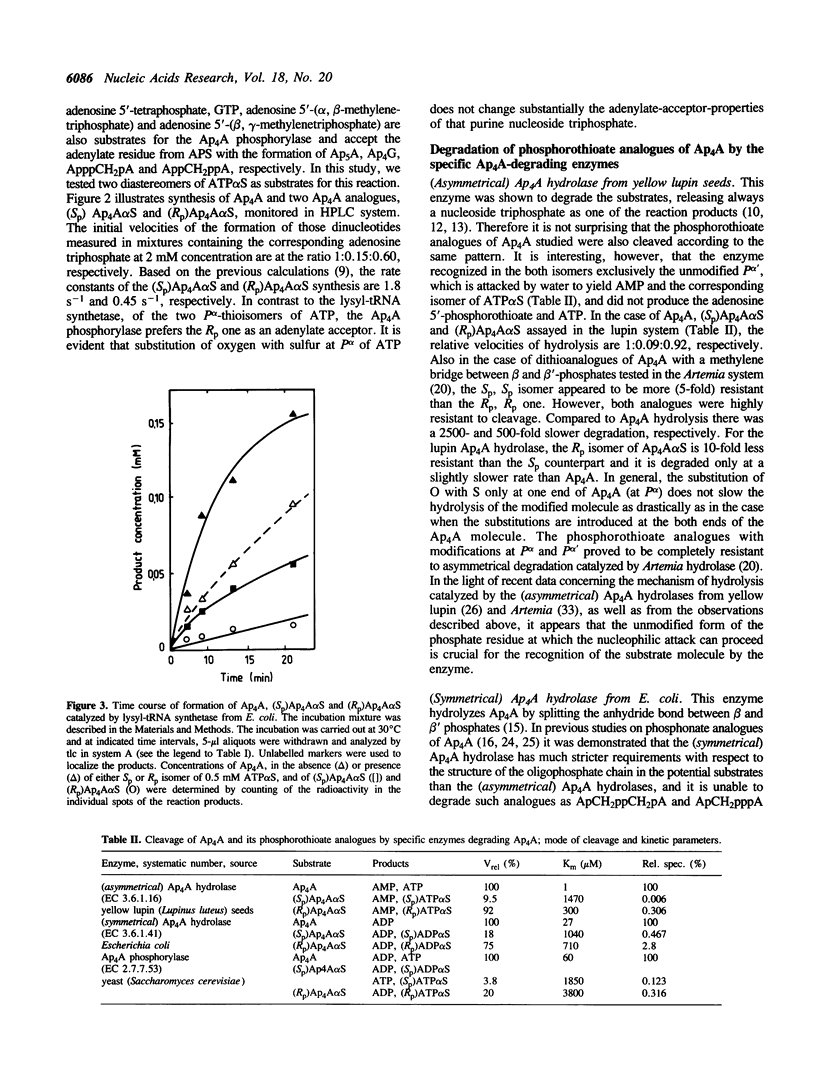
Synthesis of Sp and Rp diastereomers of Ap4A alpha S has been characterized in two enzymatic systems, the lysyl-tRNA synthetase from Escherichia coli and the Ap4A alpha, beta-phosphorylase from Saccharomyces cerevisiae. The synthetase was able to use both (Sp)ATP alpha S and (Rp)ATP alpha S as acceptors of adenylate thus yielding corresponding monothioanalogues of Ap4A,(Sp) Ap4A alpha S and (Rp)Ap4A alpha S. No dithiophosphate analogue was formed. Relative synthetase velocities of the formation of Ap4A,(Sp) Ap4A alpha S and (Rp)Ap4A alpha S were 1:0.38:0.15, and the computed Km values for (Sp)ATP alpha S and (Rp)ATP alpha S were 0.48 and 1.34 mM, respectively. The yeast Ap4A phosphorylase synthesized (Sp)Ap4A alpha S and (Rp)Ap4A alpha S using adenosine 5'-phosphosulfate (APS) as source of adenylate. The adenylate was accepted by corresponding thioanalogues of ATP. In that system, relative velocities of Ap4A, (Sp)Ap4A alpha S and (Rp)Ap4A alpha S formation were 1:0.15:0.60. The two isomeric phosphorothioate analogues of Ap4A were tested as substrates for the following specific Ap4A-degrading enzymes: (asymmetrical) Ap4A hydrolase (EC 3.6.1.17) from yellow lupin (Lupinus luteus) seeds hydrolyzed each of the analogues to AMP and the corresponding isomer of ATP alpha S; (symmetrical) Ap4A hydrolase (EC 3.6.1.41) from E. coli produced ADP and the corresponding diastereomer of ADP alpha S; and Ap4A phosphorylase (EC 2.7.7.53) from S. cerevisiae cleaved the Rp isomer only at the unmodified end yielding ADP and (Rp)ATP alpha S whereas the Sp isomer was degraded non-specifically yielding a mixture of ADP, (Sp)ADP alpha S, ATP and (Sp)ATP alpha S. For all the Ap4A-degrading enzymes, the Rp isomer of Ap4A alpha S appeared to be a better substrate than its Sp counterpart; stereoselectivity of the three enzymes for the Ap4A alpha S diastereomers is 51, 6 and 2.5, respectively. Basic kinetic parameters of the degradation reactions are presented and structural requirements of the Ap4A-metabolizing enzymes with respect to the potential substrates modified at the Ap4A-P alpha are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes L. D., Culver C. A. Isolation and characterization of diadenosine 5',5"'-P1,P4-tetraphosphate pyrophosphohydrolase from Physarum polycephalum. Biochemistry. 1982 Nov 23;21(24):6123–6128. doi: 10.1021/bi00267a015. [DOI] [PubMed] [Google Scholar]
- Biriukov A. P., Zakharova O. D., Lavrik O. I. Vliianie diadenozinoligofosfatov (Ap4A i Ap3A) i ikh fosfonatnykh analogov na kataliticheskie funktsii fenilalanil-tRNK-sintetazy iz E. coli. Bioorg Khim. 1987 Sep;13(9):1164–1169. [PubMed] [Google Scholar]
- Blackburn G. M., Taylor G. E., Thatcher G. R., Prescott M., McLennan A. G. Synthesis and resistance to enzymic hydrolysis of stereochemically-defined phosphonate and thiophosphate analogues of P1,P4-bis(5'-adenosyl) tetraphosphate. Nucleic Acids Res. 1987 Sep 11;15(17):6991–7004. doi: 10.1093/nar/15.17.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A., Coste H., Fromant M., Plateau P., Blanquet S. Yeast diadenosine 5',5'''-P1,P4-tetraphosphate alpha,beta-phosphorylase behaves as a dinucleoside tetraphosphate synthetase. Biochemistry. 1987 Jul 28;26(15):4763–4768. doi: 10.1021/bi00389a025. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. Absolute configuration of the diastereomers of adenosine 5'-O-(1-thiotriphosphate): consequences for the stereochemistry of polymerization by DNA-dependent RNA polymerase from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4798–4800. doi: 10.1073/pnas.75.10.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Traugh J. A. Phosphorylation of threonyl- and seryl-tRNA synthetase by cAMP-dependent protein kinase. A possible role in the regulation of P1, P4-bis(5'-adenosyl)-tetraphosphate (Ap4A) synthesis. J Biol Chem. 1989 Apr 5;264(10):5861–5865. [PubMed] [Google Scholar]
- Dixon R. M., Lowe G. Synthesis of (Rp,Rp)-P1,P4-Bis(5'-adenosyl)-1[17O,18O2],4[17O,18O2] tetraphosphate from (Sp,Sp)-P1,P4-Bis(5'-adenosyl)-1[thio-18O2], 4[thio-18O2]tetraphosphate with retention at phosphorus and the stereochemical course of hydrolysis by the unsymmetrical Ap4A phosphodiesterase from lupin seeds. J Biol Chem. 1989 Feb 5;264(4):2069–2074. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Romaniuk P. J., Connolly B. A. Stereochemistry of enzymic phosphoryl and nucleotidyl transfer. Methods Enzymol. 1982;87:197–212. doi: 10.1016/s0076-6879(82)87015-8. [DOI] [PubMed] [Google Scholar]
- Goerlich O., Foeckler R., Holler E. Mechanism of synthesis of adenosine(5')tetraphospho(5')adenosine (AppppA) by aminoacyl-tRNA synthetases. Eur J Biochem. 1982 Aug;126(1):135–142. doi: 10.1111/j.1432-1033.1982.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Guranowski A., Biryukov A., Tarussova N. B., Khomutov R. M., Jakubowski H. Phosphonate analogues of diadenosine 5',5'''-P1,P4-tetraphosphate as substrates or inhibitors of procaryotic and eucaryotic enzymes degrading dinucleoside tetraphosphates. Biochemistry. 1987 Jun 16;26(12):3425–3429. doi: 10.1021/bi00386a026. [DOI] [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Diadenosine 5',5'''-P1, P4-tetraphosphate alpha, beta-phosphorylase from yeast supports nucleoside diphosphate-phosphate exchange. J Biol Chem. 1986 May 5;261(13):5943–5946. [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Phosphorolytic cleavage of diadenosine 5',5'''-P1,P4-tetraphosphate. Properties of homogeneous diadenosine 5',5'''-P1,P4-tetraphosphate alpha, beta-phosphorylase from Saccharomyces cerevisiae. J Biol Chem. 1985 Mar 25;260(6):3542–3547. [PubMed] [Google Scholar]
- Guranowski A., Jakubowski H., Holler E. Catabolism of diadenosine 5',5"'-P1,P4-tetraphosphate in procaryotes. Purification and properties of diadenosine 5',5"'-P1,P4-tetraphosphate (symmetrical) pyrophosphohydrolase from Escherichia coli K12. J Biol Chem. 1983 Dec 25;258(24):14784–14789. [PubMed] [Google Scholar]
- Guranowski A., Just G., Holler E., Jakubowski H. Synthesis of diadenosine 5',5'''-P1,P4-tetraphosphate (AppppA) from adenosine 5'-phosphosulfate and adenosine 5'-triphosphate catalyzed by yeast AppppA phosphorylase. Biochemistry. 1988 Apr 19;27(8):2959–2964. doi: 10.1021/bi00408a044. [DOI] [PubMed] [Google Scholar]
- Guranowski A., Starzyńska E., Taylor G. E., Blackburn G. M. Studies on some specific Ap4A-degrading enzymes with the use of various methylene analogues of P1P4-bis-(5',5'''-adenosyl) tetraphosphate. Biochem J. 1989 Aug 15;262(1):241–244. doi: 10.1042/bj2620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler E., Holmquist B., Vallee B. L., Taneja K., Zamecnik P. Circular dichroism and ordered structure of bisnucleoside oligophosphates and their Zn2+ and Mg2+ complexes. Biochemistry. 1983 Oct 11;22(21):4924–4933. doi: 10.1021/bi00290a008. [DOI] [PubMed] [Google Scholar]
- Jakubowski H., Guranowski A. Enzymes hydrolyzing ApppA and/or AppppA in higher plants. Purification and some properties of diadenosine triphosphatase, diadenosine tetraphosphatase, and phosphodiesterase from yellow lupin (Lupinus luteus) seeds. J Biol Chem. 1983 Aug 25;258(16):9982–9989. [PubMed] [Google Scholar]
- Jakubowski H. Synthesis of diadenosine 5',5"'-P1,P4-tetraphosphate and related compounds by plant (Lupinus luteus) seryl-tRNA and phenylalanyl-tRNA synthetases. Acta Biochim Pol. 1983;30(1):51–69. [PubMed] [Google Scholar]
- Kobayashi Y., Kuratomi K. The binding activities of proteins that bind Ap4A, an alarmone, are stimulated in the presence of ethanol or phosphatidylethanolamine. Biochem Biophys Res Commun. 1989 May 15;160(3):1379–1386. doi: 10.1016/s0006-291x(89)80156-1. [DOI] [PubMed] [Google Scholar]
- Lobatón C. D., Vallejo C. G., Sillero A., Sillero M. A. Diguanosinetetraphosphatase from rat liver: Acitivity on diadenosine tetraphosphate and inhibition by adenosine tetraphosphate. Eur J Biochem. 1975 Jan 15;50(3):495–501. doi: 10.1111/j.1432-1033.1975.tb09888.x. [DOI] [PubMed] [Google Scholar]
- McLennan A. G., Prescott M., Evershed R. P. Identification of point of specific enzymic cleavage of P1,P4-bis(5'-adenosyl) tetraphosphate by negative ion FAB mass spectrometry. Biomed Environ Mass Spectrom. 1989 Jun;18(6):450–452. doi: 10.1002/bms.1200180615. [DOI] [PubMed] [Google Scholar]
- McLennan A. G., Taylor G. E., Prescott M., Blackburn G. M. Recognition of beta beta'-substituted and alpha beta,alpha'beta'-disubstituted phosphonate analogues of bis(5'-adenosyl) tetraphosphate by the bis(5'-nucleosidyl)-tetraphosphate pyrophosphohydrolases from Artemia embryos and Escherichia coli. Biochemistry. 1989 May 2;28(9):3868–3875. doi: 10.1021/bi00435a036. [DOI] [PubMed] [Google Scholar]
- Ogilvie A., Antl W. Diadenosine tetraphosphatase from human leukemia cells. Purification to homogeneity and partial characterization. J Biol Chem. 1983 Apr 10;258(7):4105–4109. [PubMed] [Google Scholar]
- Plateau P., Blanquet S. Zinc-dependent synthesis of various dinucleoside 5',5' ' '-P1,P3-Tri- or 5'',5' ' '-P1,P4-tetraphosphates by Escherichia coli lysyl-tRNA synthetase. Biochemistry. 1982 Oct 12;21(21):5273–5279. doi: 10.1021/bi00264a024. [DOI] [PubMed] [Google Scholar]
- Plateau P., Mayaux J. F., Blanquet S. Zinc(II)-dependent synthesis of diadenosine 5', 5"' -P(1) ,P(4) -tetraphosphate by Escherichia coli and yeast phenylalanyl transfer ribonucleic acid synthetases. Biochemistry. 1981 Aug 4;20(16):4654–4662. doi: 10.1021/bi00519a021. [DOI] [PubMed] [Google Scholar]
- Prescott M., Milne A. D., McLennan A. G. Characterization of the bis(5'-nucleosidyl) tetraphosphate pyrophosphohydrolase from encysted embryos of the brine shrimp Artemia. Biochem J. 1989 May 1;259(3):831–838. doi: 10.1042/bj2590831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Janeway C. M., Stephenson M. L., Zamecnik P. C. Isolation and characterization of dinucleoside tetra- and tri-phosphates formed in the presence of lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):98–105. doi: 10.1016/0006-291x(66)90416-5. [DOI] [PubMed] [Google Scholar]
- Stahl K. W., Schlimme, Eckstein F. Yeast hexokinase reaction with adenosine 5'-O-triphosphate and adenosine 5'-O-(1-thio-triphosphate) monitored by liquid chromatography. FEBS Lett. 1974 Apr 1;40(2):241–246. doi: 10.1016/0014-5793(74)80235-8. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]



