Abstract
We have developed an extremely sensitive technique, termed immuno-detection amplified by T7 RNA polymerase (IDAT) that is capable of monitoring proteins, lipids, and metabolites and their modifications at the single-cell level. A double-stranded oligonucleotide containing the T7 promoter is conjugated to an antibody (Ab), and then T7 RNA polymerase is used to amplify RNA from the double-stranded oligonucleotides coupled to the Ab in the Ab-antigen complex. By using this technique, we are able to detect the p185her2/neu receptor from the crude lysate of T6–17 cells at 10−13 dilution, which is 109-fold more sensitive than the conventional ELISA method. Single-chain Fv fragments or complementarity determining region peptides of the Ab also can be substituted for the Ab in IDAT. In a modified protocol, the oligonucleotide has been coupled to an Ab against a common epitope to create a universal detector species. With the linear amplification ability of T7 RNA polymerase, IDAT represents a significant improvement over immuno-PCR in terms of sensitivity and has the potential to provide a robotic platform for proteomics.
One of the central problems in cell biology and medicine relates to the inability to monitor cellular levels of proteins, lipids, sugars, and metabolite levels and their modifications. Traditional methodologies for protein detection and quantification include two-dimensional gel electrophoresis, mass spectrometry, and antibody (Ab) binding. Each methodology has been used to quantify protein levels from relatively large amounts of tissue, yet each suffers from a lack of sensitivity. In this regard, we believe that proteomics-oriented technology would ideally have single-cell resolution and be quantitative.
A variety of technologies have been used to improve the sensitivity of detecting these molecules. For example, the PCR technology has been combined with conventional immuno-detection methods (1). This technology, termed immuno-PCR, provides a sensitive method to detect proteins. In the general immuno-PCR approach, a linker molecule with bi-specific binding affinity for DNA and Ab is used to attach a marker DNA molecule specifically to an antigen-Ab complex, resulting in the formation of a specific antigen-Ab-DNA conjugate. The attached marker DNA can be amplified by PCR with appropriate primers. An approximate 105-fold increase in sensitivity over an alkaline phosphatase-conjugated ELISA was obtained. This approach and modifications of it have been used to detect a variety of antigens (2), including a human protooncogene protein (3) and detection of tumor necrosis factor α (4).
Although the immuno-PCR technique has some advantages over traditional methods of protein detection, including the aforementioned increase in sensitivity, there still exist several notable limitations to its use. One of the major limitations of immuno-PCR lies in the nonlinear amplification ability of PCR, which limits this technique as a quantitative detection method. Thus, there is no direct correlation between the amount of signal and the amount of protein present. Some of these problems have been overcome with a relatively isothermal rolling circle DNA amplification technique (RCA), which is an improvement over immuno-PCR (5).
We have developed a more facile and powerful method, termed immuno-detection amplified by T7 RNA polymerase (IDAT), that overcomes the limitations of the immuno-PCR technology, appears more sensitive than RCA, is far easier to use, and provides a robust quantifiable proteomics platform. T7 RNA polymerase is well known for its ability to bind to its promoter and proceed at a rate of ≈100 base/sec to create RNA molecules from substrates. Multiple T7 enzymes bind in a consecutive and progressive manner and T7 binding to RNA products does not occur. This enzymatic behavior assures a linear amplification solely dependent on the number of templates (6–8). IDAT combines the specificity of antigen–Ab interactions with the sensitivity powered by the liner amplification ability of T7 RNA polymerase. Our basic protocol configuration is similar to that used in the sandwich ELISA.
We used a single detector Ab conjugated with double-stranded oligonucleotides (ds-oligo). The ds-oligo contains the T7 promoter. RNA polymerase is then used to amplify the oligonucleotide. After electrophoresis, the presence of oligo-RNA transcripts of the appropriate molecular size demonstrates that ds-oligos are attached specifically to antigen-Ab complexes indicating the presence of specific antigen. The density of the band quantitatively reflects the absolute abundance of the Ab-bound antigen in the cell lysate because the T7 amplification procedure is linear. We also have used the same general approach in developing a fluorescence-based modification of the radiometric technique, which provides linear quantitative signals (data not shown).
The IDAT method eliminates the necessity of changing temperatures at all during immuno-PCR, which makes this method more facile and straightforward to use. Moreover we have adapted this technology by using intact Abs, single-chain fragment variable (ScFv) fragments as well as exocyclic peptide-based complementarity determining region (CDR) subunits as antigen detectors. The use of smaller Ab-binding units and fragments in addition to the already existing large single chain (reviewed in ref. 9) or cyclic peptide libraries (reviewed in ref. 10) and the use of robotic assistance makes it likely that this technology will have widespread use in research and medicine.
Materials and Methods
Cells.
B104–1-1 cells were derived from NIH 3T3 cell by expressing rat oncogenic p185neu, and the expression level of p185neu in B104–1-1 cells was previously determined by 125I-labeled anti-neu mAb-binding assay (11). NR6, negative for both epidermal growth factor receptor (EGFR) and p185neu, was a subclone from NIH 3T3. T6–17, a kind gift from J. H. Pierce (National Institutes of Health) is derived from NIH 3T3 by overexpressing the human p185her2/neu receptor (12, 13). These cell lines all were cultured in DMEM containing 10% FBS (HyClone) at 37°C in a 5% CO2 atmosphere.
Abs.
mAbs 7.16.4 and A11 are reactive with different epitopes in the extracellular domain of p185neu. The 7.16.4 Ab also binds to the human p185her2/neu form with reduced affinity (14). 1E1 is an IgG1 mAb generated against the ectodomain of human p185her2/neu and does not bind rat p185neu. rhuMAb 4D5 (Herceptin) was purchased from Genentech. The antiphosphotyrosine mAb PY99 was obtained from Santa Cruz Biotechnology.
Attachment of ds-oligo to Ab or FV or CDR.
A ds-oligo of the following sequence (GAGAGAGGATCCAAGTACTAATACGACTCACTATAGGGCCGAGAGCCGAGAAGAAAGACGTTTTTTTTT) was designed with an amine at the 5′ end to allow for covalent coupling to primary amines and a T7-promoter site (TAATACGACTCACTATAGGG) used to direct the synthesis of RNA from the cDNA template through the enzymatic activity of T7 RNA polymerase. For attachment of 1 μg of Ab to 30 μg of dscDNA, or 1 μg of CDR to 0.1 μg of dsDNA, or 1 μg of ScFv to 3 μg of dsDNA, an equal volume of 0.1% glutaraldehyde was added in 10-μl aliquot. The solution was mixed on a rotation device for 3 h at room temperature. Ethanolamine (1 M, 1/20 vol, pH 7.5) then was added. The solution was mixed for additional 2 h at room temperature. The protein-DNA complex was stored at 4°C.
RNA Amplification.
RNA amplification was performed in 96-well plates containing the first Ab-antigen complex. The following reagents were added: l × RNA amplification buffer (40 mM Tris-base, pH 7.5/7 mM MgCl2/10 mM NaCl/2 mM Spermidine/5 mM DTT/250 μM of each ATP, UTP, and GTP/5 μM CTP/0.5 μl RNAsin (Promega)/1,000 units of T7 RNA polymerase/3 μl [32P]CTP). The solution was then incubated for 4 h at 37°C. The RNA product was removed from the wells, and 3 μ1 was added to 3 μ1 of reaction stop solution (95% formamide/10 mM EDTA/0.1% bromophenol blue/0.1% xylene cyanol) and electrophoresed on a 15% denaturing polyacrylamide gel. The gel was exposed to a PhosphorImager (Molecular Dynamics) screen for 5–60 min and developed on a PhosphorImager.
Sandwich ELISA.
A total of 96-well microtiter plates (Nunc-Immuno Plate, MaxiSorp) were coated with the 1E1 (a mAb directed against the extra cellular domain of human p185her2/neu) by incubating plates overnight at 4°C with 100 μl of coating buffer (Ab concentration: 5 μg/ml). Plates then were washed three times with PBS containing 0.2% Tween 20 (PBS-T, 200 μl/well), blocked with PBS containing 0.5% FBS and 0.2% Tween 20 (200 μl/well) for 1 h at room temperature, and washed again three times with PBS-T (200 μl/well). In parallel, 100 μl of a serial dilution of T6–17 cell lysates were added to the plate and incubated for 2 h at room temperature. After this incubation step, plates were washed with PBS-T six times (200 μl/well) and incubated with humanized anti-p185her2/neu Ab 4D5 (150 ng/ml, 50 μl/well) for 2 h at room temperature. Subsequently, plates were washed six times and incubated with 50 μl of anti-human IgG-horseradish peroxidase (Zymed; final dilution 1:10,000) for another 2 h at room temperature. Enzymatic reactions were carried out at room temperature by adding 3,3′,5,5′-tetramethylbenzidine (TMB) (2.5 mM each in 0.1 M phosphate-citrate buffer, pH 5.0) (100 μl of each reagent/well). Reactions were stopped after 15–60 min by the addition of 50 μl of 1 M H2SO4. Color development was measured at 450 nm by using the Tecan microplate reader.
Immunoblotting Procedures.
Subconfluent cells in 10-cm dishes cells were washed twice with cold PBS and solubilized with the lysis buffer (1% Triton X-100/1% deoxycholate/0.1% SDS/0.15 M NaCl/0.01 M sodium phosphate, pH 7.4/1% aprotinin/1 mM PMSF/2 mM EDTA/10 mM sodium pyrophosphate/400 μM sodium orthovanadate/10 mM iodoacetamide) buffer. Proteins were separated by proper concentration of SDS/PAGE and transferred to nitrocellulose membranes (Nitrobind, Micron Separations, Westboro, MA). Membranes were incubated overnight with the blocking buffer (0.5% nonfat milk and 5% goat serum in PBS). For immunoblotting (Western blot) analysis, Abs were diluted in PBS containing 0.1% nonfat milk and 1% goat serum. All polyclonal sera and secondary horseradish peroxidase-conjugated Abs (Roche Molecular Biochemicals) were used at a 1:5,000 dilution. Bands were visualized by using ECL assay (Amersham Pharmacia).
Results
IDAT Is More Sensitive than Conventional Detection Methods Such as Western Blot and ELISA.
We illustrated the power of this approach by using the erbB receptor system we have been studying (15, 16). ErbB receptors are type 1 tyrosine kinases and are involved in cell growth differentiation and transformation. p185her2/neu is overexpressed in a number of human tumors including breast, ovarian, and pancreatic neoplasms (17–19). This receptor family develops transforming receptor ensembles by forming homo-oligomeric or hetero-oligomeric assemblies (20–22).
We began these efforts by using the humanized mAb 4D5 (trade name Herceptin from Genentech) and conjugated it with a ds-oligo sequence (see detailed sequence in Materials and Methods). 4D5 was derived against the human p185her2/neu (23). This Ab recognizes the native structure of p185her2/neu and has been used in immunoprecipitation and fluorescence-activated cell sorter analysis. Initially we coated a microplate with a second mAb 1E1, which is also specific for p185her2/neu but recognizes a different epitope (data not shown). A serial dilution of p185her2/neu-expressing T6–17 cell lysate was then incubated with the capture Ab 1E1. After extensive washing to remove the unbound antigen, 4D5-ds-oligo was added to the plate.
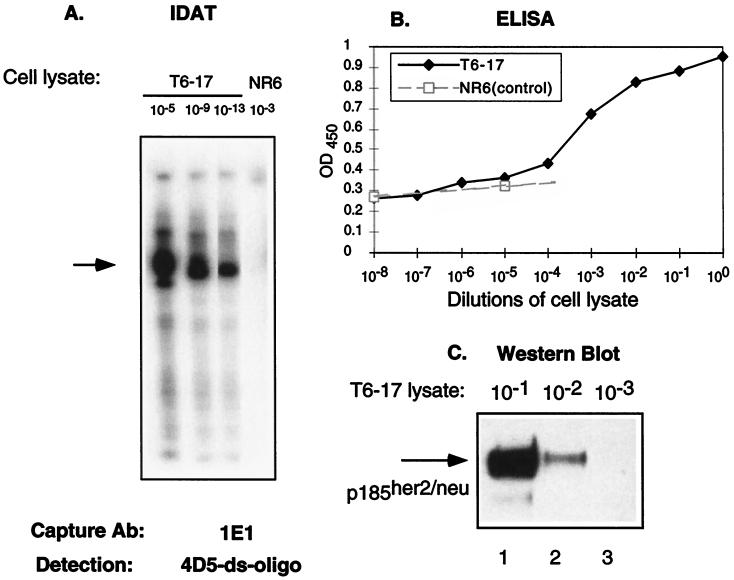
T7 RNA polymerase amplification with radioactive α-[32P]ATP was performed in the same plate. After running a denaturing urea-PAGE, we were able to detect signals from lysate at <10−13 dilution (Fig. 1A). We repeated this experiment several times and observed positive signals at 10−14, but not at 10−16 dilution (data not shown). In a parallel experiment, when using the conventional double-sandwich ELISA method with the same set of mAb (1E1 and 4D5), we found the detection limit for T6–17 cell lysate is 10−4 dilution (Fig. 1B). Similarly, when cell lysates were used for a Western blot analysis visualized by the widely used enhanced chemiluminescence reaction, the p185her2/neu band could be detected only in lanes of >10−2 dilution of lysates (Fig. 1C). Because only one-tenth of the amplification reaction in the IDAT was loaded for electrophoresis, and with exposure time ranging from 5 to 60 min, we calculated that IDAT was >109-fold higher in sensitivity than the most sensitive ELISA technique available.
Figure 1.
A comparison of the sensitivity of several assays. (A) IDAT assay in which lysates (1 mg/ml) from T6–17 or NR6 (p185her2/neu negative) cells were diluted as indicated and captured by the anti-p185her2/neu Ab 1E1. The ds-oligo-conjugated mAb 4D5 was used to detect the antigen and perform the amplification assay. Although we detected a positive band in T6–19 cells at 10−13 dilution, no positive band was noticeable in the control NR6 cells at 10−3 dilution. It should be noted that this type of RNA amplification can lead to smaller-sized signals because of premature T7 termination and RNA degradation. Such smaller-sized signals are apparent in some figures. (B) A conventional sandwich ELISA assay with 1E1 and 4D5 was performed on cell lysates. An anti-human IgG-horseradish peroxidase (Zymed) was used as the secondary Ab. Results from NR6 cell lysates also were included to show the baseline of the ELISA assay. The ELISA assay detects p185her2/neu readily only at 10−4, 10−3, 10−2, 10−1, and 100 dilutions. (C) Western blot assay. T6–17 cell lysate (lane 1, 10 μl; lane 2, 1 μl; lane 3, 0.1 μl, equal to 10−1, 10−2, and 10−3 dilutions, respectively, as in A and B) were loaded for 6% PAGE. A polyclonal anti-p185her2/neu Ab NEU from Santa Cruz (200 μg/ml) was used for Western blot at 1:2,000 dilution.
ScFv and CDR Peptides Also Can Be Used as the Detection Molecules.
Abs contain two heavy and two light chains. The antigen binding (or variable) regions of an Ab, each comprise a light and a heavy chain variable subdomain, and are generally known as the Fv regions. Fv fragments can be produced in mammalian cells (24, 25) or plants (26) by use of recombinant DNA technology. Martineau et al. (27) also have devised an expression system that enabled production of functional Fv fragments in Escherichia coli.
Previously we derived a mAb 7.16.4 against rat p185neu (15). A ScFv construct of 7.16.4 (designated as 7.16.4ScFv) was developed later (28). In this construct, the Fv region of the heavy chain and light chain region were joined by a (gly4-Ser)5 linker. Because the ScFv7.16.4 contained a polyhistidine tag, it was readily purified over Ni-nitrilotriacetic acid resin. After purification, the binding of 7.16.4ScFv to cell-bound p185 ectodomains was confirmed by fluorescence-activated cell sorter analysis on B104–1-1 cells, in both direct binding and then with competitive binding against the mAb 7.16.4 (data not shown).
Also recently, AHNP, a constrained exocyclic peptide designed from the CDR3.H region of the anti-human p185her2/neu Ab 4D5, was described. AHNP bound to p185her2/neu and mimics the growth-inhibitory effects of 4D5 (29). To explore the possibility of using the ScFv and CDR cyclic peptide as detection molecules, we also coupled the ScFv7.16.4 and AHNP to ds-oligo.
Conjugation with ds-oligo did not change the binding affinity of the CDR detection molecules with their antigens as determined by simultaneous plasmon resonance analysis (data not shown). Because 7.16.4ScFv and mAb 7.16.4 have comparable binding affinity against the p185neu receptor, they were used at similar molar concentration in the IDAT assay. However, ANHP was used at higher concentrations because its known affinity is lower for p185her2/neu than 4D5.
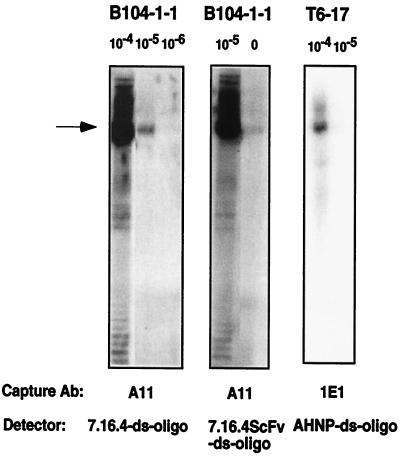
As shown in Fig. 2, both ds-oligo coupled 7.16.4ScFv and AHNP were able to detect antigens, rat p185neu from B104–1-1 cells and human p185her2/neu from T6–17 cells, respectively.
Figure 2.
Lysates from different cell lines (1 mg/ml) were diluted as indicated and captured by the anti-rat p185neu Ab A11 or the anti-human p185her2/neu Ab 1E1. The ds-oligo-conjugated mAb 7.16.4, single-chain 7.16.4ScFv, or the CDR3 peptide AHNP were used to detect the antigen and perform the amplification assay.
Universal Antigen Detector.
Coupling ds-oligo to a mAb directed against a general epitope on ScFv or CDR mimetics can create universal detection molecules. Because the ds-oligo have to be coupled directly to individual detection molecules (mAb or ScFv) as described above, we envisioned that it would be a time-consuming procedure if our purpose was to detect hundreds or thousands of antigens in a mass screening chip-based proteomic assay. In addition, variation in the coupling efficiency might complicate the interpretation of the amplification results. An alternative strategy to circumvent this problem is to couple ds-oligo to a mAb that is reactive against a general epitope embedded in the primary detection molecules. An example is the polyHis-tag in the 7.16.4ScFv initially designed for the purification of the protein. Fig. 3A demonstrates the results of the modified protocol.
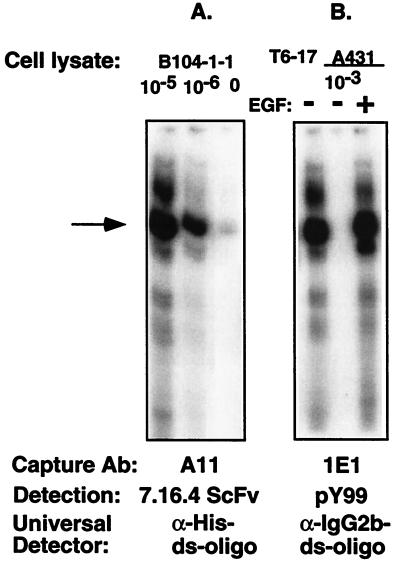
Figure 3.
Lysates from different cell lines (1 mg/ml) were diluted as indicated, and receptors were captured by the anti-rat p185neu Ab A11 or the anti-human p185her2/neu Ab 1E1. The 7.16.4ScFv and pY99 (a monoclonal antiphosphotyrosine) were used to probe the rat p185neu or the phosphorylated proteins, followed by the detection with the ds-oligo-conjugated anti-polyHis tag Ab or the anti-IgG2b subtype Ab, respectively. Although the p185her2/neu is constitutively phosphorylated in T6–17 cells, it is transactivated in A431 cells only after EGF stimulation. EGF stimulates heteromer formation of EGFR and p185her2/neu, which forms an active kinase complex.
After p185neu receptors were captured on the A11 coated on the plate, free 7.16.4ScFv was added, followed by extensive washing and then by incubation with T7 DNA-conjugated anti-His Ab. After T7 polymerase amplification, we detected specific RNA bands from 10−6 dilution of the lysate isolated from the cells. The sensitivity was even greater than that seen in the basic protocol with 7.16.4-oligo as the detection molecule (Fig. 2).
Application of IDAT in the Detection of Posttranslation Modifications.
The complexity of a tissue's protein complement is increased through posttranslational modification of existing proteins. It is critical to monitor these modifications because many processes, such as the phosphorylation of specific residues, are related to the functional status of the protein. Here we determined whether IDAT can detect the phosphorylation of the p185her2/neu receptor induced by epidermal growth factor (EGF) treatment and transphosphorylation by the EGF receptor (EGFR). Previously we established a signaling model in which, upon EGF stimulation, EGFRs homodimerize then heterodimerize with and trans-activate p185, resulting in the phosphorylation of specific tyrosine residues on the endodomain of the p185 receptor (20, 30–32).
We analyzed lysates from the A431 cell line, which overexpress both EGFR as well as p185her2/neu. After EGFR stimulation, the p185her2/neu receptor becomes trans-phosphorylated. The p185her2/neu polypeptide in the cell lysate was captured in a 96-well plate by 1E1, a new mAb that was recently developed against p185her2/neu. PY99, an IgG2b type of anti-phosphorylated Tyr Ab, was then used to detect phosphorylated receptors.
A second Ab, anti-IgG2b, coupled with ds-oligo, was used to probe the antigen-Ab sandwich complex. As shown in Fig. 5, A431 cells stimulated with EGF produced a positive band, that was not observed in cells without EGF treatment. T6–17 cells that overexpress p185her2/neu and have active tyrosine complexes of p185her2/neu homodimers also showed a positive band, indicating constitutive phosphorylation of the overexpressed p185 receptor complexes, as shown (33). These data indicate that IDAT is capable of detecting the functional status of a protein by analyzing even subtle posttranslational modifications.
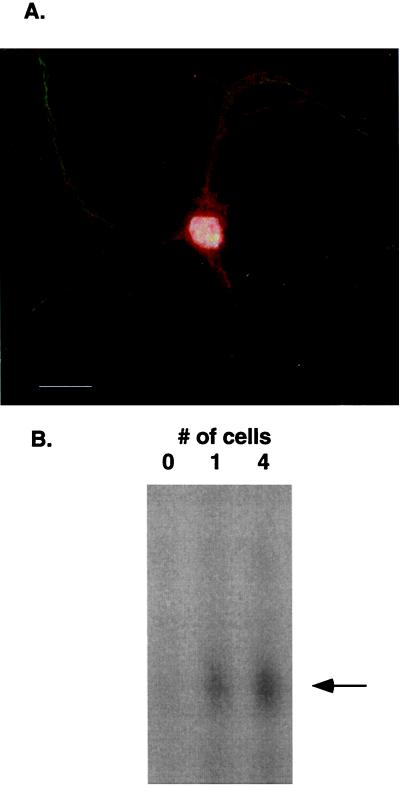
Figure 5.
(A) Rat hippocampal neurons in primary cell culture (36) were fixed in 4% paraformaldehyde in 100 mM Na cacodylate or PBS with 5 mM MgCl2 for 15 min at room temperature and permeablized in 0.1% Triton X-100 in PBS for 15 min at room temperature. Nonspecific interactions were blocked by incubation in 10% normal goat serum, 0.5% nonfat dried milk, 0.1% gelatin, 0.1% Tween-20 in PBS for 2 h at room temperature. Primary Ab was added at 1:150 dilution in blocking solution and incubated overnight at 4°C. Cy3-conjugated secondary Ab was used to visualize 7.16.4 staining (red). Dendritic processes are immuno-stained with MAP2 (green) whereas nuclei are counterstained with 4′,6-diamidino-2-phenylindole (white). (B) These are the IDAT results for lysates isolated from one cell or pooled from four individual cells. The IDAT procedure was as described; 0 cell means that media alone were aspirated into the pipette.
Serum Detection of p185her2/neu Ectodomain Shed at Early Stages of her2/neu Tumor Growth.
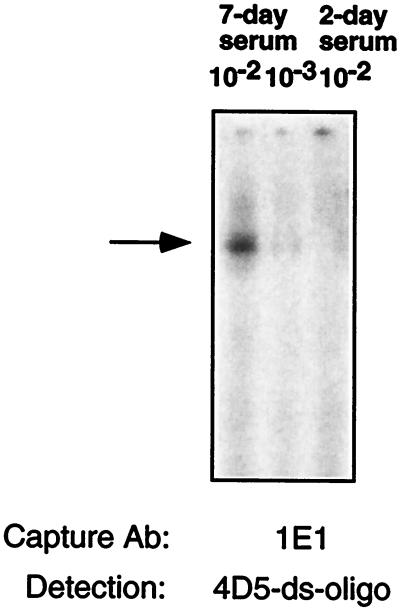
Several years ago it was noted that rodents carrying large p185-driven tumors shed the p185 receptor that could be detected in the sera (data not shown). This observation was extended by others to humans and is sometimes used in testing blood for evidence of p185 expressing tumors in advanced tumor-bearing states (reviewed in ref. 34). We wondered whether IDAT was sensitive enough to identify shed p185 in tumor-bearing mice. We injected one million T6–17 cells into the back of nude mice and analyzed the presence of shed p185 receptor fragments in 0.2-μl aliquots of sera taken 48 h, 7 days, and 14 days after tumor implantation. As shown in Fig. 4, we could detect a signal as early as 7 days. In other experiments with fresh serum samples, p185 receptor fragments can be detected from serum of day 2 after tumor implantation (data not shown).
Figure 4.
Serum from a nude mouse carrying p185her2/neu-derived tumors was first collected and diluted by 1:5 in PBS. The serum-PBS (1 μl) was then further diluted as indicated and captured by the anti-p185her2/neu Ab 1E1. The 10−2 dilution contains 0.2 μl of original serum. The ds-oligo-conjugated mAb 4D5 was used to detect the antigen and perform the amplification assay.
Detection of p185neu from Single Rat Hippocampal Pyramidal Neurons in Primary Cell Culture.
The mRNA complement from single cells can be harvested and quantified by using the antisense RNA (aRNA) amplification methodology (35). Although dilution studies of tissue homogenates suggested that we had single cell resolution of protein antigens (Fig. 1A), we wondered whether we also could achieve single-cell resolution by using the protein lysate isolated from a single cell with the IDAT methodology. Using the 7.16.4 mAb that is specific for rat p185neu, we performed immunohistochemistry on rat hippocampal neurons in primary cell culture to show that p185neu was present in these neurons. There is clear immunostaining of the cell body and dendrites of this primary neurons (Fig. 5A), confirming earlier results (15, 16). Next we preformed the IDAT assay on protein isolated from individual cells and groups of cells whose cytoplasmic extract was harvested by a microcapillary tube approach after visualization with microscopy. Using the anchoring Ab A11 and the 7.16.4 detector, we were able to visualize p185neu in the cell lysates from single cells as well as that from four pooled cells (Fig. 5B). The autoradiographic exposure for this gel was 3 h with 14% of the amplified sample loaded onto the gel. These data illustrate the robustness of the IDAT technology in detecting proteins that are present in single cells.
Discussion
A sensitive protein detection method that has direct application to the field of chip-based proteomics has been developed. By using T7 RNA polymerase as a signal generator, IDAT assays possess linear amplification and potential precise quantification, which are desirable in the development of biological and medical assays. Finally, this approach has been used with both radiolabels and fluorescence supporting its resilience and versatility.
The specific binding between any antigens and their Abs or Ab derivatives is the only limiting parameter of IDAT. ScFv and CDR species as well as intact Abs have been used. The extraordinary diversity of ScFv and cyclic CDR peptide libraries offers the opportunity to create binding moieties for virtually any molecule. Differences in Ab affinities have limited the use of some Abs for protein detection, yet the potent T7 amplification procedure associated with IDAT facilitate the use of even these low affinity Abs.
The advantages of using universal detection molecules in our protocol are many. First, virtually any cellular antigen can be detected without having coupled each mAb with a ds-oligo. Without the universal probe, the method would only be useful to look at one or several particular antigens at a time. The universal probe, on the other hand, allows for the detection of any cellular or fluid residing antigen that can be bound with available Abs or their fragments. In addition, with slight modification in the protocol, different proteins can be detected simultaneously in a single gel electrophoresis lane if oligonucleotides of different sizes are attached to detection Abs/ScFvs/CDR peptides.
We have demonstrated that IDAT is a versatile technique that is applicable in the identification of protein antigens as well as posttranslational modification of polypeptides or (by extension, although not shown here) any structure such as sugars or lipids at the single-cell level of detection. In addition, IDAT may have other potential applications, including the analysis of protein interactions and the detection of small molecules. For example, ligand peptides can be used as detection molecules on tissue samples to identify the expression of specific receptors, or verse visa. With available Abs or binding proteins, small molecules, such as toxins or drug metabolites, can be detected from water, food, or body fluid, etc.
Immuno-PCR in general has sensitivity from 2 to 3 orders of magnitude higher than ELISA. We used total cell lysates in the IDAT assay and demonstrated 1011- and 109-fold increase in the sensitivity over enhanced chemiluminescence-Western blot assay and ELISA assay, respectively, indicating that IDAT is perhaps the most sensitive assay system developed to date. Because our studies have shown that dilutions of >1013 still lead to obvious signals, it is likely that we are able to detect even a few copies of antigens in a mixture. Its ease of use and facile chemistry also distinguish it from the HPLC-, two-dimensional chromatography-, and mass spectrometry-based methods of protein detection, which are not as sensitive as IDAT and far more cumbersome and time consuming.
An array of epitopes recognized by the Fvs or CDR fragments can be shown to identify larger polypeptides or can be used to identify motifs in supernatants, fluids, extracts of cells or bacteria, or any other eukaryotic organism. The actual identity of the polypeptides an organic molecule or sugar structures then can be helped by computer-aided analysis of databases using the binding of several epitopes by scFvs as a guide. For example, binding by distinct scFv species might identify a sugar molecule as having particular carbohydrate side chains, therefore, suggesting that it may belong to a particular family of complex sugars. Alternatively, it may be possible to attach the DNA template to detect specific sugars.
In this way, the IDAT form of proteomics might allow definition and identification of many if not all molecules in a cell at any one particular time. Finally, the adaptation of fluorescence detection or RNA product hybridization should facilitate the development of robotics methods to create a high-throughput protein quantification methodology, perhaps as a chip-based system.
Acknowledgments
This work was supported by grants awarded to M.I.G. from the Abramson Cancer Institute and to J.E. from the National Institutes of Health.
Abbreviations
- IDAT
immuno-detection amplified by T7 RNA polymerase
- CDR
complementarity determining region
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ScFv
single chain fragment variable
- ds-oglio
double-stranded oligonucleotide
References
- 1.Sano T, Smith C L, Cantor C R. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 2.Ruzicka V, Marz W, Russ A, Gross W. Science. 1993;260:698–699. doi: 10.1126/science.8480182. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Fisher R J, Papas T S. Nucleic Acids Res. 1993;21:6038–6039. doi: 10.1093/nar/21.25.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanna P P, Weiss F, Samson M E, Bloom F E, Pich E M. Proc Natl Acad Sci USA. 1995;92:272–275. doi: 10.1073/pnas.92.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schweitzer B, Wiltshire S, Lambert J, O'Malley S, Kukanskis K, Zhu Z, F, Kingsmore S F, Lizardi P M, Ward D C. Proc Natl Acad Sci USA. 2000;97:10113–10119. doi: 10.1073/pnas.170237197. . (First Published August 22, 2000, 10.1073/pnas.170237197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Gelder R N, von Zastrow M E, Yool A, Dement W C, Barchas J D, Eberwine J H. Proc Natl Acad Sci USA. 1990;87:1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee M, Yang R, Hubbell E, Berno A, Huang X C, Stern D, Winkler J, Lockhart D J, Morris M S, Fodor S P. Science. 1996;274:610–614. doi: 10.1126/science.274.5287.610. [DOI] [PubMed] [Google Scholar]
- 8.Luo L, Salunga R C, Guo H, Bittner A, Joy K C, Galindo J E, Xiao H, Rogers K E, Wan J S, Jackson M R, Erlander M G. Nat Med. 1999;5:117–122. doi: 10.1038/4806. [DOI] [PubMed] [Google Scholar]
- 9.Barth S, Weidenmuller U, Tur M K, Schmidt M F, Engert A. J Mol Biol. 2000;301:751–757. doi: 10.1006/jmbi.2000.4038. [DOI] [PubMed] [Google Scholar]
- 10.Scott C P, Abel-Santos E, Wall M, Wahnon D C, Benkovic S J. Proc Natl Acad Sci USA. 1999;96:13638–13643. doi: 10.1073/pnas.96.24.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokai Y, Myers J N, Wada T, Brown V I, LeVea C M, Davis J G, Dobashi K, Greene M I. Cell. 1989;58:287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 12.Di Fiore P, Pierce J H, Kraus M H, Segatto O, King C R, Aaronson S A. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 13.Di Marco E, Pierce J H, Knicley C L, Di Fiore P P. Mol Cell Biol. 1990;10:3247–3252. doi: 10.1128/mcb.10.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Wang Q, Kajino K, Greene M I. DNA Cell Biol. 2000;19:253–263. doi: 10.1089/10445490050021168. [DOI] [PubMed] [Google Scholar]
- 15.Drebin J A, Stern D F, Link V C, Weinberg R A, Greene M I. Nature (London) 1984;312:545–548. doi: 10.1038/312545a0. [DOI] [PubMed] [Google Scholar]
- 16.Qian X, O'Rourke D M, Zhao H, Greene M I. Oncogene. 1996;13:2149–2157. [PubMed] [Google Scholar]
- 17.Slamon D J, Clark G M. Science. 1988;240:1795–1798. doi: 10.1126/science.3289120. [DOI] [PubMed] [Google Scholar]
- 18.Williams T M, Weiner D B, Greene M I, Maguire H C., Jr Pathobiology. 1991;59:46–52. doi: 10.1159/000163614. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J A, Weiner D B, More K F, Kokai Y, Williams W V, Maguire H C, Jr, LiVolsi V A, Greene M I. Oncogene. 1989;4:81–88. [PubMed] [Google Scholar]
- 20.Wada T, Qian X L, Greene M I. Cell. 1990;61:1339–1347. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 21.Stern D F, Kamps M P. EMBO J. 1988;7:995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzahar E, Yarden Y. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 23.Hudziak R M, Lewis G D, Winget M, Fendly B M, Shepard H M, Ullrich A. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brocks B, Rode H J, Klein M, Gerlach E, Dubel S, Little M, Pfizenmaier K, Moosmayer D. Immunotechnology. 1997;3:173–184. doi: 10.1016/s1380-2933(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 25.Li E, Pedraza A, Bestagno M, Mancardi S, Sanchez R, Burrone O. Protein Eng. 1997;10:731–736. doi: 10.1093/protein/10.6.731. [DOI] [PubMed] [Google Scholar]
- 26.Fiedler U, Phillips J, Artsaenko O, Conrad U. Immunotechnology. 1997;3:205–216. doi: 10.1016/s1380-2933(97)00014-6. [DOI] [PubMed] [Google Scholar]
- 27.Martineau P, Jones P, Winter G. J Mol Biol. 1998;280:117–127. doi: 10.1006/jmbi.1998.1840. [DOI] [PubMed] [Google Scholar]
- 28.Peterson N C, Greene M I. DNA Cell Biol. 1998;17:1031–1040. doi: 10.1089/dna.1998.17.1031. [DOI] [PubMed] [Google Scholar]
- 29.Park B W, Zhang H T, Wu C, Berezov A, Zhang X, Dua R, Wang Q, Kao G, O'Rourke D M, Greene M I, Murali R. Nat Biotechnol. 2000;18:194–198. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 30.Qian X L, Decker S J, Greene M I. Proc Natl Acad Sci USA. 1992;89:1330–1334. doi: 10.1073/pnas.89.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokai Y, Dobashi K, Weiner D B, Myers J N, Nowell P C, Greene M I. Proc Natl Acad Sci USA. 1988;85:5389–5393. doi: 10.1073/pnas.85.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murali R, Brennan P J, Kieber-Emmons T, Greene M I. Proc Natl Acad Sci USA. 1996;93:6252–6257. doi: 10.1073/pnas.93.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian X, Dougall W C, Hellman M E, Greene M I. Oncogene. 1994;9:1507–1514. [PubMed] [Google Scholar]
- 34.Sias P E, Kotts C E, Vetterlein D, Shepard M, Wong W L. J Immunol Methods. 1990;132:73–80. doi: 10.1016/0022-1759(90)90400-p. [DOI] [PubMed] [Google Scholar]
- 35.Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, Zettel M, Coleman P. Proc Natl Acad Sci USA. 1992;89:3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchhalter J R, Dichter M A. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]