Abstract
Frontotemporal dementia is commonly associated with parkinsonism in several sporadic (i.e., progressive supranuclear palsy, corticobasal degeneration) and familial neurodegenerative disorders (i.e., frontotemporal dementia associated with parkinsonism and MAPT or progranulin mutations in chromosome 17). The clinical diagnosis of these disorders may be challenging in view of overlapping clinical features, particularly in speech, language, and behavior. The motor and cognitive phenotypes can be viewed within a spectrum of clinical, pathologic, and genetic disorders with no discrete clinicopathologic correlations but rather lying within a dementia–parkinsonism continuum. Neuroimaging and cerebrospinal fluid analysis can be helpful, but the poor specificity of clinical and imaging features has enormously challenged the development of biological markers that could differentiate these disorders premortem. This gap is critical to bridge in order to allow testing of novel biological therapies that may slow the progression of these proteinopathies.
Keywords: Frontotemporal dementia, Frontotemporal lobar degeneration, Parkinsonism, Corticobasal syndrome, Progressive supranuclear palsy, Corticobasal degeneration
Introduction
Parkinsonism is characterized by the presence of slow (bradykinesia) and/or low-amplitude movements (hypokinesia) associated with either rigidity, tremor at rest, or postural instability. The parkinsonism may precede, coincide, or follow the behavioral or language-predominant cognitive impairments characteristic of frontotemporal dementia (FTD). FTD with parkinsonism are part of a growing number of pathologically and phenotypically separate frontotemporal lobar degenerations (FTLD). The clinical phenotypes include the “classical” presentation of progressive supranuclear palsy (PSP, Steele–Richardson–Olszewski syndrome), corticobasal syndrome, progressive akinesia and freezing of gait, and a number of additional clinical variants, representing a range of distinct pathologies (Table 1). The underlying pathologies of these phenotypes include PSP, corticobasal degeneration (CBD), Alzheimer’s disease (AD), tau-positive neuronal and glial inclusions FTLD (FTDP-tau), and tau-negative but ubiquitin-positive neuronal inclusions that are also TDP-43 positive (FTDP-U or FTDP-TDP; Mackenzie et al. 2009).
Table 1.
Parkinsonian phenotypes and relationship with FTLD
| Major motor features | Association with FTD/pathology | |
|---|---|---|
| FTD-P | Parkinsonian features are more common in FTDP-17T/MAPT than in FTDP-17U/PGRN | Strong–early/tauopathy |
| PSP (classical) | Postural instability and falls within the first year of disease, slowing of vertical saccades or supranuclear gaze palsy, pseudobulbar palsy, parkinsonism minimally or unresponsive to levodopa. Median survival is 6 years. | Strong–early/tauopathy Familial (FTDP-17) Sporadic H1 haplotype |
| PSP-PAGF/PPFG | Freezing of gait or speech, with no rigidity or tremor, with no sustained response to levodopa, and no dementia or ophthalmoplegia within the first 5 years. Median survival is 10 years. | Weak–late/tauopathy |
| PSP-PNFA and CBS-PNFA | Severe language deficits, primarily PNFA or apraxia of speech, accompany a hypokinetic-rigid syndrome suggestive of PSP (PSP-PNFA) or CBS (CBS-PNFA). | Strong–early/tauopathy |
| CBS | Markedly asymmetric parkinsonism with ideomotor apraxia, myoclonus, dystonic posture, alien limb syndrome, and sensory or visual neglect. | Moderate–late/tauopathy Familial (FTDP-17) sporadic H1 haplotype |
| FTD-MND | Rapidly progressive frontal dementia, aphasia, parkinsonism unresponsive to levodopa, and weakness of limbs and orofacial muscles. | Strong–early/TDP-43 proteinopathy (without PGRN mutation). |
| Perry syndrome | Levodopa-responsive parkinsonism, vertical gaze palsy, hypoventilation, weight loss, and depression or psychiatric symptoms. | Unclear/TDP-43 proteinopathy |
PAGF pure akinesia with gait freezing, PPFG primary progressive freezing gait, PNFA progressive nonfluent aphasia, MND motor neuron disease
PSP and CBD are the most common FTLD-tau motor phenotypes (Mackenzie et al. 2009; Cairns et al. 2007; Kovacs et al. 2008). MAPT and PGRN mutations may account for up to 40% of inherited cases of FTDP-tau and FTDP-TDP, respectively. FTD with motor neuron disease (MND), a clinical phenotype corresponding to FTDP-TDP pathology (Table 1), may present with rapidly progressive parkinsonism (disease course between 1 and 3 years; Cairns et al. 2007; Josephs et al. 2005).
Inherited FTLD-tau is associated with frontotemporal dementia and parkinsonism linked to chromosome 17 and MAPT gene mutations (FTDP-17T/MAPT; Hutton et al. 1998). Inherited FTLD-TDP comprises FTDP associated with progranulin (PGRN) gene mutations (FTDP-17U/PGRN; Baker et al. 2006), as well as Perry syndrome associated with dynactin 1 mutations (Farrer et al. 2009), inclusion body myopathy with Paget’s disease of the bone and frontotemporal dementia, associated with valosin-containing protein (VCP) gene mutations (Watts et al. 2004), and familial amyotrophic lateral sclerosis associated with TAR DNA-binding protein-43 (TARDBP) gene mutations (Rutherford et al. 2008). Parkinsonism has not been reported as a feature of the latter two. Mutations in either MAPT or PGRN account for 2–10% of all cases and 10–20% of familial cases (Rohrer et al. 2009). Parkinsonism in carriers of mutations in chromatin modifying protein 2B (FTD-3/CHMP2B) and fused in sarcoma (ALS-FTD/FUS), which more often causes amyotrophic lateral sclerosis (ALS) and psychosis, is very rare and has therefore not been well characterized.
Clinical Features
The classical PSP phenotype is characterized by a symmetric distribution of parkinsonian deficits, whereas asymmetry is characteristic of the corticobasal syndrome (CBS; Table 1). Early frontal executive disturbances and apathy are typically observed early in PSP, which overlaps with other FTDs (Yatabe et al. 2011). Similarly, while early vertical supranuclear palsy is typical of PSP, it can be seen in CBD. However, vertical supranuclear gaze palsy precedes the development of horizontal gaze palsy in PSP, whereas both horizontal and vertical supranuclear gaze palsy tend to occur simultaneously in CBD, typically preceded by an increase in saccade latency rather than a decrease in speed as well as some degree of ocular motor apraxia (Vidailhet et al. 1994; Rivaud-Pechoux et al. 2000). Greatly diminished blink rate and eyelid “apraxia,” a difficulty or slowness when opening or closing the eyelids, accompanied by compensatory elevation of the eyebrows and frontalis muscle overactivity, probably indicative of focal dystonia (Krack and Marion 1994), are also more common in PSP but potentially overlapping with pathology-proven CBD.
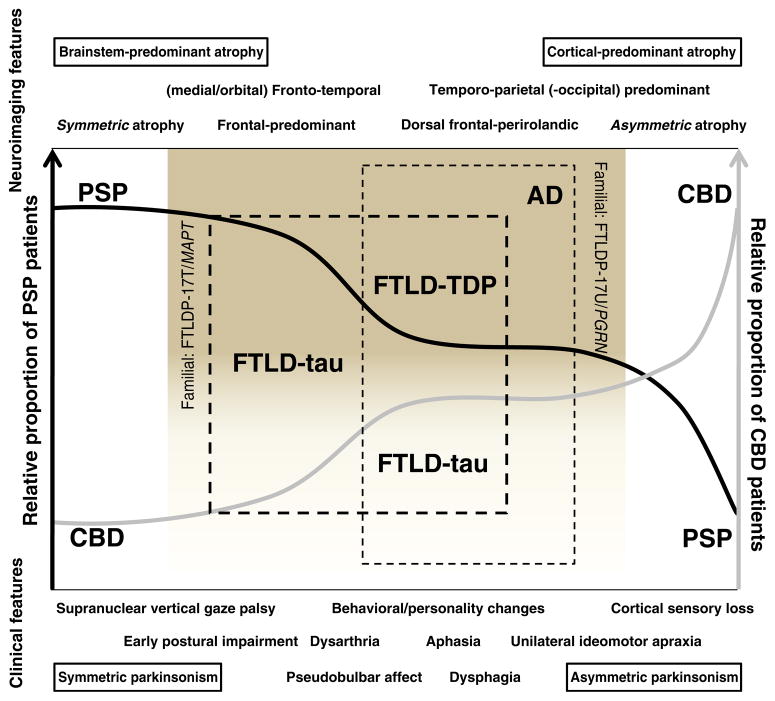
Major overlapping clinical deficits between PSP, CBD, and other FTDs are speech, swallowing, and language disorders (Fig. 1). PSP patients typically develop a hypokinetic–spastic dysarthria, but language impairments may also occur in the form of (fluent or nonfluent) aphasia with perseveration and anomia (Esmonde et al. 1996; Josephs and Duffy 2008). Prominent early or severe (especially nonfluent) aphasia and swallowing difficulties may also be present in CBS cases due to CBD pathology. Apraxia of speech, in particular, is strongly associated with underlying tau pathology (FTLD-Tau, CBD, and PSP) (Josephs et al. 2006a). Some clinical features may favor a specific tauopathy over another in the appropriate premortem clinical scenario. Clinicopathologic case series suggest that speech impairments in the form of isolated dysarthria favor a diagnosis of PSP but in the form of nonfluent aphasia or speech/orobuccal apraxia predict CBD (Table 2; Graham et al. 2003; Ozsancak et al. 2004).
Fig. 1.
The overlap between PSP, CBD, and FTLD. The diagnostic certainty of PSP (black line) and CBD (gray line) varies depending on the relative presence or absence of clinical (bottom) and neuroimaging features (upper), and can be diagrammatically represented as belonging to a clinicopathologic spectrum. The “classical” clinical presentation of PSP (early falls, supranuclear vertical gaze palsy) can occur in a small proportion of patients with pathology-proven CBD (leftmost end of the proposed spectrum). Less characteristic presentations, or with co-occurrence of behavioral or personality changes or bulbar/pseudobulbar features may fall within the spectrum of FTLD, most often FTLD-tau (thick hashed line). Conversely, the typical phenotype of CBD (unilateral ideomotor apraxia, cortical sensory loss) can be present in a small proportion of patients with pathology proven PSP (right end of the diagram) or, particularly if behavioral or language abnormalities coexist, in patients with such pathologies as AD (the most common non-CBD etiology in CBS; thin hashed line), FTLD-tau, or FTLD-TDP. Neuroimaging patterns in the left end of the diagrammatic spectrum tend to show brainstem-predominant (PSP) and symmetric atrophy (FTLD-tau), whereas in the right end of the spectrum tend to show asymmetric (FTLD-TPD, particularly due to PGRN mutations) and posterior predominant patterns of atrophy (in particular, the posterior cortical atrophy pattern is a feature of some forms of CBD and AD). As a caveat, however, no pattern of atrophy can reliably distinguish FTLD-tau from FTLD-TDP
Table 2.
Overlapping clinical features between PSP and CBS and the frequency of the reported pathologies
| PSP | CBS | |
|---|---|---|
| Cognitive impairment | Frontal dysexecutive, early | Frontal dysexecutive, late |
| Speech | Dysarthria, early | Dysarthria, late; apraxia of speech |
| Language | Fluent perserverative | Nonfluent (PNFA) |
| Saccades | Normal latency but slow velocity and decreased amplitude (hypometric) | Delayed latency but normal velocity and amplitude (non-hypometric) |
| Motor phenotype | Typically symmetric akinesia with axial-predominant rigidity, but lateralized featuresa may predominate | Typically lateralized features, but symmetric akinesia with axial-predominant rigidity may predominate |
| Common pathologies (approx. frequency) | PSP, 80% | CBD, 35% |
| CBD, 10% | AD, 20% | |
| FTLD-Tau, 10% | PSP, 15% | |
| FTLD-TDP, 15% | ||
| FTLD-Tau, 15% |
Lateralized motor features mostly apply to unilateral dystonia or myoclonus; lateralized cognitive features mainly apply to unilateral ideomotor apraxia, cortical sensory signs, or visual neglect.
The bulk of these observations suggest that most phenotypes are in general poorly predictive of the underlying pathology (Josephs et al. 2006b). CBS can include a diverse, non-CBD range of neuropathologies including PSP and most notably AD (Shelley et al. 2009), particularly when episodic memory complaints are prominent (see Bradley Boeve’s, “The multiple phenotypes of corticobasal syndrome and corticobasal degeneration,” this issue). Conversely, although the classical PSP phenotype predicts underlying PSP pathology more often than other pathologies (Josephs et al. 2006b), it can also be the expression of CBD (Table 2; Ling et al. 2010; Kouri et al. 2011). Minor deviations from the classical PSP phenotype may indicate an alternative diagnosis. For instance, patients with FTDP-17T/MAPT (+16 mutation) may present with a PSP phenotype at a younger age (40s) and without falls within the first year from symptom onset (Morris et al. 2003).
The two best understood forms of autosomal dominant FTDP exhibit broad though overlapping differences (Table 3). Parkinsonism in FTDP linked to chromosome 17 with tau pathology (FTDP-17T/MAPT) is typically tremorless and may exhibit early but rarely sustained response to levodopa (Tsuboi et al. 2002). It also tends to develop at a younger age, about a decade earlier, than in FTDP linked to chromosome 17 but with ubiquitin and TDP-43-positive pathology (FTDP-17U/PGRN) (Boeve and Hutton 2008). A number of MAPT mutations have been reported among those with early (N279K, delN296, S305S, +11) (Soliveri et al. 2003; Pastor et al. 2001; Wszolek et al. 2001; Skoglund et al. 2008) or late (P301S, N296H, +3, +12, +14, +16; Baba et al. 2007; Iseki et al. 2001; Rohrer et al. 2011) parkinsonism associated with FTLDP-17T/MAPT, with a picture most often resembling PSP. Similarly, several mutations in PGRN have been reported among those with parkinsonism associated with FTDP-17U/PGRN, with a phenotype most often suggestive of CBS, with or without language abnormalities (c.26C>A, g.2988_2989delCA, P439_R440fsX6, IVS1+1 G–>A, +1, +11; Wider et al. 2008; Gabryelewicz et al. 2010; Boeve et al. 2006). Psychiatric symptoms and language impairment, particularly in the form of progressive nonfluent aphasia (PNFA), are more common among patients with PGRN mutations (see Fujioka and Wszolek, this issue).
Table 3.
FTDP-17T/MAPT vs. FTDP-17U/PGRN
| FTDP-17T/MAPT | FTDP-17U/PGRN | |
|---|---|---|
| Age | Younger, 40s | Older, >50 years |
| Predominant clinical phenotype | Parkinsonism and personality change are more common | Language abnormalities, parkinsonism less common (except for CBS)a |
| Most common presenting deficits | Behavioral/personality changes, semantic impairment | Anomia, apathy (or disinhibition), apraxia |
| Penetrance | Nearly 100% (false sporadic cases are rare) | Age dependent: 90% reached by 70 years |
| Disease duration | Mean, 5 years | Mean, 7 years |
| Range, 3–10 years | Range, 1–15 years | |
| Common clinical presentations | bvFTD, CBS, PSP, AD | PNFA, CBS, AD, PDD/DLB |
| Response to levodopa | Commonly present but rarely sustained | Rarely present |
| Distribution of atrophy | Anteromedial temporal lobe and orbitofrontal region; caudate | Inferior frontal, temporal, and inferior parietal lobe |
| Symmetry of atrophy | Symmetric | Asymmetric. Asymmetry becomes greater over time |
Although pathology of FTDP-17U/PGRN is always FTLD-TDP, MND is exceptionally rare among PGRN carriers
bvFTD behavioral variant of frontotemporal dementia, PNFA progressive nonfluent aphasia, PDD Parkinson’s disease dementia, DLB dementia with Lewy bodies
Hypoventilation, slowness of vertical saccades, and weight loss can be features of Perry syndrome. Unlike CBD and PSP, Perry syndrome patients can be strikingly levodopa responsive and demonstrate axial and craniocervical L-dopa-induced dyskinesia (Newsway et al. 2010). Finally, FTD and parkinsonim may even be due to α-synuclein pathology; thus, disorders such as Parkinson’s disease dementia, dementia with Lewy bodies, and multiple system atrophy may be misdiag-nosed as FTD, decreasing the power of finding relevant biomarkers in clinically defined cohorts.
Laboratory Studies
Lacking validated biomarkers, only postmortem pathology can definitively ascertain the diagnosis. A neurological examination yielding features suggestive of the classical PSP phenotype or even CBS is reasonably predictive of tau-positive pathology. Brain MRI may assist in delineating a pattern of regional atrophy that involves the dorsolateral or inferior frontal, anterolateral temporal, and parietal regions disproportionate to other brain regions. CBD is associated with asymmetric atrophy in dorsal prefrontal and perirolandic cortex, corpus callosum, striatum, and brainstem (Kouri et al. 2011; Lee et al. 2011). PSP is associated with atrophy of the midbrain, which yields the image of a “hummingbird or a standing king penguin” in a midsagittal view on brain MRI (Groschel et al. 2006).
Magnetic resonance spectroscopy may demonstrate reduced N-acetylaspartate (NAA)/creatine–phosphocreatine (Cre) ratio in the brainstem, centrum semiovale, frontal and precentral cortex, and reduced NAA/Choline (Cho) in the lentiform nucleus in PSP patients compared to controls, whereas CBS patients have reduced NAA/Cre in the centrum semiovale and reduced NAA/Cho in the lentiform nucleus and parietal cortex compared to controls (Tedeschi et al. 1997). This technique is not helpful in distinguishing PSP from CBS or from FTD and is currently not recommended for application in individual patients. Patterns of regional glucose metabolism using 18F-Fluorodeoxyglucose PET scans have been shown to be helpful in identifying CBS and PSP cases, but the technique is not widely available, and further validation studies are needed (Eckert et al. 2005). Such is also the case for reduction in PET measures of striatal dopamine D2 receptor density using 76Br-bromospiperone or 11C-raclopride (Baron et al. 1986; Brooks et al. 1992).
Unlike the symmetric pattern of frontotemporal brain atrophy of patients with FTDP-17T/MAPT, patients with FTDP-17U/PGRN show asymmetric frontotemporal and parietal atrophy (Rohrer et al. 2010; Kelley et al. 2009). Caudate atrophy tends to be more common in patients with FTDP-17T/MAPT (Kim et al. 2007) and ALS-FTD/FUS compared to other genetic FTLDs (Josephs et al. 2010). Currently, mutation type and pathology trump clinical phenotypes in predicting the topographical distribution of atrophy (Whitwell et al. 2010). Transcranial sonography is reported to distinguish between PSP and CBS, but at present, there are no studies in which the diagnosis made with imaging has been compared to pathology.
If available, PIB PET imaging may help confirm if the CBS is due to a focal form of AD. Similarly, cerebrospinal fluid (CSF) levels of beta amyloid and tau may help differentiate AD from the other disorders. Of potentially more immediate use is the measurement of CSF tau 33 kDa/55 kDa ratio by immunoprecipitation and Western blot analysis. This ratio is significantly lower in PSP compared to other neurodegenerative disorders, including the other four-repeat tauopathy, CBD (Borroni et al. 2008). This CSF tau ratio, which represents truncated tau production and selectively affects brainstem neuron susceptibility, would need to be validated in other FTD cohorts to refine its role as a biomarker of the PSP variant of FTLD.
Measurement of serum progranulin using a commercial ELISA has been recently suggested as a cost-effective means of predicting PGRN mutations (Schofield et al. 2010). Four patients with PGRN mutations confirmed on DNA testing (2/17 behavioral FTD, 2/8 CBS) had abnormally low serum progranulin levels. The sensitivity and specificity of this test remain to be determined. Additional potential biological markers are sorely needed.
Genetic Investigations
Only in cases where there is strong family history or when age at onset is less than 50 years are commercially available genetic tests recommended. Most cases of FTLD associated with parkinsonism are tauopathies, and therefore, MAPT mutations are recommended as first step in genetic investigations (Goldman et al. 2011). When the presentation includes CBS with PNFA (especially if there is asymmetric parietal atrophy identifiable on brain MRI), PGRN mutations may be pursued first (Guerreiro et al. 2008; Spina et al. 2007). Additional clinical scenarios may be considered to refine the sequence of additional genetic testing if no MAPT or PGRN mutations are found (Goldman et al. 2011). The presence of MND should discourage testing for either of these genes. Together, the known FTLD genes explain less than 50% of the familial cases, suggesting that additional genes await discovery. Currently, commercially available genetic testing in the USA and Europe is limited to MAPT, PGRN, VCP, and TARDBP (the latter two are not recommended in the setting of FTD with parkinsonism).
There is a genetic susceptibility to PSP and MAPT conferred by tau H1 haplotype. More recently, vascular endothelial growth factor haplotypes have been reported to increase risk of FTD, CBS, and PSP (Borroni et al. 2010). Investigation of haplotypes are not currently recommended as part of the diagnostic work up of individual patients.
Conclusions
The overlapping clinical and pathological features render clinicians at a disadvantage in diagnosing and counseling patients with a variety of parkinsonian disorders associated with FTD. The multiplicity of phenotypes that can arise within the same tau- and ubiquitin-based pathologies (or even within the same MAPT and PGRN genotypes), the delay in the presentation of telltale signs, the large gap in our knowledge on the genetic contribution of over half of FTLDs, and the lack of validated biological markers are substantial obstacles on the road ahead. Nevertheless, when carefully characterized, clinical features can be helpful in steering the diagnostic work up and refining the probable ante-mortem diagnosis of these disorders. It is hoped that needed diagnostic markers be soon developed so properly diagnosed patients can be included in novel therapeutic trials that could decrease specific protein aggregation and slow disease progression.
Footnotes
Conflicts of Interest The authors report no conflicts of interest.
Disclosures Dr. Espay is supported by a K23 Mentored Career Development Award (1K23MH092735). Dr. Litvan is partially funded by R01 PAS-03-092 NIA “Genetic and environmental risk factors for PSP.”
Contributor Information
Alberto J. Espay, Department of Neurology, Movement Disorders Center, UC Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA
Irene Litvan, Email: i.litvan@louisville.edu, Department of Neurology, Division of Movement Disorders Center, University of Louisville, Louisville, KY, USA. Division of Movement Disorders, Department of Neurology, University of Louisville School of Medicine, Frazier Rehab Neuroscience Institute, 220 Abraham Flexner Way, Suite 1503, Louisville, KY 40202, USA.
References
- Baba Y, Baker MC, Le BI, et al. Clinical and genetic features of families with frontotemporal dementia and parkinsonism linked to chromosome 17 with a P301S tau mutation. J Neural Transm. 2007;114(7):947–950. doi: 10.1007/s00702-007-0632-9. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Baron JC, Maziere B, Loc’h C. Loss of striatal [76Br] bromospiperone binding sites demonstrated by positron tomography in progressive supranuclear palsy. J Cereb Blood Flow Metab. 1986;6(2):131–136. doi: 10.1038/jcbfm.1986.26. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Hutton M. Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN) Arch Neurol. 2008;65(4):460–464. doi: 10.1001/archneur.65.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Baker M, Dickson DW, et al. Frontotemporal dementia and parkinsonism associated with the IVS1+1G->A mutation in progranulin: a clinicopathologic study. Brain. 2006;129(Pt 11):3103–3114. doi: 10.1093/brain/awl268. [DOI] [PubMed] [Google Scholar]
- Borroni B, Malinverno M, Gardoni F, et al. Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy. Neurology. 2008;71(22):1796–1803. doi: 10.1212/01.wnl.0000335941.68602.39. [DOI] [PubMed] [Google Scholar]
- Borroni B, Del BR, Goldwurm S, et al. VEGF haplotypes are associated with increased risk to progressive supranuclear palsy and corticobasal syndrome. J Alzheimers Dis. 2010;21(1):87–94. doi: 10.3233/JAD-2010-091615. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, et al. Striatal D2 receptor status in patients with Parkinson’s disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol. 1992;31(2):184–192. doi: 10.1002/ana.410310209. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114(1):5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T, Barnes A, Dhawan V, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26(3):912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Esmonde T, Giles E, Xuereb J, et al. Progressive supranuclear palsy presenting with dynamic aphasia. J Neurol Neurosurg Psychiatr. 1996;60(4):403–410. doi: 10.1136/jnnp.60.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ, Hulihan MM, Kachergus JM, et al. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41(2):163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryelewicz T, Masellis M, Berdynski M, et al. Intra-familial clinical heterogeneity due to FTLD-U with TDP-43 proteinopathy caused by a novel deletion in progranulin gene (PGRN) J Alzheimers Dis. 2010;22(4):1123–1133. doi: 10.3233/JAD-2010-101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JS, Rademakers R, Huey ED, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76(5):475–483. doi: 10.1212/WNL.0b013e31820a0d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NL, Bak T, Patterson K, et al. Language function and dysfunction in corticobasal degeneration. Neurology. 2003;61(4):493–499. doi: 10.1212/01.wnl.0000081230.09863.ed. [DOI] [PubMed] [Google Scholar]
- Groschel K, Kastrup A, Litvan I, et al. Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology. 2006;66(6):949–950. doi: 10.1212/01.wnl.0000203342.77115.bf. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Santana I, Bras JM, et al. Novel progranulin mutation: screening for PGRN mutations in a Portuguese series of FTD/CBS cases. Mov Disord. 2008;23(9):1269–1273. doi: 10.1002/mds.22078. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Iseki E, Matsumura T, Marui W, et al. Familial frontotemporal dementia and parkinsonism with a novel N296H mutation in exon 10 of the tau gene and a widespread tau accumulation in the glial cells. Acta Neuropathol. 2001;102(3):285–292. doi: 10.1007/s004010000333. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Curr Opin Neurol. 2008;21(6):688–692. doi: 10.1097/WCO.0b013e3283168ddd. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Knopman DS, Whitwell JL, et al. Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology. 2005;65(4):645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006a;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006b;66(1):41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Parisi JE, et al. Caudate atrophy on MRI is a characteristic feature of FTLD-FUS. Eur J Neurol. 2010;17 (7):969–975. doi: 10.1111/j.1468-1331.2010.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BJ, Haidar W, Boeve BF, et al. Prominent phenotypic variability associated with mutations in progranulin. Neurobiol Aging. 2009;30(5):739–751. doi: 10.1016/j.neurobiolaging.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Rabinovici GD, Seeley WW, et al. Patterns of MRI atrophy in tau positive and ubiquitin positive frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatr. 2007;78(12):1375–1378. doi: 10.1136/jnnp.2006.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri N, Murray ME, Hassan A, et al. Neuropathologic features of corticobasal degeneration presenting as corticobasal syndrome or Richardson syndrome. Brain. 2011 doi: 10.1093/brain/awr234. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Majtenyi K, Spina S, et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67(10):963–975. doi: 10.1097/NEN.0b013e318187a80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Marion MH. “Apraxia of lid opening,” a focal eyelid dystonia: clinical study of 32 patients. Mov Disord. 1994;9 (6):610–615. doi: 10.1002/mds.870090605. [DOI] [PubMed] [Google Scholar]
- Lee SE, Rabinovici GD, Mayo MC, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, O’Sullivan SS, Holton JL, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133(Pt 7):2045–2057. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117 (1):15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HR, Osaki Y, Holton J, et al. Tau exon 10 +16 mutation FTDP-17 presenting clinically as sporadic young onset PSP. Neurology. 2003;61(1):102–104. doi: 10.1212/01.wnl.0000072325.27824.a5. [DOI] [PubMed] [Google Scholar]
- Newsway V, Fish M, Rohrer JD, et al. Perry syndrome due to the DCTN1 G71R mutation: a distinctive levodopa responsive disorder with behavioral syndrome, vertical gaze palsy, and respiratory failure. Mov Disord. 2010;25(6):767–770. doi: 10.1002/mds.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsancak C, Auzou P, Dujardin K, et al. Orofacial apraxia in corticobasal degeneration, progressive supranuclear palsy, multiple system atrophy and Parkinson’s disease. J Neurol. 2004;251(11):1317–1323. doi: 10.1007/s00415-004-0530-0. [DOI] [PubMed] [Google Scholar]
- Pastor P, Pastor E, Carnero C, et al. Familial atypical progressive supranuclear palsy associated with homozigosity for the delN296 mutation in the tau gene. Ann Neurol. 2001;49(2):263–267. doi: 10.1002/1531-8249(20010201)49:2<263::aid-ana50>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Rivaud-Pechoux S, Vidailhet M, Gallouedec G, et al. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2000;54(5):1029–1032. doi: 10.1212/wnl.54.5.1029. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73 (18):1451–1456. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Modat M, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53(3):1070–1076. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Paviour D, Vandrovcova J, et al. Novel L284R MAPT mutation in a family with an autosomal dominant progressive supranuclear palsy syndrome. Neurodegener Dis. 2011;8(3):149–152. doi: 10.1159/000319454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford NJ, Zhang YJ, Baker M, et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4(9):e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield EC, Halliday GM, Kwok J, et al. Low serum progranulin predicts the presence of mutations: a prospective study. J Alzheimers Dis. 2010;22(3):981–984. doi: 10.3233/JAD-2010-101032. [DOI] [PubMed] [Google Scholar]
- Shelley BP, Hodges JR, Kipps CM, et al. Is the pathology of corticobasal syndrome predictable in life? Mov Disord. 2009;24 (11):1593–1599. doi: 10.1002/mds.22558. [DOI] [PubMed] [Google Scholar]
- Skoglund L, Viitanen M, Kalimo H, et al. The tau S305S mutation causes frontotemporal dementia with parkinsonism. Eur J Neurol. 2008;15(2):156–161. doi: 10.1111/j.1468-1331.2007.02017.x. [DOI] [PubMed] [Google Scholar]
- Soliveri P, Rossi G, Monza D, et al. A case of dementia parkinsonism resembling progressive supranuclear palsy due to mutation in the tau protein gene. Arch Neurol. 2003;60(10):1454–1456. doi: 10.1001/archneur.60.10.1454. [DOI] [PubMed] [Google Scholar]
- Spina S, Murrell JR, Huey ED, et al. Corticobasal syndrome associated with the A9D progranulin mutation. J Neuropathol Exp Neurol. 2007;66(10):892–900. doi: 10.1097/nen.0b013e3181567873. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Litvan I, Bonavita S, et al. Proton magnetic resonance spectroscopic imaging in progressive supranuclear palsy, Parkinson’s disease and corticobasal degeneration. Brain. 1997;120(Pt 9):1541–1552. doi: 10.1093/brain/120.9.1541. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Baker M, Hutton ML, et al. Clinical and genetic studies of families with the tau N279K mutation (FTDP-17) Neurology. 2002;59(11):1791–1793. doi: 10.1212/01.wnl.0000038909.49164.4b. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouider-Khouja N, et al. Eye movements in parkinsonian syndromes. Ann Neurol. 1994;35(4):420–426. doi: 10.1002/ana.410350408. [DOI] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, et al. Inclusion body myopathy associated with Paget disease of bone and frontotem-poral dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36(4):377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010;75(24):2212–2220. doi: 10.1212/WNL.0b013e31820203c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, Uitti RJ, Wszolek ZK, et al. Progranulin gene mutation with an unusual clinical and neuropathologic presentation. Mov Disord. 2008;23(8):1168–1173. doi: 10.1002/mds.22065. [DOI] [PubMed] [Google Scholar]
- Wszolek ZK, Tsuboi Y, Uitti RJ, et al. Progressive supranuclear palsy as a disease phenotype caused by the S305S tau gene mutation. Brain. 2001;124(Pt 8):1666–1670. doi: 10.1093/brain/124.8.1666. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Hashimoto M, Kaneda K, et al. Neuropsychiatric symptoms of progressive supranuclear palsy in a dementia clinic. Psychogeriatrics. 2011;11(1):54–59. doi: 10.1111/j.1479-8301.2010.00352.x. [DOI] [PubMed] [Google Scholar]