Abstract
Objectives
Clinical models incompletely predict outcomes following coronary artery bypass grafting. Novel molecular technologies may identify biomarkers to improve risk stratification. We examined whether metabolic profiles can predict adverse events in patients undergoing coronary artery bypass grafting.
Methods
The study population comprised 478 subjects from the CATHGEN biorepository of patients referred for cardiac catheterization who underwent coronary artery bypass grafting after enrollment. Targeted mass spectrometry-based profiling of 69 metabolites was performed in frozen, fasting plasma samples collected prior to surgery. Principal-components analysis and Cox proportional hazards regression modeling were used to assess the relation between metabolite factor levels and a composite outcome of post-coronary artery bypass grafting myocardial infarction, need for percutaneous coronary intervention, repeat coronary artery bypass grafting, or death.
Results
Over a mean follow-up of 4.3 ± 2.4 years, 126 subjects (26.4%) suffered an adverse event. Three principal-components analysis-derived factors were significantly associated with adverse outcome in univariable analysis: short-chain dicarboxylacylcarnitines (factor 2, P=0.001); ketone-related metabolites (factor 5, P=0.02); and short-chain acylcarnitines (factor 6, P=0.004). These three factors remained independently predictive of adverse outcome after multivariable adjustment: factor 2 (adjusted hazard ratio 1.23; 95% confidence interval [1.10-1.38]; P<0.001), factor 5 (1.17 [1.01-1.37], P=0.04), and factor 6 (1.14 [1.02-1.27], P=0.03).
Conclusions
Metabolic profiles are independently associated with adverse outcomes following coronary artery bypass grafting. These profiles may represent novel biomarkers of risk that augment existing tools for risk stratification of coronary artery bypass grafting patients and may elucidate novel biochemical pathways that mediate risk.
Introduction
Coronary heart disease remains the leading cause of death in the United States. Coronary artery bypass grafting (CABG) is the preferred treatment option for the vast majority of patients with left main or three-vessel coronary artery disease. Beneficial outcomes following CABG, which is the most widely studied operation in history, are well established. However, progression of native vessel atherosclerosis and vein graft failure contribute significantly to continued morbidity and mortality following CABG long-term. For example, up to 10% of patients undergoing CABG require a subsequent medical or surgical revascularization procedure within three years after operation.1 Repeat CABG occurs in 11% of patients by 10 years after initial CABG.2 Furthermore, 14-36% of patients have a new post-operative myocardial infarction (MI) within 10 years after their index CABG,3,4 with a significant resultant decrease in survival.3
Unfortunately, clinical models incompletely predict risk for adverse events following CABG. For example, the EUROSCORE model has a c-statistic of 0.79 and others have a similar model fit.5 Further, most currently available risk models predict peri-operative risk but do not describe longer-term risk. Thus, there remains a gap in our risk prediction models for adverse events after CABG. A better understanding of risk could lead to alternate and/or adjunctive peri-operative strategies or use of alternative initial revascularization strategies to improve outcome in patients at high risk for adverse events following CABG. Metabolic profiling, the systematic study of the small molecule byproducts of cellular metabolism, has been used to identify novel cardiovascular biomarkers to improve risk stratification and better understand the mechanisms of several disease processes.6 In one recent study from our group, a cluster of small-medium chain dicarboxylated acylcarnitine metabolites was found to be predictive of imminent cardiovascular events (death/MI).7 We hypothesized that targeted metabolic profiling would identify novel biomarkers of risk for adverse events after CABG, as well as potentially provide a better understanding of disease pathophysiology.
Methods
Study Population
The study population was selected from patients enrolled in the CATHGEN biorepository of >9000 subjects at Duke University Medical Center (DUMC), a biorepository of clinical and follow-up data and blood samples collected from patients presenting for cardiac catheterization. Details of the biorepository have been published.8 In brief, fasting blood samples were collected in EDTA tubes via an arterial sheath at the time of cardiac catheterization, prior to the administration of supplemental heparin (if given). Blood tubes were then chilled to 4°C, centrifuged within 30 minutes of collection, separated into aliquots, and frozen at −80°C. Demographics, medical history, angiographic data and longitudinal follow-up for CATHGEN patients were collected through the Duke Databank for Cardiovascular Disease (DDCD), which has archived this information on all patients undergoing cardiac procedures at DUMC since 1969. Follow-up includes annual determination of mortality, MI, hospitalizations and coronary revascularization procedures. Vital status is confirmed through the National Death Index and the Social Security Death Index.
Metabolic profiling had been performed on a total of 3899 unique subjects from December 2006 to March 2010.7,8 For this study, we selected all subjects with metabolic profiling who underwent CABG at any time after their index CATHGEN cardiac catheterization. The CATHGEN biorepository and this substudy were approved by the Duke University Institutional Review Board (IRB). Prior to collection of blood samples, all study subjects provided written informed consent to participate.
Laboratory Methods
The levels of 45 acylcarnitines, 15 amino acids, ketones, β-hydroxybutyrate, and total free fatty acids were quantitatively determined on frozen, fasting plasma samples using previously reported methods with known coefficients of variation.7,9,10 The laboratory (Sarah W. Stedman Nutrition and Metabolism metabolomics core laboratory) was blinded to event status, and samples were randomly numbered with respect to event status. Acylcarnitines and amino acids were profiled using a mass spectrometry-based protocol.9,11 Proteins were first removed by precipitation with methanol. Aliquots of supernatants were then dried and esterified with hot, acidic methanol (acylcarnitines) or n-butanol (amino acids). Analysis was done by tandem mass spectroscopy with a Quattro Micro instrument (Waters Corp, Milford, MA). Quantification of the “targeted” intermediary metabolites was facilitated by addition of mixtures of known quantities of stable-isotope internal standards as previously described.7,10,12
Statistical Analysis
The primary endpoint of the study was occurrence of any event at any time after CABG from within a composite adverse event, defined as incident percutaneous coronary intervention (PCI), MI, repeat CABG, or death.
Principal components analysis (PCA) was used, given the co-linearity of the metabolites, to reduce the burden of adjustment for multiple comparisons. Metabolites with > 25% of values equaling “0” (below the lower limits of quantification) were not analyzed (C6 and C7-DC acylcarnitines). Varimax rotation was used to produce identifiable factors. Factors with an eigenvalue ≥ 1 were retained based on the commonly used Kaiser criterion.13 Metabolites with a ∣factor load∣ ≥ 0.4 were reported as comprising a given factor. Scoring coefficients were constructed and used to calculate factor scores for each individual (weighted sum of the standardized metabolites within that factor, weighted on the factor loading for each metabolite.)
Cox proportional hazard regression modeling was used to assess the association of factor levels with time to first occurrence of any component of the composite of myocardial infarction, percutaneous coronary intervention, repeat CABG, or death after the index CABG. Both univariable and multivariable models, adjusted for age, sex, race, dyslipidemia, diabetes, renal disease, smoking, body mass index, baseline ejection fraction, number of diseased coronary vessels at time of cardiac catheterization, and pre-procedural heparin use at time of sample collection, were performed. The multivariable models also were adjusted for all factors significant in univariable analyses (P<0.05). Clinical variables with P<0.10 were retained in the final model. For these time-to-event analyses, the day of CABG was considered the baseline time zero. Kaplan-Meier curves adjusted for all variables retained in the final model were constructed to visualize the relation of those factors that were significantly associated with the composite event. Factor levels were divided into tertiles for visualization purposes.
Statistical analysis was performed by authors D.M.C., A.A.S. and S.H.S. using SAS version 9.1 (Cary, NC).
Results
Of 478 subjects who had metabolic profiling of baseline samples collected at the time of cardiac catheterization and subsequently had CABG, 126 (26.4%) experienced the composite clinical endpoint post-CABG over a mean follow-up time of 4.3 ±2.4 years after CABG. Nineteen (4.0%) suffered MI, 28 (5.9%) had PCI, 4 (0.8%) had repeat CABG, and 75 (15.7%) died (first events). The median time from enrollment in the CATHGEN cardiac catheterization study to CABG was 4 (interquartile range 2,49) days. Baseline characteristics of the study population according to event or no event during follow-up are presented in Table 1.
Table 1.
Baseline Characteristics of the Study Population.
| Overall (N=478) |
No Event Subjects (N=352) |
Event Subjects (N=126) |
P | |
|---|---|---|---|---|
| Age (mean [SD]) | 61 ± 11 | 61 ± 11 | 62 ± 12 | 0.33 |
| Sex (% female) | 159 (33%) | 111 (32%) | 48 (38%) | 0.18 |
| Race (% white) | 355 (74%) | 263 (75%) | 92 (73%) | 0.71 |
| Diabetes (%) | 170 (36%) | 114 (32%) | 56 (44%) | 0.02 |
| Dyslipidemia (%) | 309 (65%) | 227 (65%) | 82 (65%) | 0.91 |
| Renal Disease (%) | 12 (3%) | 5 (1%) | 7 (6%) | 0.02 |
| Current Smoker (%) | 262 (55%) | 188 (53%) | 74 (58%) | 0.30 |
| BMI (mean [SD]) | 30 ± 7 | 30 ± 6 | 30 ± 7 | 0.69 |
| EF (mean [SD]) | 54 ± 14 | 55 ± 14 | 52 ± 15 | 0.03 |
| Number of Diseased Coronary Vessels (mean [SD]) |
2.3 ± 0.8 | 2.3 ± 0.8 | 2.5 ± 0.8 | 0.02 |
| Pre-Procedure Heparin Use (%) |
116 (24%) | 77 (22%) | 39 (31%) | 0.05 |
SD=standard deviation; BMI=body mass index; EF=ejection fraction
PCA identified 12 metabolic factors (Table 2), residing in biologically plausible clusters as previously reported.7,12,14 In univariable time-to-event analyses, levels of several factors were significantly associated with time to the composite event (Table 3). These included factor 2, consisting of short-chain dicarboxylacylcarnitines (hazard ratio [HR] 1.19, 95% confidence interval [1.07-1.33], P=0.001); factor 5, consisting of ketone-related metabolites (HR 1.21 [1.04-1.41], P=0.02); and factor 6, consisting of short-chain acylcarnitines (HR 1.20 [1.06-1.35], P=0.004).
Table 2.
Principal Components Analysis
| Factor | Name | Individual Components | Eigen- Value |
Variance |
|---|---|---|---|---|
| 1 | Medium-Chain Acylcarnitines |
C8, C10, C10:1, C10-OH/C8-DC, C12, C12:1, C14, C14:1, C14:1-OH/C12:1-DC, C14:2, C16:1, C16:1-OH/C14:1-DC, C16:2, C18:1-OH/C16:1-DC |
15.69 | 0.26 |
| 2 | Short Chain Dicarboxylacyl carnitines |
Ci4-DC/C4-DC, C5-DC, C6-DC, C6:1- DC/C8:1-OH, C8:1, C8:1-DC, C10:1, C10:2, C10:3, C10-OH/C8-DC, C12-OH/C10-DC, citrulline |
5.72 | 0.09 |
| 3 | Long-Chain Dicarboxylacyl carnitines |
C12-OH/C10-DC, C14-OH/C12-DC, C16- OH/C14-DC, C18-OH/C16-DC, C18:1- OH/C16:1-DC, C20:1-OH/C18:1-DC, C20- OH/C18-DC, C20 |
4.41 | 0.07 |
| 4 | Long Chain Acylcarnitines |
C16, C16:1-OH/C14:1-DC, C18,C18:1, C18:2, C20:4 |
3.17 | 0.05 |
| 5 | Ketone-Related | Ketones, Hbut, C4-OH, C2, alanine (-) | 2.83 | 0.05 |
| 6 | Short-Chain Acylcarnitines |
C2, C3, C4/Ci4 C5s | 2.52 | 0.04 |
| 7 | Branched-Chain Amino Acids |
leucine/isoleucine, valine, phenylalanine, ornithine |
2.25 | 0.04 |
| 8 | Urea Cycle Amino Acids |
methionine, serine, histidine, citrulline | 1.90 | 0.03 |
| 9 | Amino Acids | glycine, histidine, phenylalanine, C5:1 | 1.74 | 0.03 |
| 10 | Various | aspartic acid/asparagine, C5:1(-), C5-OH/C3- DC |
1.49 | 0.02 |
| 11 | Medium-Chain Acylcarnitines |
C8:1, C10:3 | 1.27 | 0.02 |
| 12 | Various | C22, NEFA | 1.11 | 0.02 |
NEFA: non-esterified fatty acids; Hbut: β-hydroxybutyrate; (-): negative factor load for this metabolite on this factor
Table 3.
Relationship Between Metabolite Factor Levels and Adverse Events
| Factor | Name | Factor Levels – No Event Subjects (N=352) |
Factor Levels – Event Subjects (N=126) |
Hazard Ratio [95% Confidence Interval] |
P* |
|---|---|---|---|---|---|
| 1 | Medium-Chain Acylcarnitines | −0.02 ± 1.1 | 0.06 ± 0.74 | 1.07 [0.94-1.23] | 0.30 |
| 2 |
Short Chain Dicarboxyl-
acylcarnitines |
−0.09 ± 0.85 | 0.24 ± 1.3 | 1.19 [1.07-1.33] | 0.001 |
| 3 | Long-Chain Dicarboxyl- acylcarnitines |
−0.04 ± 0.81 | 0.12 ± 1.39 | 1.11 [0.99-1.24] | 0.08 |
| 4 | Long Chain Acylcarnitines | 0.03 ± 1.00 | −0.09 ± 0.99 | 0.90 [0.75-1.08] | 0.25 |
| 5 | Ketone-Related | −0.07 ± 0.92 | 0.19 ± 1.17 | 1.21 [1.04-1.41] | 0.02 |
| 6 | Short-Chain Acylcarnitines | −0.07 ± 0.71 | 0.19 ± 1.52 | 1.20 [1.06-1.35] | 0.004 |
| 7 | Branched-Chain Amino Acids | −0.01 ± 0.98 | 0.03 ± 1.06 | 1.02 [0.85-1.21] | 0.87 |
| 8 | Urea Cycle Amino Acids | 0.03 ± 0.97 | −0.09 ± 1.08 | 0.91 [0.72-1.13] | 0.39 |
| 9 | Amino Acids | −0.001 ± 1.01 | 0.002 ± 0.98 | 1.15 [0.96-1.38] | 0.13 |
| 10 | Various | −0.04 ± 0.99 | 0.10 ± 1.03 | 1.02 [0.86-1.20] | 0.86 |
| 11 | Medium-Chain Acylcarnitines | −0.05 ± 0.95 | 0.14 ± 1.12 | 1.16 [0.99-1.37] | 0.07 |
| 12 | Various | −0.02 ± 0.98 | 0.05 ± 1.05 | 1.07 [0.90-1.27] | 0.48 |
Metabolite factors significantly associated with time-to-event are highlighted in bold.
P-value for univariate Cox proportional hazards regression model of association of factor level with time-to-event.
After multivariable adjustment for clinical variables and significant factors in univariable analyses, these three factors remained significantly associated with risk of adverse events: factor 2 (HR 1.23 [1.10-1.38], P<0.001); factor 5 (HR 1.17 [1.01-1.37], P=0.04); factor 6 (HR 1.14 [1.02-1.27], P=0.03) (Table 4). The clinical variables ejection fraction (P=0.05) and pre-procedure heparin use (P=0.06) were also retained in the final model. Kaplan-Meier curves demonstrating the relationship of factor 2 levels and adverse outcome after CABG are presented in Figure 1, showing a linear relation between metabolite factor levels and outcomes, with higher metabolite factor levels associated with a higher probability of adverse events. Kaplan-Meier curves demonstrating the relation of factors 5 and 6 with adverse event are presented in Figures 2 and 3.
Table 4.
Multivariable Model for Time to Adverse Event
| Variable | Hazard Ratio (95% Confidence Interval) | Adjusted P* |
|---|---|---|
| Factor 2 | 1.23 (1.10 – 1.38) | < 0.001 |
| Factor 5 | 1.17 (1.01 – 1.37) | 0.04 |
| Factor 6 | 1.14 (1.02 – 1.27) | 0.03 |
| Ejection fraction | 0.99 (0.98 – 1.00) | 0.05 |
| Pre-procedure heparin use | 1.43 (0.98 – 2.10) | 0.06 |
adjusted for age, sex, race, hyperlipidemia, diabetes, renal disease, smoking, body mass index, ejection fraction, number of diseased coronary vessels, pre-procedural heparin use, and factors significant in univariable analyses (factors 2, 5, 6)
Figure 1. Kaplan-Meier curve for the relationship between factor 2 levels and adverse events.
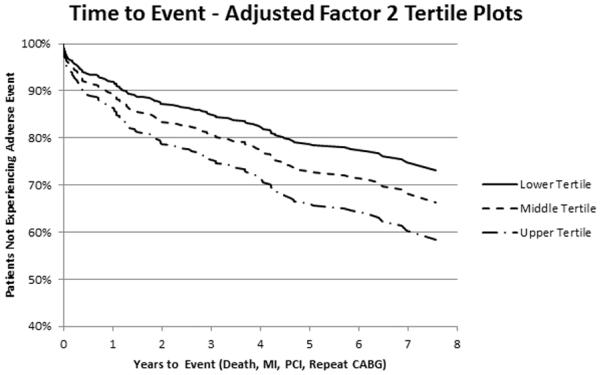
Displayed is an adjusted Kaplan-Meier curve of the relationship between tertiles of metabolite factor 2 and event-free survival after CABG, adjusted for all variables retained in the final multivariable model, demonstrating a linear relationship between factor 2 levels and decreased event-free survival.
Figure 2. Kaplan-Meier curve for the relationship between factor 5 levels and adverse events.
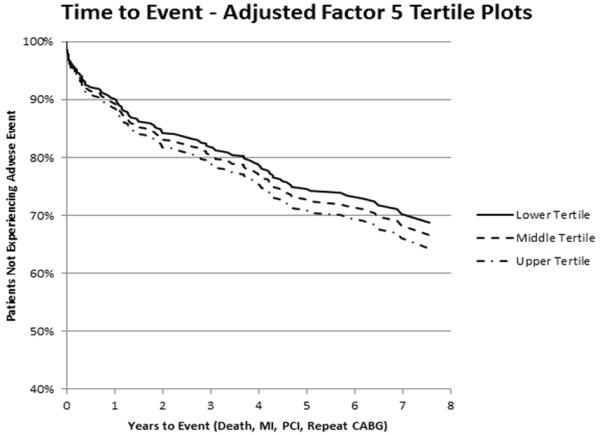
Displayed is an adjusted Kaplan-Meier curve of the relationship between tertiles of metabolite factor 5 and event-free survival after CABG, adjusted for all variables retained in the final multivariable model, demonstrating a linear relationship between factor 5 levels and decreased event-free survival.
Figure 3. Kaplan-Meier curve for the relationship between factor 6 levels and adverse events.
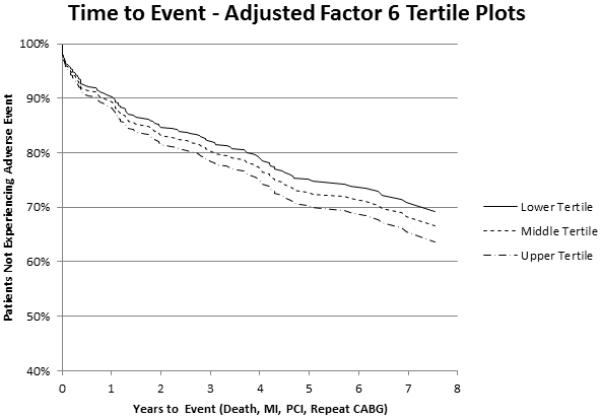
Displayed is an adjusted Kaplan-Meier curve of the relationship between tertiles of metabolite factor 6 and event-free survival after CABG, adjusted for all variables retained in the final multivariable model, demonstrating a linear relationship between factor 6 levels and decreased event-free survival.
Discussion
To our knowledge, this is the first report of an association between baseline plasma metabolic profiles and outcomes following CABG. In this cohort, elevated levels of short-chain dicarboxylacylcarnitines, ketone-related metabolites, and short-chain acylcarnitines were predictive of a composite endpoint of MI, repeat revascularization, or death at any time following CABG. This predictive capacity held true after adjustment for numerous clinical covariates known to be associated with cardiovascular events.
Our group has previously observed that elevated levels of short-chain dicarboxylacylcarnitines are independently associated with a higher risk of death and incident MI for those undergoing cardiac catheterization.7,14 Additionally, we have previously shown that adding metabolic profiles to clinical risk assessment tools can significantly improve risk stratification and help reclassify intermediate risk CAD patients.7 The results of the current study extend those findings into this specific subgroup of CAD patients suffering adverse events after CABG, patients with a more severe disease burden in a group with a more homogeneous degree of CAD severity.
Dicarboxylated acylcarnitines can be derived by ω-oxidation of fatty acids via microsomal cytochrome p450 enzymes. Long-chain dicarboxylated fatty acyl CoAs are shortened mainly via peroxisomal oxidation,15 and when converted to the cognate acylcarnitines are diagnostic for several inborn errors of metabolism.16 Hence, factor 2 may reflect changes in flux through the microsomal cytochrome p450 and/or peroxisomal oxidation systems. However, dicarboxylated acylcarnitines can also be derived from CoA esters generated during oxidation of amino acids or other metabolic fuels in the mitochondria. Thus, the pathways by which these metabolites are generated and their biological significance remain to be investigated.
Short chain acylcarnitines (factor 6) also are products of fatty acid oxidation. Similar to dicarboxylacylcarnitines, elevated systemic levels of short chain acylcarnitines can be indicative of inborn errors of metabolism. For example, butyrylcarnitine is elevated in patients with short-chain acyl-CoA dehydrogenase (SCAD) deficiency. The elevated levels of factor 6 in our adverse event population may also be related to errors in fatty acid oxidation.
In addition to acylcarnitines, a group of ketone-related metabolites were predictive of adverse outcome in our study. During normal conditions the heart preferentially oxidizes fatty acids for energy. However, during periods of metabolic stress, such as during an ischemic event, glucose becomes the predominant source of fuel, which is protective.17,18 Failure of a cell to increase glucose uptake may result in cellular dysfunction.19 Increased levels of ketones: (1) correlate with the level of cardiac dysfunction;20 (2) increase reactive oxygen species production;17 and (3) decrease glucose uptake.17 These mechanisms may explain the increased levels of ketone-related metabolites in patients experiencing adverse events after CABG.
There are potential implications of these findings. First, with respect to clinical applicability, our results suggest that metabolic profiling of patients undergoing CABG could provide health care providers an additional tool for identifying patients at higher risk for adverse events, thereby facilitating personalized tailoring of the revascularization and post-procedure management of these patients. For example, patients with higher pre-operative plasma levels of short-chain dicarboxylacylcarnitines may benefit from total arterial revascularization, post-operative clopidogrel use (as opposed to aspirin), and closer post-discharge follow-up. While we are optimistic about such a role of these novel biomarkers, such applications necessitate further studies including further validation and prospective outcome studies. Second, specific metabolites and metabolic pathways may provide insight into novel mechanisms of disease in patients who experience adverse events after CABG. We have previously observed that metabolic profiles are heritable12 and genetic variation has been shown to be associated with metabolic traits;21 thus, the identified metabolic profiles may actually be reporting on underlying genetic variation increasing risk for adverse events after CABG. Both of these implications provide fertile opportunity for further study.
Limitations of this study are worth noting. First, although blood was drawn on all study subjects prior to CABG, there was potential variability in the clinical states of these patients at the time of sampling. For example, patients experiencing non-ST segment elevation myocardial infarctions (NSTEMI) may have different metabolite profiles compared to patients undergoing elective cardiac catheterization. However, we did adjust for pre-procedural heparin use as a surrogate, since most patients with NSTEMI would be treated with heparin prior to cardiac catheterization. Second, although mass spectrometry is a readily available technology, quantification of the plasma concentrations of these metabolites involved the use of a specialized center experienced in performing these assays. Many clinical laboratories may not have the capabilities to perform these assays in a timely fashion at this time. Work is ongoing to translate these findings into more readily available clinical tools, including determining the optimal time to obtain these metabolite profiles preoperatively. Other limitations include the bias of not having enrolled unstable patients who were unable to undergo the consenting process due to their instability (i.e. ST segment elevation MI), and the non-randomized selection of patients.
In summary, we have identified metabolic profiles composed of short-chain dicarboxylacylcarnitines, ketone-related metabolites, and short-chain acylcarnitines that predict adverse outcomes following CABG independent of standard clinical predictors of risk. These findings suggest that metabolites may be significant cardiovascular risk biomarkers, which may shed light on novel biochemical pathways of disease and supplement clinical risk assessment models in patients undergoing CABG.
Acknowledgements
We would like to thank the patients who participated in CATHGEN and Elaine Dowdy for her study coordination; and Melissa Hurdle, Amanda House, and Becky Church for sample collection.
Funding Sources: This study was supported by funding from (1) NIH grant R01HL095987-01 (SH Shah); (2) the Measurement to Understand Re-Classification of Disease of Cabarrus and Kannapolis (MURDOCK) Study through a gift to Duke University from the David H. Murdock Foundation; (3) NIH grant 1UL1 RR024128-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research; its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at [http://www.ncrr.nih.gov/]. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview\-translational.asp; (4) Division of Cardiovascular and Thoracic Surgery, Duke University Medical Center; (5) American Heart Association (SH Shah).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Shizuta S, Ehara N, et al. Long-Term Outcomes of Coronary-Artery Bypass Graft Surgery Versus Percutaneous Coronary Intervention for Multivessel Coronary Artery Disease in the Bare-Metal Stent Era. Circulation. 2008 Sep 30;118(14 Suppl):S199–209. doi: 10.1161/CIRCULATIONAHA.107.735902. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove DM, Loop FD, Lytle BW, Gill CC, Golding LA, Gibson C, et al. Predictors of reoperation after myocardial revascularization. J Thorac Cardiovasc Surg. 1986;92:811. [PubMed] [Google Scholar]
- 3.Sergeant PT, Blackstone EH, Meyns BP. Does arterial revascularization decrease the risk of infarction after coronary artery bypass grafting? Ann Thorac Surg. 1998 Jul;66(1):1–10. doi: 10.1016/s0003-4975(98)00394-4. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 4.Peduzzi P, Detre K, Murphy ML, Thomsen J, Hultgren H, Takaro T. Ten-year incidence of myocardial infarction and prognosis after infarction. Department of Veterans Affairs; [DOI] [PubMed] [Google Scholar]
- 5.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999 Jul;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 6.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010 Nov 11;5(11):e1395. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010 Apr;3(2):207–14. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 8.Shah SH, Granger CB, Hauser ER, Kraus WE, Sun JL, Pieper K, et al. Reclassification of cardiovascular risk using integrated clinical and molecular biosignatures: Design of and rationale for the Measurement to Understand the Reclassification of Disease of Cabarrus and Kannapolis (MURDOCK) Horizon 1 Cardiovascular Disease Study. Am Heart J. 2010 Sep;160(3):371–379. e2. doi: 10.1016/j.ahj.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 9.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004 Mar;10(3):268–74. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 10.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009 Apr;9(4):311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cell Metab. 2009 Jun;9(6):565–6. Erratum in: [Google Scholar]
- 11.Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem. 1995 Jan;41(1):62–8. [PubMed] [Google Scholar]
- 12.Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Haynes C, Stevens RD, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5:258. doi: 10.1038/msb.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20:141–151. [Google Scholar]
- 14.Shah SH, Sun JL, Pieper K, Crosslin DR, Haynes C, Bain JR, et al. Plasma Metabolic Profiles Predict Future Cardiovascular Events. Circulation. 2009 Nov;120:S466–S467. [Google Scholar]
- 15.Ferdinandusse S, Denis S, Van Roermund CW, Wanders RJ, Dacremont G. Identification of the peroxisomal beta-oxidation enzymes involved in the degradation of long-chain dicarboxylic acids. J Lipid Res. 2004;45(6):1104–1111. doi: 10.1194/jlr.M300512-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW. Synthesis of dicarboxylic acylcarnitines. Chem Phys Lipids. 2004;129(2):161–171. doi: 10.1016/j.chemphyslip.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier A, Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2007 May;292(5):E1325–32. doi: 10.1152/ajpendo.00186.2006. [DOI] [PubMed] [Google Scholar]
- 18.Depre C, Vanoverschelde JLJ, Taegtmeyer H. Glucose for the heart. Circulation. 1999 Feb 2;99(4):578–88. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Aleem S, St Louis JD, Hughes GC, Lowe JE. Metabolic changes in the normal and hypoxic neonatal myocardium. Ann N Y Acad Sci. 1999 Jun 30;874:254–61. doi: 10.1111/j.1749-6632.1999.tb09240.x. [DOI] [PubMed] [Google Scholar]
- 20.Lommi J, Kupari M, Koskinen P, Naveri H, Leinonen H, Pulkki K, et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 21.Illig T, Gieger C, Zhai G, Römisch-Margl W, Wang-Sattler R, Prehn C, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010 Feb;42(2):137–41. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]


