Abstract
Objective
Oxidized low-density lipoprotein (oxLDL) modulates intracellular redox status and induces apoptosis in endothelial cells. However, the signal pathways and molecular mechanism remain unknown. In this study, we investigated the role of manganese superoxide dismutase (Mn-SOD) on oxLDL-induced apoptosis via c-Jun NH2-terminal kinase (JNK)-mediated ubiquitin/proteasome pathway.
Methods and Results
OxLDL induced JNK phosphorylation that peaked at 30 minutes in human aortic endothelial cells. Fluorescence-activated cell sorting analysis revealed that oxLDL increased mitochondrial superoxide production by 1.88±0.19-fold and mitochondrial membrane potential by 18%. JNK small interference RNA (siJNK) reduced oxLDL-induced mitochondrial superoxide production by 88.4% and mitochondrial membrane potential by 61.7%. OxLDL did not affect Mn-SOD mRNA expression, but it significantly reduced Mn-SOD protein level, which was restored by siJNK. Immunoprecipitation by ubiquitin antibody revealed that oxLDL increased ubiquitination of Mn-SOD, which was inhibited by siJNK. OxLDL-induced caspase-3 activities were also attenuated by siJNK but were enhanced by Mn-SOD small interfering RNA. Furthermore, overexpression of Mn-SOD abrogated oxLDL-induced caspase-3 activities.
Conclusion
OxLDL-induced JNK activation regulates mitochondrial redox status and Mn-SOD protein degradation via JNK-dependent ubiquitination, leading to endothelial cell apoptosis.
Keywords: manganese superoxide dismutase, mitochondrial redox status, oxidized low-density lipoprotein, ubiquitination
Circulating low-density lipoprotein (LDL) enters the vascular wall, where it undergoes oxidative modifications.1,2 Oxidized LDL (oxLDL) mediates a host of endothelial functions, including endothelial redox status3 and apoptosis at a high concentration.4–6 Emerging evidence reveals that oxLDL induces mitochondrial reactive oxygen species production7–9 and apoptosis in vascular cells, including endothelial cells.10–14 However, the precise mechanisms whereby oxLDL induces mitochondrial reactive oxygen species production and apoptosis remain unknown.
Mitogen-activated protein kinase cascades play an important role in regulating endothelial function.15 Of several mitogen-activated protein kinases, c-Jun NH2-terminal kinase (JNK) is an important signaling molecule expressed in 3 isoforms, namely, JNK1, JNK2, and JNK3. Both JNK1 and JNK2 are ubiquitously distributed, whereas JNK3 is present in neuronal tissue.16 JNK expression and activation were upregulated in atherosclerotic lesions.17 The JNK inhibitor SP600125 reduced superoxide production and restored nitric oxide release in coronary arteries.18 JNK2 was activated in the aorta from apolipoprotein E (apoE−/−) mice fed a hypercholesterolemic diet, and atheroma formation was significantly reduced in apoE−/− JNK2(−/−) double-knockout mice compared to apoE−/− mice.19
In this study, we provide evidence that oxLDL induced mitochondrial superoxide production and endothelial apoptosis via JNK activation. Specifically, immunoprecipitation Western blot assay revealed that oxLDL-activated JNK increased ubiquitination of manganese superoxide dismutase (Mn-SOD), resulting in protein degradation. Furthermore, knockdown of JNK or overexpression of Mn-SOD attenuated oxLDL-mediated endothelial apoptosis. We hereby demonstrate a molecular mechanism whereby oxLDL regulates mitochondrial redox status and apoptosis via JNK-mediated ubiquitination of Mn-SOD.
Materials and Methods
Cell Culture
Human aortic endothelial cells (HAEC) were purchased from Cell Applications and cultured on a gelatin-coated dish with endothelial growth medium (Cell Applications) with 4% heat-inactivated fetal bovine serum (Gibco) at 37°C in a 5% CO2 atmosphere. All experiments were performed within 9 passages (please see Data Supplement available online at http://atvb.ahajournals.org).
LDL Preparation and Oxidation
Venous blood was obtained from fasting adult human volunteers, with Institutional Review Board approval from the Atherosclerosis Research Unit at the University of California, Los Angeles. The technique used for separating LDL was similar to that described previously (available online at http://atvb.ahajournals.org).20
Adenoviral Vectors
Human Mn-SOD adenoviral vector (Ad-Mn-SOD) was generously provided by Dr Donald Heistad at the University of Iowa. The technique used for making Mn-SOD adenoviral vector was described previously.21
Western Blot Analysis
HAEC were harvested and the entire cell extracts were prepared as described previously.22
SiRNA Transfection
The small interfering RNA (siRNA) target sequence for JNK1 was 5′-AAG CCG ACC ATT TCA GAA TCA-3′, JNK2 was 5′-AAG CCG TCC TTT TCA GAA CCA-3′, and Mn-SOD (SOD2) was 5′-CAG GCC TGA TTA TCT AAA AGC-3′.
Mitochondrial Membrane Potential
Mitochondrial membrane potential (Δψm) was measured using the cationic fluorescent dye, tetramethylrhodamine methyl ester (Molecular Probes).23,24
Conversion of Tetramethylrhodamine Methyl Ester Fluorescent Intensity to Voltage
Mitochondrial membrane potential (Δψ m) was established by probing the fluorescent intensity as described previously.25
Flow Cytometry Analysis to Quantify Mitochondrial Superoxide Production
Mitochondrial superoxide (mtO2•−) production was measured using MitoSOX Red (Molecular Probes).
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was isolated using TRIzol (Invitrogen). RNA was reverse-transcribed by iScript cDNA synthesis kit (BioRad), followed by polymerase chain reaction amplification using the quantitative polymerase chain reaction Master Mix (Applied Biological Materials).
Immunoprecipitation Assay
Ubiquitinated Mn-SOD was detected by using immunoprecipitation Western blot analysis.
Cleaved Caspase-3 Activity Assay
Cleaved caspase-3 activities were measured by using enzyme-linked immunosorbent assay kit (Cell Signaling Technology) according to manufacturer’s instructions.
Statistical Analysis
Data are expressed as mean±SD and compared among separate experiments. Comparisons of multiple values were made by ANOVA, and statistical significance for pair-wise comparison was determined using the Tukey test. P<0.05 is considered statistically significant.
Results
OxLDL Induced JNK Activation
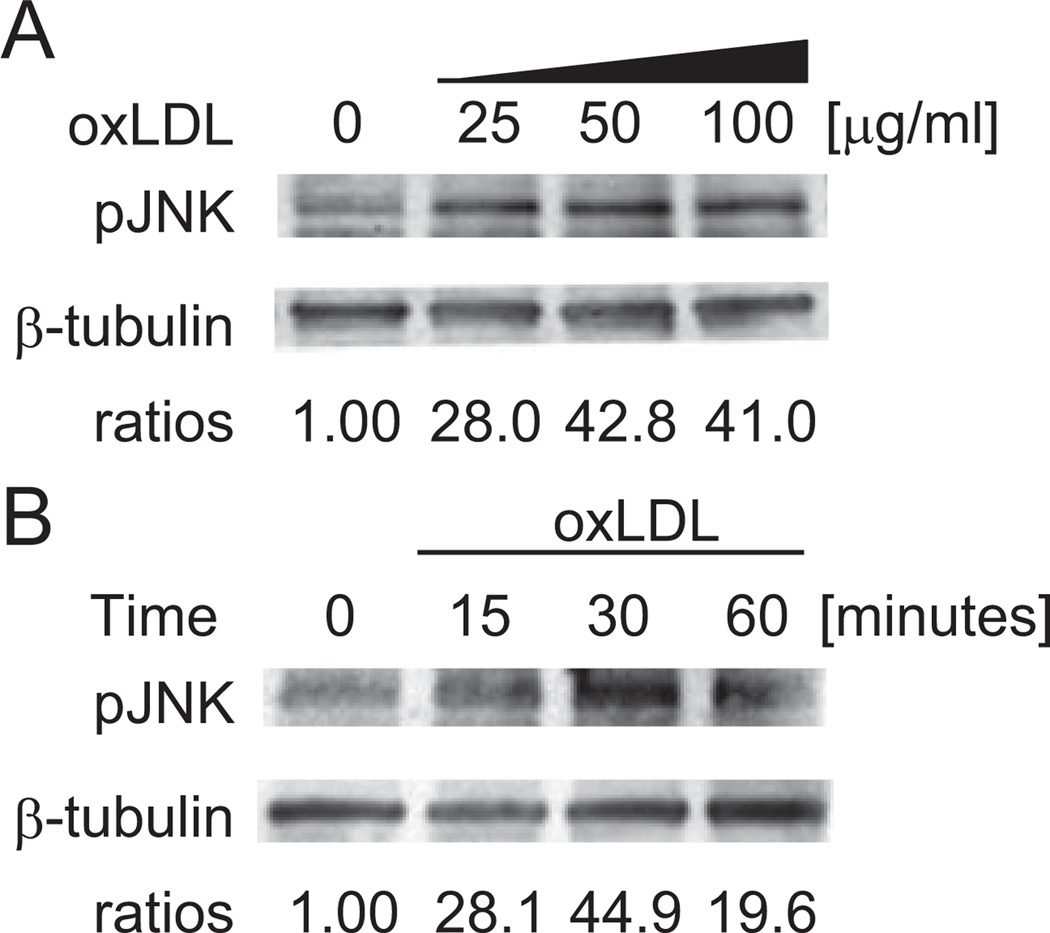
HAEC were treated with oxLDL at 25, 50, and 100 µg/mL for 30 minutes (Figure 1A). JNK phosphorylation was dose-dependent. Next, HAEC were treated with 50 µg/mL of oxLDL for 15, 30, and 60 minutes (Figure 1B). JNK activation peaked at 30 minutes. Hence, oxLDL induced JNK activation in time-dependent and dose-dependent manners.
Figure 1.
Time- and dose-dependent induction of JNK phosphorylation by oxLDL in human endothelial cells. Western blot analysis was performed to determine JNK phosphorylation in response to treatment of HAEC with (A) 25, 50, and 100 µg/mL of oxLDL for 30 minutes and (B) 50 µg/mL of oxLDL over the course of 1 hour. HAEC were lysed at the indicated time, and 10 µg of the entire cell protein was used to detect phosphorylated JNK. β-tubulin was used as a loading control. The blots were representative of 3 independent experiments with similar results.
JNK Influenced Mitochondrial Superoxide Production and Membrane Potential
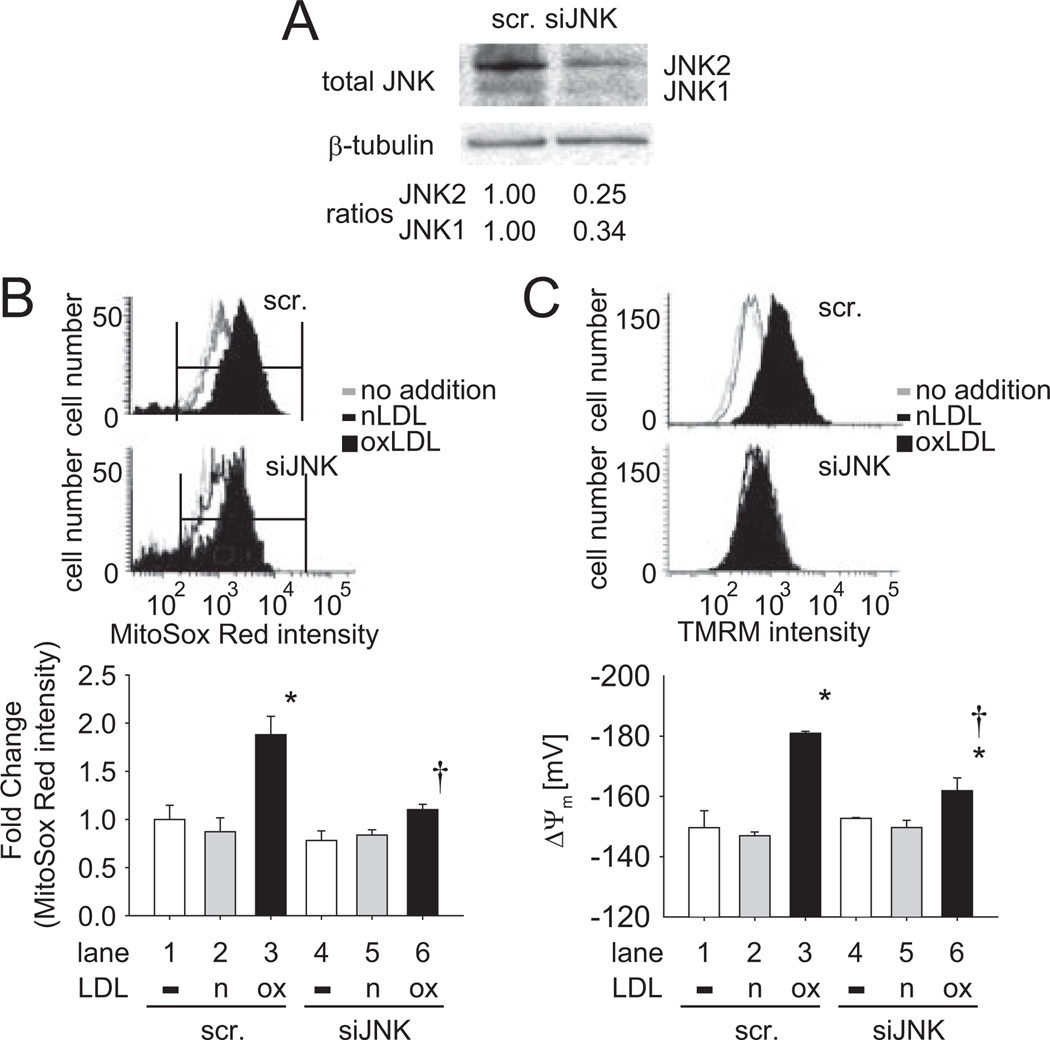
To assess whether oxLDL induced endothelial mitochondrial superoxide (mtO2•−) generation and change in mitochondrial membrane potential (Δψm) via JNK pathway, we silenced JNK1 and JNK2 gene expression with siRNA (siJNK). The effect of siRNA was confirmed by Western blot analysis (Figure 2A). JNK1 protein level was decreased by 66% and JNK2 protein level was decreased by 75% after siJNK transfection as compared to scramble siRNA. In response to oxLDL at 50 µg/mL for 1 hour, MitoSOX Red intensity, a probe for mtO2•−, was significantly increased by 1.88±0.19-fold (n=3; P<0.05; lane 1 vs 3), and this increase was reversed by siJNK (88.4% reduced; n=3; P<0.05; lane 3 vs 6; Figure 2B). Similarly, mitochondrial membrane potential (Δψm), as converted from the tetramethylrhodamine methyl ester intensity, was significantly increased by 18%, from −149.60±5.64mV to −180.97±0.64mV (n=3; P<0.05; lane 1 vs 3) in response to oxLDL. This increase was attenuated by siJNK (61.7% reduction; n=3; P<0.05; lane 3 vs 6; Figure 2C). Native LDL had no effect on mtO2•− and Δψm (lanes 2 and 5 in Figure 2B and 2C; n=3; P>0.05). Hence, oxLDL-activated JNK influenced mitochondrial redox status.
Figure 2.
SiJNK attenuated oxLDL-induced mitochondrial redox status. HAEC were transfected with siJNK or scramble siRNA for 48 hours. A, Cell lysate was used to verify the efficiency of siJNK on the protein level of JNK. The blots were representative of 2 independent experiments with similar results. B, Cells were preincubated with 5 µmol/L MitoSox Red for 10 minutes at 37°C. Next, cells were cultured in the absence or presence of 50 µg/mL of oxLDL for 1 hour. Cells were fixed by 1% paraformaldehyde and fluorescent intensity was measured by fluorescence-activated cell sorting (FACS). Top, typical histograms were showed in the absence and presence of LDL. Bottom, Bar graph was quantified from gated area shown in the hisograms. *P<0.05 vs in the absence of oxLDL and in the presence of scramble siRNA (lane 1). †P<0.05 vs in the presence of oxLDL and in the presence of scramble siRNA (lane 3). C, Cells were exposed to 50 µg/mL of oxLDL for 1 hour and tetramethylrhodamine methyl ester (TMRM+) intensity was measured by FACS. The fluorescence intensity was converted to mitochondrial membrane polarization (Δψm) as described (available online at http://atvb.ahajournals.org). *P<0.05 vs in the absence of oxLDL and in the presence of scramble siRNA (lane 1). †P<0.05 vs in the presence of oxLDL and in the presence of scramble siRNA (lane 3). These data represented the means from triplicates of 2 independent experiments. Scr, scramble; n, native LDL; ox, oxLDL.
OxLDL Decreased Mn-SOD Protein Levels Via JNK Activation
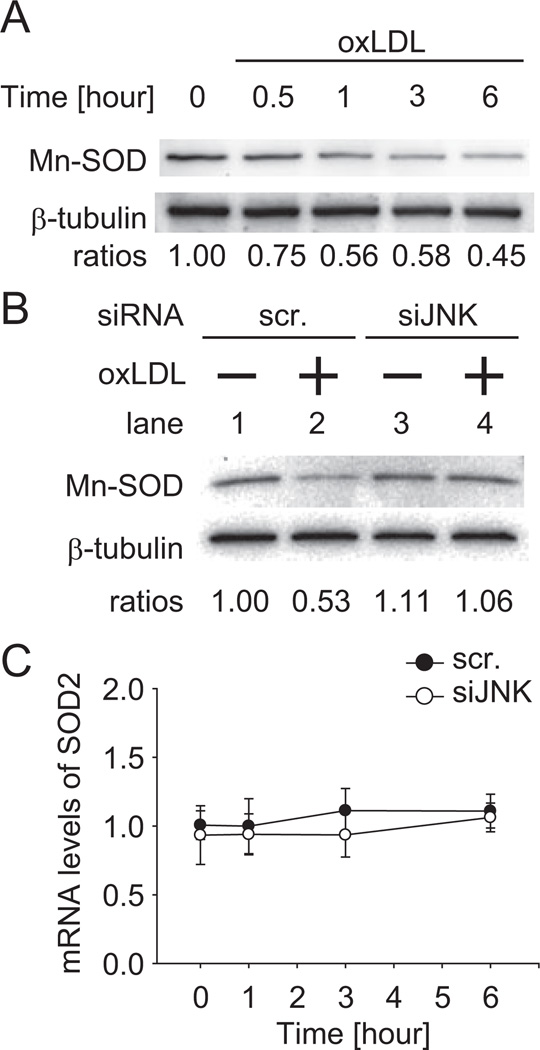
Mn-SOD is localized in the mitochondrial matrix, where mtO2•− is dismutated to hydrogen peroxide.26 We assessed whether oxLDL-induced JNK activation regulated Mn-SOD levels. In the presence of oxLDL, Mn-SOD protein levels decreased in a time-dependent manner (Figure 3A). We next examined the effect of siJNK on oxLDL regulation of Mn-SOD protein (Figure 3B). In the scramble siRNA-transfected HAEC, Mn-SOD was decreased by 47% after 6 hours of oxLDL treatment (50 µg/mL; lane 1 vs 2), whereas in the siJNK-transfected HAEC, Mn-SOD protein levels were unchanged with oxLDL treatment. However, Mn-SOD mRNA expression remained unchanged in the presence or absence of siJNK1 and siJNK2 (siJNK) over the course of 6 hours of oxLDL treatment (black circle, scramble siRNA; white circle, siJNK; n=3; P>0.05; Figure 3C). Thus, oxLDL significantly decreased Mn-SOD protein levels via JNK activation.
Figure 3.
OxLDL decreased Mn-SOD protein level via JNK. A, HAEC were incubated with 50 µg/mL of oxLDL at 30 minutes and 1, 3, and 6 hours; 10 µg entire cell protein was prepared and Mn-SOD levels were assessed. B, SiJNK or scramble siRNA transfected cells were incubated in the absence or presence of 50 µg/mL of oxLDL for 6 hours; 10 µg entire cell protein was prepared and Mn-SOD levels were assessed. These blots were representative of 2independent experiments with identical results. C, Cells were treated with 50 µg/mL of oxLDL over 6 hours (black circle, with scramble siRNA; white circle, with siJNK). Total RNA was prepared and the relative expression levels of mRNA were determined by quantitative real-time polymerase chain reaction. The data represented the means from triplicates of 2 independent experiments. The error bars represented the standard deviation of the mean.
OxLDL Induced Ubiquitination of Mn-SOD Via JNK Activation
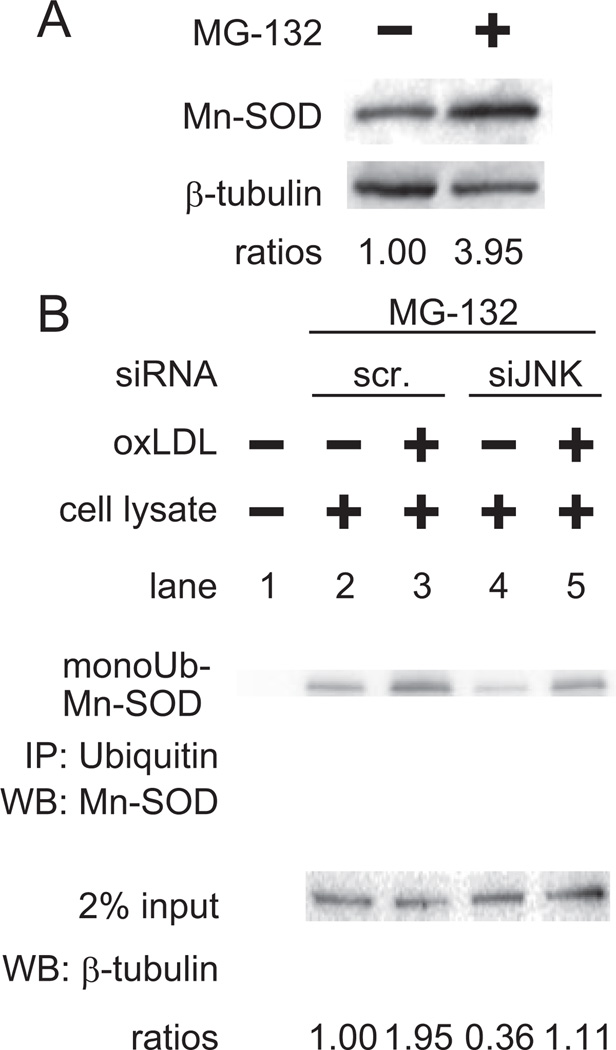
To assess whether the proteasome would degrade Mn-SOD at steady state, we treated HAEC with a proteasome inhibitor, MG-132, for 6 hours. Mn-SOD protein accumulated in the presence of 10 µmol/L of MG-132 (Figure 4A). Next, we assessed the effects of oxLDL on ubiquitination of Mn-SOD protein via JNK activation. Immunoprecipitation was performed by using ubiquitin antibody, followed by Western blot assay for Mn-SOD (Figure 4B). In the scramble siRNA-transfected HAEC, oxLDL induced ubiquitination of Mn-SOD (lane 2 vs 3), whereas in the siJNK transfected cells both basal and oxLDL-induced ubiquitination were attenuated (lane 2 vs 4 and lane 3 vs 5). These findings indicate that JNK modulated ubiquitination of Mn-SOD and subsequent protein degradation.
Figure 4.
OxLDL induced ubiquitination of Mn-SOD via JNK. A, Proteasomal degradation was assessed by determining Mn-SOD accumulation in the presence of the proteasome inhibitor, MG-132. HAEC were treated with 10 µmol/L of MG-132 for 6 hours; 10 µg entire cell lysate was prepared and the Mn-SOD was detected by Western blot analysis. B, Effects of siJNK on Mn-SOD ubiquitination. Cells were transfected with siJNK or scramble siRNA for 48 hours. Cells were preincubated with 10µmol/L of MG-132 for 1 hour. Next, cells were treated with 50 µg/mL of oxLDL for 6 hours; 500 µg entire cell lysate was prepared, endogenous ubiquitinated proteins were immunoprecipitated, and Mn-SOD protein was assessed by Western blot analysis; 2% of input protein was used as a loading control. These blots were representative of 2 independent experiments with identical results.
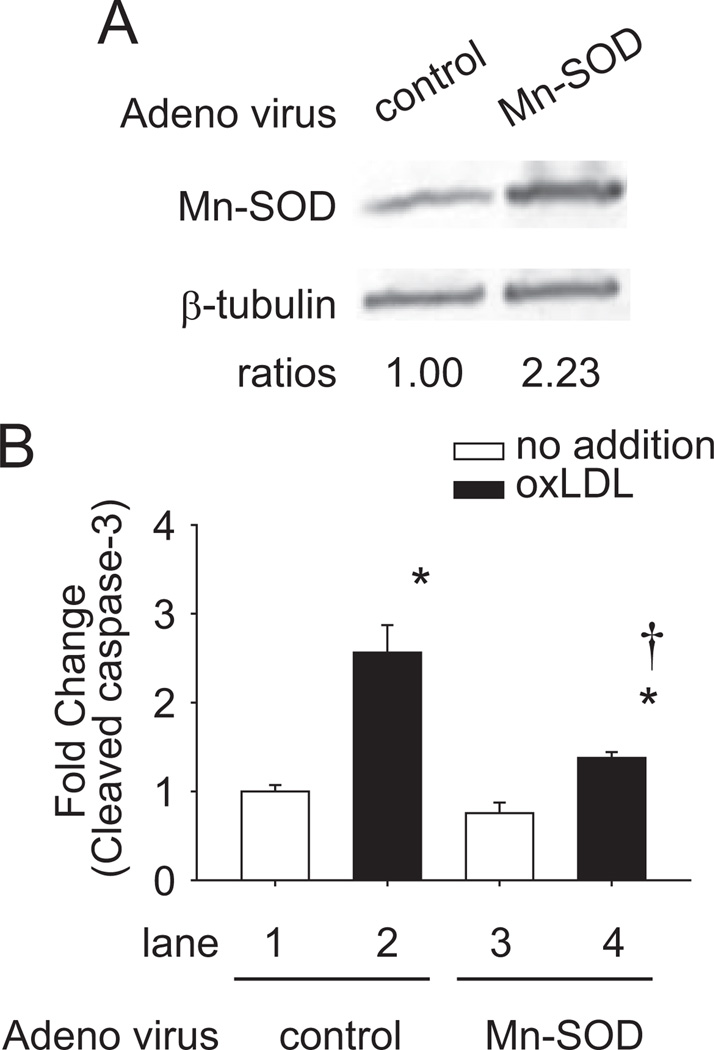
Mn-SOD Mitigated OxLDL-Induced Apoptosis
We also assessed whether overexpression of Mn-SOD attenuated oxLDL-induced apoptosis. Mn-SOD protein levels were upregulated by >2-fold after Mn-SOD adenoviral vector infection at multiplicity of infection of 15 for 48 hours (Figure 5A). Mn-SOD adenoviral vector or Ad-control–infected HAEC were treated with 100 µg/mL of oxLDL for 24 hours (Figure 5B). Caspase-3 activities were measured by enzyme-linked immunosorbent assay kit. In the Ad-control–infected HAEC, oxLDL induced a 2.56±0.31-fold increase in caspase-3 activity (n=3; P<0.05; lane 1 vs 2) (white block, no oxLDL; black block, with oxLDL), whereas in the Mn-SOD adenoviral vector-infected HAEC, oxLDL-induced caspase-3 activity was significantly attenuated by 76% (n=3; P<0.05; lane 2 vs 4).
Figure 5.
Mn-SOD inhibited oxLDL-induced apoptosis. HAEC were infected with human Mn-SOD adenoviral vector (Ad-Mn-SOD) or Ad-control at multiplicity of infection of 15 for 48 hours. A, Cell lysate was used to verify the efficiency of Ad-Mn-SOD on the Mn-SOD protein level. The blots were representative of 2 independent experiments with similar results. B, Effects of Ad-Mn-SOD on cell apoptosis. Cells were treated with 100 µg/mL of oxLDL for 24 hours (white block, no oxLDL; black block, with oxLDL). The cell lysates were collected and the caspase-3 activities were measured by enzyme-linked immunosorbent assay kit. These data represented the means from triplicates of 2 independent experiments. *P<0.05 vs in the absence of oxLDL and in the presence of Ad-control (lane 1). †P<0.05 vs in the presence of oxLDL and in the presence of Ad-control (lane 2).
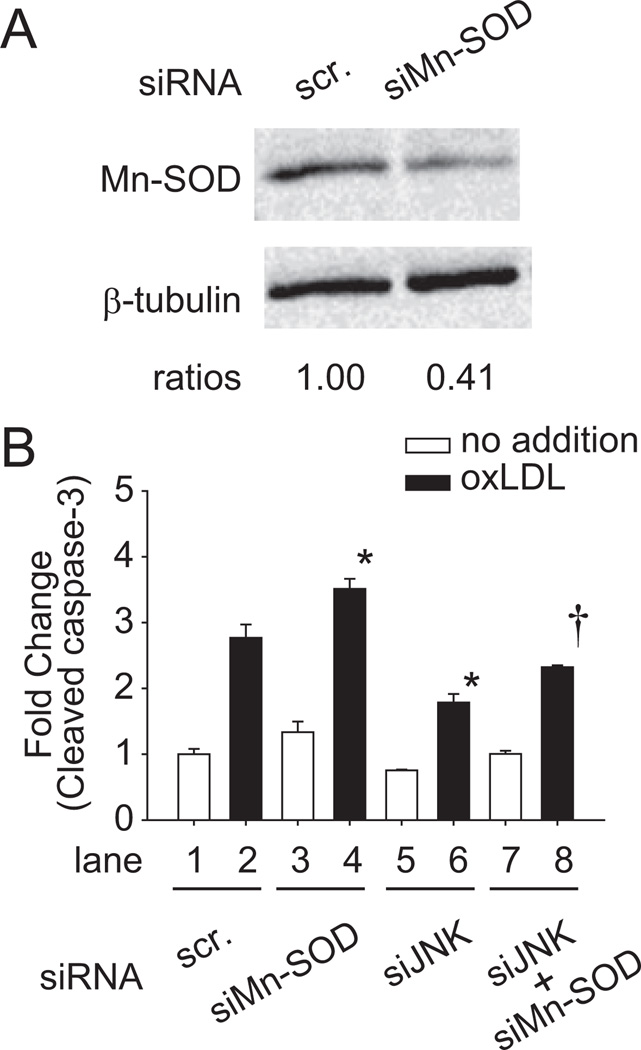
Mn-SOD and JNK Influenced Cleaved Caspase-3 Activities
We further assessed whether silencing JNK or Mn-SOD affected oxLDL-induced apoptosis. Mn-SOD small interference (siMn-SOD) transfection decreased Mn-SOD protein levels by 59% (Figure 6A). HAEC transfected with siJNK or siMn-SOD were treated with 100 µg/mL of oxLDL for 24 hours to induce apoptosis (Figure 6B). Caspase-3 activities were measured by using enzyme-linked immunosorbent assay kit. Of all of the conditions, oxLDL significantly induced caspase-3 activities (n=3; P<0.05; lane 1 vs lane 2, 4, 6, and 8; white block, no oxLDL; black block, with oxLDL). OxLDL induced a 2.77±0.20-fold increase in the caspase-3 activities in the scramble siRNA-transfected HAEC (lane 2). Caspase-3 activity was further increased by 3.51±0.15-fold (lane 4) in siMn-SOD–transfected HAEC. SiJNK significantly reduced oxLDL-induced caspase-3 activity by 55% (lane 6, 1.79±0.13-fold; lane 2 vs 6, n=3; P<0.05). This effect was abrogated by cotransfecting HAEC with siJNK and siMn-SOD (lane 8, 2.32±0.03-fold; lane 6 vs 8, n=3; P<0.05). These results supported the notion that oxLDL induced HAEC apoptosis via JNK-mediated ubiquitination and degradation of Mn-SOD (Figure 3B).
Figure 6.
The interaction of JNK and Mn-SOD regulated apoptosis in response to oxLDL. A, HAEC were transfected with siMn-SOD or scramble siRNA. After 48 hours of transfection, cell lysate was extracted and analyzed to verify the efficiency of siMn-SOD. The blots were representative of 2 independent experiments with similar results. B, Cells were transfected with siJNK, siMn-SOD, or scramble siRNA for 48 hours. Cells were incubated in the absence or presence of 100 µg/mL of oxLDL for 24 hours (white block, no oxLDL; black block, with oxLDL). The cell lysates were collected and the caspase-3 activities were measured by enzyme-linked immunosorbent assay kit. *P<0.05 vs in the presence of oxLDL and in the presence of scramble siRNA (lane 2). †P<0.05 vs in the presence of oxLDL and in the presence of siJNK (lane 6). The data were the means from triplicates of 2 independent experiments.
Discussion
This study presents evidence that oxLDL regulates mitochondrial redox status and apoptosis via JNK activation. We demonstrate that oxLDL increased mitochondrial superoxide (mtO2•−) production and mitochondrial membrane potential (ψΔm), which were significantly reduced by siJNK (Figure 2). We further demonstrate that JNK stimulated ubiquitination and degradation of Mn-SOD (Figure 4). Moreover, knockdown of JNK or overexpression of Mn-SOD significantly reduced oxLDL-induced caspase-3 activities (Figures 5 and 6). Hence, oxLDL-induced JNK activation regulates mitochondrial redox status and Mn-SOD protein degradation via JNK-dependent ubiquitination leading to endothelial cell apoptosis.
Emerging evidence supports the role of JNK activation in oxidative stress and atherosclerosis. OxLDL is implicated in activation of JNK in macrophages, and uptake of oxidatively modified LDL binds to the scavenge receptors of macrophages.27 Ricci et al19 showed that macrophages lacking JNK2 displayed suppressed foam cell formation and apoE−/− and JNK2−/− double-knockout mice had less atherosclerosis compared to apoE−/− mice. They further showed that macrophage-restricted deletion of JNK2 was sufficient to decrease atherogenesis and that pharmacological inhibition of JNK activity efficiently reduced plaque formation.
Zhou et al28 reported that hydrogen peroxide-activated JNK transmigrated into the mitochondria. Our current studies demonstrated that oxLDL increased mtO2•−, and Δψm also induced endothelial cell apoptosis via JNK activation.
In this study, we showed that JNK induces mitochondrial oxidative stress by decreasing Mn-SOD protein levels. During oxidative phosphorylation, ≈1.5% to 2% of electrons leak out from complex I and III to form superoxide anion (O2•−).26,29 Mn-SOD in the mitochondrial matrix dismutates O2•− to hydrogen peroxide. In the hippocampus, Mn-SOD activities were significantly reduced in apoE−/− mice compared to the control mice.30 In tissue culture studies, Mn-SOD protein level was upregulated in response to atheroprotective shear stress, and Mn-SOD overexpression downregulated oxidative modification of LDL.31 In this study, we demonstrate that overexpression of Mn-SOD rendered an antiapoptotic effect against oxLDL-induced caspase-3 activity (Figure 5B, lane 4 vs 2). Knockdown of Mn-SOD accentuated oxLDL-induced caspase-3 activity compared to scramble siRNA (Figure 6B, lane 4 vs 2), whereas siJNK abrogated the caspase-3 activity (lane 6 vs 2). Also, siMn-SOD interfered with the effect of siJNK (lane 8 vs 6). Previous studies using cardiomyocytes demonstrated an antagonistic role between JNK and Mn-SOD in cell apoptosis,32 suggesting the importance of Mn-SOD regulation in apoptosis. The interplay between JNK and Mn-SOD is further supported by findings in JNK2−/− mice.33 Osto et al33 reported that the levels of extracellular superoxide dismutase and Mn-SOD were decreased in wild-type mice fed a high-cholesterol diet but not in JNK2−/− mice fed a high-cholesterol diet.
Several receptors mediate oxLDL uptake in the vascular wall.34–37 CD36 and lectin-like oxidized LDL receptor are expressed on activated human large vessel endothelial cells38–39 and are implicated in mediating oxLDL-induced endothelial cell apoptosis.40,41 Bao et al42 showed that oxLDL induced lectin-like oxidized LDL receptor-1 and downregulated SOD activity in endothelial cells, supporting the notion that oxLDL-activated JNK induced ubiquitination and degradation of SOD protein in our study. However, further study is required to assess the role of receptors in oxLDL-induced endothelial apoptosis via Mn-SOD degradation.
Multiple signal pathways have been implicated in oxLDL-induced endothelial cell dysfunction. We observed that JNK inhibitor, but not ERK or P38 kinase inhibitor, inhibited oxLDL-induced Mn-SOD downregulation, although these 3 inhibitors significantly attenuated oxLDL-induced apoptosis (Supplementary Figure I, available online at http://atvb.ahajournals.org). These results suggest that mitogen-activated protein kinase ERK and p38 are also involved in oxLDL-induced apoptosis of endothelial cells, but only JNK regulated endothelial apoptosis via Mn-SOD ubiquitination/degradation process.
The novel finding in this study was that JNK regulated Mn-SOD levels by increasing its ubiquitination, resulting in protein degradation. Several lines of evidence supported that JNK modulated protein stability via ubiquitination. Cho et al43 reported that JNK induced Nrf2 degradation via the ubiquitination pathway. JNK also inhibited ubiquitination and stabilized specific proteins, including p21,44 p53,45 and Sp1.46 In our study, silencing JNK significantly decreased ubiquitination of Mn-SOD, resulting in a decrease level of Mn-SOD protein degradation. JNK has been reported to mediate phosphorylation and activation of E3 ubiquitin ligase. Gao et al47 reported that activation of the JNK mitogen-activated protein kinase cascade after T-cell stimulation accelerated degradation of c-Jun and JunB through phosphorylation-dependent activation of the E3 ligase Itch. However, the E3 ligase specific for Mn-SOD protein remains to be defined. The precise mechanism whereby JNK regulates Mn-SOD ubiquitination will be of great interest for future investigation. In summary, we demonstrate the important role of oxLDL-induced JNK in regulating mitochondrial redox status and apoptosis.
Supplementary Material
Acknowledgments
The authors express gratitude to the Atherosclerosis Research Unit at UCLA for providing the native and oxidized LDL. The authors are grateful to Dr Donald Heistad of Carver College of Medicine at the University of Iowa for providing the Mn-SOD adenoviral vector.
Sources of Funding
These studies were supported by American Heart Association (GIA 0655051Y to T.K.H.) and National Institutes of Health (HL-068689 and HL-083015 to T.K.H.).
Footnotes
Disclosures
None.
References
- 1.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 3.Cominacini L, Pasini A, Garbin U, Evangelista S, Crea AE, Tagliacozzi D, Nava C, Davoli A, LoCascio V. Zofenopril inhibits the expression of adhesion molecules on endothelial cells by reducing reactive oxygen species. Am J Hypertens. 2002;15:891–895. doi: 10.1016/s0895-7061(02)02995-3. [DOI] [PubMed] [Google Scholar]
- 4.Martinet W, Kockx MM. Apoptosis in atherosclerosis: focus on oxidized lipids and inflammation. Curr Opin Lipidol. 2001;12:535–541. doi: 10.1097/00041433-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Harada-Shiba M, Kinoshita M, Kamido H, Shimokado K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J Biol Chem. 1998;273:9681–9687. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- 6.Dimmeler S, Haendeler J, Galle J, Zeiher AM. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases. A mechanistic clue to the ‘response to injury’ hypothesis. Circulation. 1997;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- 7.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 8.Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H852–H861. doi: 10.1152/ajpheart.00015.2005. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zhao J, Xu J, Zhao B, Zhang Y, Zhang S, Miao J. Protective effects of a benzoxazine derivative against oxidized LDL-induced apoptosis and the increases of integrin beta4, ROS, NF-kappaB and P53 in human umbilical vein endothelial cells. Bioorg Med Chem Lett. 2009;19:2896–2900. doi: 10.1016/j.bmcl.2009.03.070. [DOI] [PubMed] [Google Scholar]
- 11.de Nigris F, Franconi F, Maida I, Palumbo G, Anania V, Napoli C. Modulation by alpha- and gamma-tocopherol and oxidized low-density lipoprotein of apoptotic signaling in human coronary smooth muscle cells. Biochem Pharmacol. 2000;59:1477–1487. doi: 10.1016/s0006-2952(00)00275-6. [DOI] [PubMed] [Google Scholar]
- 12.Deng T, Zhang L, Ge Y, Lu M, Zheng X. Redistribution of intracellular calcium and its effect on apoptosis in macrophages: Induction by oxidized LDL. Biomed Pharmacother. 2009;63:267–274. doi: 10.1016/j.biopha.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Li HL, Wang AB, Zhang R, Wei YS, Chen HZ, She ZG, Huang Y, Liu DP, Liang CC. A20 inhibits oxidized low-density lipoprotein-induced apoptosis through negative Fas/Fas ligand-dependent activation of caspase-8 and mitochondrial pathways in murine RAW264.7 macrophages. J Cell Physiol. 2006;208:307–318. doi: 10.1002/jcp.20665. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J, Cui R, Chen CH, Du J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148:2085–2094. doi: 10.1210/en.2006-1709. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohit AA, Martin JH, Miller CA. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995;14:67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 17.Metzler B, Hu Y, Dietrich H, Xu Q. Increased expression and activation of stress-activated protein kinases/c-Jun NH(2)-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol. 2000;156:1875–1886. doi: 10.1016/S0002-9440(10)65061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, Hersberger M, Eriksson U, Eberli FR, Becher B, Boren J, Chen M, Cybulsky MI, Moore KJ, Freeman MW, Wagner EF, Matter CM, Luscher TF. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Weintraub NL, Rios CD, Chappell DA, Zwacka RM, Engelhardt JF, Oberley LW, Yan T, Heistad DD, Spector AA. Overexpression of human superoxide dismutase inhibits oxidation of low-density lipoprotein by endothelial cells. Circ Res. 1998;82:1289–1297. doi: 10.1161/01.res.82.12.1289. [DOI] [PubMed] [Google Scholar]
- 22.Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Floryk D, Houstek J. Tetramethyl rhodamine methyl ester (TMRM) is suitable for cytofluorometric measurements of mitochondrial membrane potential in cells treated with digitonin. Biosci Rep. 1999;19:27–34. doi: 10.1023/a:1020193906974. [DOI] [PubMed] [Google Scholar]
- 24.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Beebe T, Cui J, Rouhanizadeh M, Ai L, Wang P, Gundersen M, Takabe W, Hsiai TK. Pulsatile shear stress increased mitochondrial membrane potential: implication of Mn-SOD. Biochem Biophys Res Commun. 2009;388:406–412. doi: 10.1016/j.bbrc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 27.Katayama I, Hotokezaka Y, Matsuyama T, Sumi T, Nakamura T. Ionizing radiation induces macrophage foam cell formation and aggregation through JNK-dependent activation of CD36 scavenger receptors. Int J Radiat Oncol Biol Phys. 2008;70:835–846. doi: 10.1016/j.ijrobp.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 29.Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–389. doi: 10.1016/s0076-6879(03)61019-0. [DOI] [PubMed] [Google Scholar]
- 30.Ramassamy C, Krzywkowski P, Averill D, Lussier-Cacan S, Theroux L, Christen Y, Davignon J, Poirier J. Impact of apoE deficiency on oxidative insults and antioxidant levels in the brain. Brain Res Mol Brain Res. 2001;86:76–83. doi: 10.1016/s0169-328x(00)00268-0. [DOI] [PubMed] [Google Scholar]
- 31.Ai L, Rouhanizadeh M, Wu JC, Takabe W, Yu H, Alavi M, Li R, Chu Y, Miller J, Heistad DD, Hsiai TK. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. Am J Physiol Cell Physiol. 2008;294:C1576–C1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 33.Osto E, Matter CM, Kouroedov A, Malinski T, Bachschmid M, Camici GG, Kilic U, Stallmach T, Boren J, Iliceto S, Luscher TF, Cosentino F. c-Jun N-terminal kinase 2 deficiency protects against hypercholesterolemia-induced endothelial dysfunction and oxidative stress. Circulation. 2008;118:2073–2080. doi: 10.1161/CIRCULATIONAHA.108.765032. [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler SW, Parthasarathy S, Carew TE, Steinberg D, Witztum JL. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity . N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 36.Curtiss LK. Reversing atherosclerosis? N Engl J Med. 2009;360:1144–1146. doi: 10.1056/NEJMcibr0810383. [DOI] [PubMed] [Google Scholar]
- 37.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Mehta JL. Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ Res. 2009;104:566–568. doi: 10.1161/CIRCRESAHA.109.194209. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Renier G. The oral anti-diabetic agent, gliclazide, inhibits oxidized LDL-mediated LOX-1 expression, metalloproteinase-9 secretion and apoptosis in human aortic endothelial cells. Atherosclerosis. 2009;204:40–46. doi: 10.1016/j.atherosclerosis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Yang JH, Burns AR, Chen HH, Tang D, Walterscheid JP, Suzuki S, Yang CY, Sawamura T, Chen CH. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res. 2009;104:619–627. doi: 10.1161/CIRCRESAHA.108.190116. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 42.Bao M, Lou Y. Isorhamnetin prevents endothelial cell injuries from oxidized LDL via activation of p38MAPK. Eur J Pharmacol. 2006;547:22–30. doi: 10.1016/j.ejphar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Cho MK, Kim WD, Ki SH, Hwang JI, Choi S, Lee CH, Kim SG. Role of Galpha12 and Galpha13 as novel switches for the activity of Nrf2, a key antioxidative transcription factor. Mol Cell Biol. 2007;27:6195–6208. doi: 10.1128/MCB.02065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Y, Chen H, Qiao B, Liu Z, Luo L, Wu Y, Yin Z. c-Jun NH2-terminal kinase decreases ubiquitination and promotes stabilization of p21(WAF1/CIP1) in K562 cell. Biochem Biophys Res Commun. 2007;355:263–268. doi: 10.1016/j.bbrc.2007.01.146. [DOI] [PubMed] [Google Scholar]
- 45.Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu J, Ong CN, Shen HM. Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilization. Mol Cancer Ther. 2007;6:1338–1347. doi: 10.1158/1535-7163.MCT-06-0638. [DOI] [PubMed] [Google Scholar]
- 46.Chuang JY, Wang YT, Yeh SH, Liu YW, Chang WC, Hung JJ. Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Mol Biol Cell. 2008;19:1139–1151. doi: 10.1091/mbc.E07-09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.