Abstract
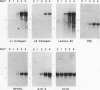
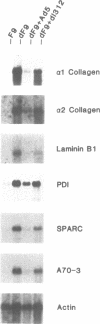
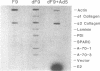
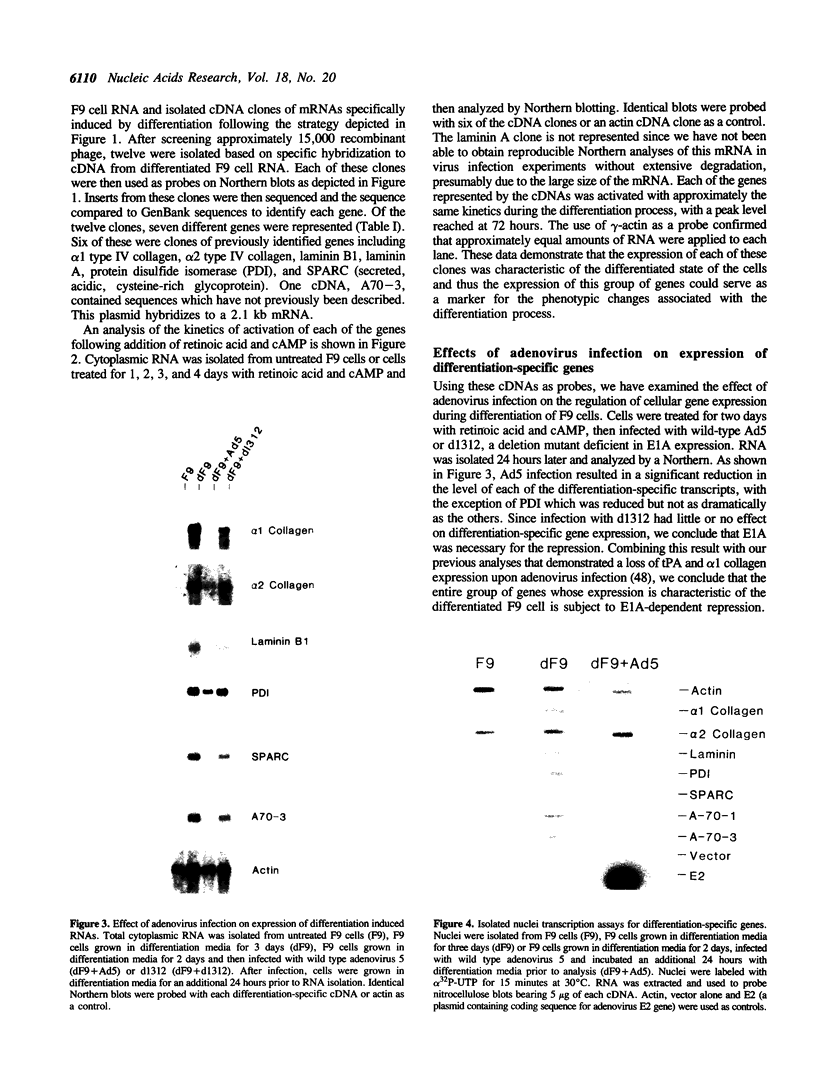
Previous experiments have demonstrated a link between transcriptional regulatory mechanisms acting during F9 cell differentiation and transcription control by the adenovirus E1A gene. We have isolated a number of differentiation-specific genes by cDNA cloning to determine if E1A exerts a coordinated control over differentiation specific gene expression. The mRNAs encoded by these cDNAs were undetectable or only barely detectable in undifferentiated cells but then rose in concentration upon differentiation. Analysis of transcription rates in isolated nuclei revealed that all but one of the genes was transcriptionally regulated during differentiation. Interestingly, alpha 2-type IV collagen expression was activated by a post-transcriptional mechanism since the gene was transcribed in both undifferentiated and differentiated cells whereas the cytoplasmic mRNA was undetectable in undifferentiated cells but rose in abundance in parallel with other regulated transcripts. Adenovirus infection of differentiated F9 cells reduced the cytoplasmic mRNA levels of each of the differentiation specific genes to near that found in the undifferentiated cell. Of those genes that were transcriptionally activated by differentiation, adenovirus infection specifically inhibited transcription. In contrast, although the alpha 2 collagen mRNA levels were reduced by adenovirus infection similar to the other mRNAs, the control was post-transcriptional since transcription of the gene was unaffected. Thus, the mechanism for loss of gene expression mediated by E1A reflects the mechanism by which the gene was activated during differentiation. Based on these results we suggest that E1A controls the expression of the F9 cell phenotype by targeting a regulatory activity acting early in the differentiation program.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bergman Y., Shavit D. Regulation of the Ig kappa-chain enhancer by the adenovirus E1A gene products. Repression in lymphoid cells, activation in fibroblasts. J Immunol. 1988 Mar 15;140(6):2073–2080. [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Carlock L. R., Jones N. C. Transformation-defective mutant of adenovirus type 5 containing a single altered E1a mRNA species. J Virol. 1981 Dec;40(3):657–664. doi: 10.1128/jvi.40.3.657-664.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémisi C., Duprey P. A labile inhibitor blocks endo A gene transcription in murine undifferentiated embryonal carcinoma cells. Nucleic Acids Res. 1987 Aug 11;15(15):6105–6116. doi: 10.1093/nar/15.15.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M. E1A 13S and 12S mRNA products made in Escherichia coli both function as nucleus-localized transcription activators but do not directly bind DNA. Mol Cell Biol. 1985 Oct;5(10):2653–2661. doi: 10.1128/mcb.5.10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Linney E. Polyoma mutants that productively infect F9 embryonal carcinoma cells do not rescue wild-type polyoma in F9 cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1479–1483. doi: 10.1073/pnas.79.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J., Heijneker H. L. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. doi: 10.1038/251687a0. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Chambon P. Repression of the immunoglobulin heavy chain enhancer by the adenovirus-2 E1A products. Science. 1985 Dec 20;230(4732):1391–1394. doi: 10.1126/science.2999984. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddurah-Daouk R., Lillie J. W., Daouk G. H., Green M. R., Kingston R., Schimmel P. Induction of a cellular enzyme for energy metabolism by transforming domains of adenovirus E1a. Mol Cell Biol. 1990 Apr;10(4):1476–1483. doi: 10.1128/mcb.10.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Helfman D. M., Williams J. G., Hogan B. L. Isolation of cDNA clones for basal lamina components: type IV procollagen. Nucleic Acids Res. 1983 Sep 24;11(18):6199–6209. doi: 10.1093/nar/11.18.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Schiavi S. C., Huse W., Johnson G. L., Ruley H. E. myc and E1A oncogenes alter the responses of PC12 cells to nerve growth factor and block differentiation. Oncogene. 1987;1(4):361–367. [PubMed] [Google Scholar]
- Montell C., Courtois G., Eng C., Berk A. Complete transformation by adenovirus 2 requires both E1A proteins. Cell. 1984 Apr;36(4):951–961. doi: 10.1016/0092-8674(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Definition and mapping of adenovirus 2 nuclear transcription. Methods Enzymol. 1980;65(1):768–785. doi: 10.1016/s0076-6879(80)65072-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Isolation and analysis of nuclear RNA. Methods Enzymol. 1987;152:234–241. doi: 10.1016/0076-6879(87)52025-0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Regulation of early adenovirus gene expression. Microbiol Rev. 1987 Dec;51(4):419–430. doi: 10.1128/mr.51.4.419-430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988 Jan 25;263(3):1563–1569. [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Segal S., Khoury G. Differentiation as a requirement for simian virus 40 gene expression in F-9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5611–5615. doi: 10.1073/pnas.76.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Kitchener K., Kao H. T., Hickey E., Weber L., Voellmy R., Heintz N., Nevins J. R. Selective induction of human heat shock gene transcription by the adenovirus E1A gene products, including the 12S E1A product. Mol Cell Biol. 1987 Aug;7(8):2884–2890. doi: 10.1128/mcb.7.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J. Differential regulation of viral and cellular genes in F9 mouse embryonal carcinoma cells. Nucleic Acids Res. 1987 Nov 25;15(22):9379–9395. doi: 10.1093/nar/15.22.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Ziff E. B. Repression of insulin gene expression by adenovirus type 5 E1a proteins. Mol Cell Biol. 1987 Mar;7(3):1164–1170. doi: 10.1128/mcb.7.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C., Harlow E. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 1987 Jul;6(7):2027–2035. doi: 10.1002/j.1460-2075.1987.tb02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Webster K. A., Muscat G. E., Kedes L. Adenovirus E1A products suppress myogenic differentiation and inhibit transcription from muscle-specific promoters. Nature. 1988 Apr 7;332(6164):553–557. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- Winberg G., Shenk T. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 1984 Aug;3(8):1907–1912. doi: 10.1002/j.1460-2075.1984.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. S., Raychaudhuri P., Jakoi L., Nevins J. R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989 Feb;9(2):578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. S., Weigel R., Hiebert S., Nevins J. R. Adenovirus E1A-mediated negative control of genes activated during F9 differentiation. Mol Cell Biol. 1989 Jul;9(7):3109–3113. doi: 10.1128/mcb.9.7.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]