Abstract
Low back pain is one of the most common ailments in the general population, which tends to increase in severity along with aging. While few patients have severe enough symptoms or underlying pathology to warrant surgical intervention, in those select cases treatment choices remain controversial and reimbursement is a substancial barrier to surgery. The object of this study was to examine outcomes of discogenic back pain without radiculopathy following minimally-invasive lateral interbody fusion. Twenty-two patients were treated at either one or two levels (28 total) between L2 and 5. Discectomy and interbody fusion were performed using a minimallyinvasive retroperitoneal lateral transpsoas approach. Clinical and radiographic parameters were analyzed at standard pre- and postoperative intervals up to 24 months. Mean surgical duration was 72.1 minutes. Three patients underwent supplemental percutaneous pedicle screw instrumentation. Four (14.3%) stand-alone levels experienced cage subsidence. Pain (VAS) and disability (ODI) improved markedly postoperatively and were maintained through 24 months. Segmental lordosis increased significantly and fusion was achieved in 93% of levels. In this series, isolated axial low back pain arising from degenerative disc disease was treated with minimally-invasive lateral interbody fusion in significant radiographic and clinical improvements, which were maintained through 24 months.
1. Introduction (Succinct)
Intervertebral disc degeneration in the spine is natural process of aging and in many cases is asymptomatic [1]. However, low back pain (LBP) is strongly associated with lumbar disc degeneration [2]. LBP is one of the most common reasons for physician visits and loss of workplace productivity worldwide, thus the issue encompasses important clinic and socioeconomic consequences.
Conservative (nonoperative) care for LBP, while covering many different modalities, generally includes treatment with NSAIDs, weak opioids, and exercise therapy [3]. When extensive conservative therapies fail to adequately manage LBP, lumbar fusion is on possible surgical option, though its use remains controversial, as reported in the literature [4–8].
The objective of this work was to evaluate minimally invasive lateral interbody fusion in the surgical treatment of lumbar discogenic pain, and to perform a literature review of degenerative disc disease and its treatment in the literature.
2. Methods
Data were collected through retrospective review of prospectively collected clinical and radiographic registry at a single institution. Inclusion in the current study included consecutively treated patients with degenerative disc disease presenting with discogenic low back pain without radicular symptoms, after failing at least 6 months of conservative care. Discogenic pain was assessed by clinical examination [9], such as centralization phenomenon and pain during standing, and radiological signs of degeneration [10], such as black discs and endplate modifications. Provocative discography was not routinely used in making diagnostic conclusions. Patients with idiopathic/degenerative scoliosis or grade II/III/IV spondylolisthesis were excluded from the study. A psychological screening [11] was performed preoperatively, to assess psychosocial features, patient understanding and to adapt patient expectations according to the surgical objective.
Patients were treated via the minimally invasive, lateral retroperitoneal transpsoas approach [12]. The surgical procedure was performed with patients in a true 90° lateral decubitus position and the table was flexed to increase the distance between the iliac crest and the rib cage. Retroperitoneal blunt was used to dissect through the psoas muscle, using progressive dilators and an expandable retractor to expose the lateral surface of the spine. Real-time directional electromyography (EMG) with discrete-threshold responses was used in all cases (NeuroVision JJB System, NuVasive Inc, San Diego, CA). Wide discectomies were performed with release of the contralateral annulus while preserving the anterior and posterior longitudinal ligaments. Interbody spacers were placed on the lateral and posterolateral borders of the apophyseal ring to increase contact with strong cortical bone [13, 14], to restore disc height, sagittal and coronal plane alignment [15–18], and to indirectly decompress the neural structures [19]. The interbody grafts were made from polyetheretherketone and filled with recombinant human BMP-2 (Infuse, Medtronic Sofamor Danek, Memphis, TN), silicate substituted calcium phosphate (Actifuse ABX, Apatech, Hertfordshire, England), calcium sodium phosphate cement (Graftys HBS, Graftys, Aix-en-Provence, France), or hydroxyapatite (HAP-91, Implamed, Sao Paulo, Brazil).
Clinical evaluations were performed by a clinical and included a physical exam for lower extremity motor and sensory function and self-assessed questionnaires using the Oswestry disability index (ODI) and visual analogue scale (VAS) for back and leg pain. Evaluations were performed preoperatively and at 1 and 6 weeks, 3, 6, 12, and 24 months postoperative. Minimum follow-up for inclusion in the current analysis was 24 months postoperatively.
Bony fusion was assessed by two spine surgeons and two spine researchers in CT scans and dynamic X-rays. Fusion was considered complete when translational motion was <3 mm, angular motion was <5°, and >50% of disc space showed complete bony bridging.
Statistical analyses included descriptive statistics to characterize baseline variables and paired t-testing to evaluate differences in mean outcome variables from pre- to postoperative time points. Statistical analyses were performed using SPSS software (SPSS, Version 10, SPSS, Chicago, Ill, USA) and statistical significance was evaluated at P < 0.05.
3. Results
From 220 patients that underwent lateral interbody fusion for degenerative disc disease between August 2007 and December 2009, 22 (10%) patients met inclusion-exclusion criteria (mean age 57.6 years, range 32–85; mean BMI 28.9, SD 7.9; 50% female) with 28 spine levels treated. One- and two-level procedures were performed in 16 (73%) and 6 (27%) cases, respectively. Levels treated included L2-3, L3-4, and/or L4-5.
Surgical procedures were performed in an average of 72.1 minutes (range 40–110 min) with an average blood loss of less than 50cc. The average hospital discharge was 21 hours (range 8–44 hours). Intraoperative complications included one instance of anterior longitudinal ligament rupture, which resulted in the placement of posterior pedicle screws. No other intraoperative complications were observed. Three patients (5 spine levels) required supplemental percutaneous pedicle screw instrumentation for grade I spondylolisthesis with instability, while other cases (23 spine levels) were performed as stand-alone interbody constructs.
Four stand-alone levels experienced cage subsidence (14.3%) by 6-week followup. These patients experienced transient axial back pain (persisting several months) and in one (4.5%) case radiculopathy arose, which required a foraminotomy 12 months postoperative.
Clinical outcomes improved postoperatively (Figure 1 and Table 1). LBP, assessed by VAS, showed a 44.2% improvement at the first postoperative visit (1 week) further improving to a 70.1% reduction at final followup. Disability was also significantly lowered as early as one week following surgery (24% improvement in ODI) and was further lowered until last followup, when a 52.5% improvement was observed (Figure 1).
Figure 1.
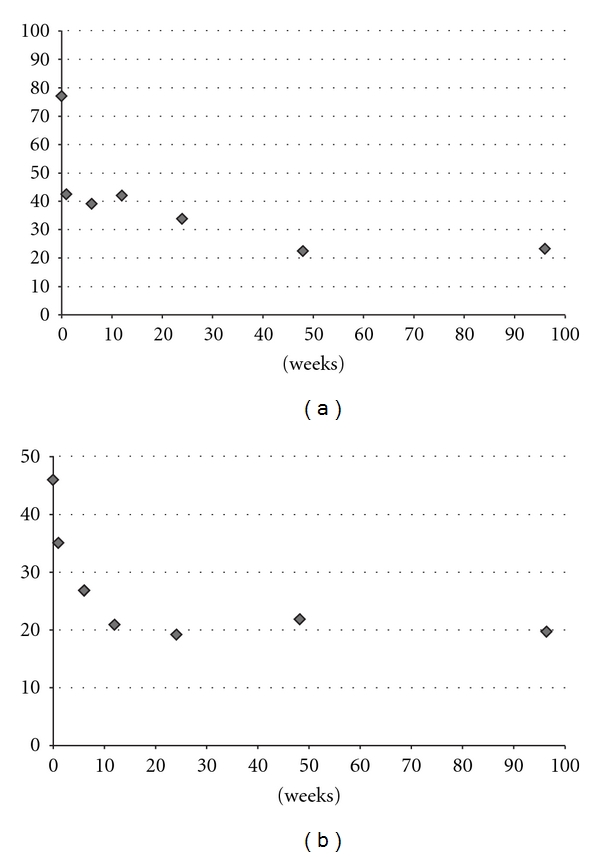
Clinical outcomes. (a) VAS back pain scores, all postoperative results are statistically significant compared to baseline (P < 0.003). (b) ODI scores, results are statistically significant since 1-week followup (P < 0.04) and in other postoperative visits (P < 0.001) compared to baseline.
Table 1.
Clinical and radiological results.
| Preop | 6 weeks | P value | 24 months | P value | |
|---|---|---|---|---|---|
| VAS (cm) | 7.7 ± 2.4 | 4.3 ± 2.2 | 0.001* | 2.3 ± 1.9 | <0.001* |
| ODI (%) | 46 ± 19 | 27 ± 14 | <0.001* | 19.6 ± 13 | 0.003* |
| Segmental Lordosis (degrees) | 12.2° ± 7.4° | — | — | 16.7° ± 6.5° | 0.031* |
| Fusion | — | — | — | 92.9% (26/28) | — |
P Values are referent to comparison to Preop values. *Statistically significant.
Index level lordosis significantly changed from a mean preoperative value of 12.2° (7.4° SD) to 16.7° (6.5° SD) at final followup (P = 0.032). Bony fusion was observed in 92.9% (26/28) of total lumbar levels treated (exemplified in Figures 2 and 3).
Figure 2.
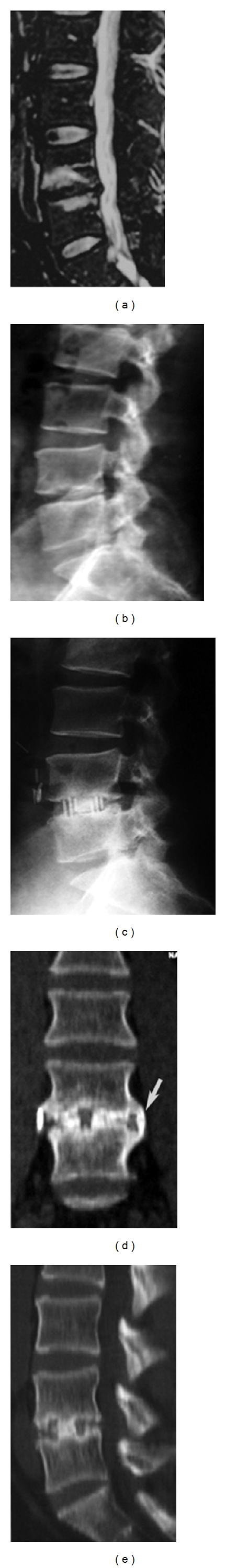
Case example number 1. Male, 54 years old, 7-year pain history which used to get worst by end of the day, refractory to physiotherapy and chiropractic. VAS scores-preoperative 8; 1-week 2; 24-month 1. Patient underwent an L4L5 stand-alone lateral interbody fusion. (a) Preoperative sagittal MRI. (b) Preoperative lateral orthostatic X-ray. (c) 24-month lateral orthostatic X-ray. (d) 24-month computed tomography coronal reconstruction, arrow shows fusion sentinel sign. (e) 24-month computed tomography sagittal reconstruction.
Figure 3.
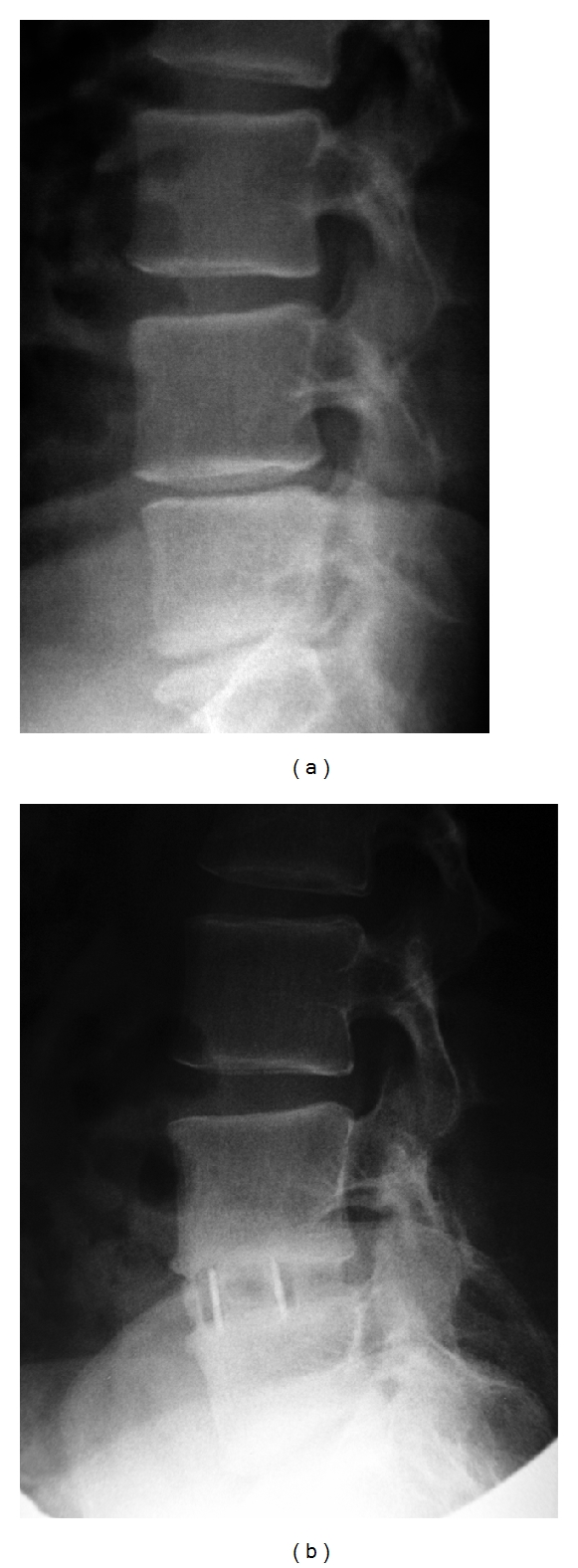
Case example number 2. Male, 58 years old, long history of lumbar axial pain and recurrent crisis event. VAS scores-preoperative 6; 1-week 3; 24-month 1. Patient underwent an L4L5 stand-alone lateral interbody fusion using rh-BMP. (a) Preoperative lateral orthostatic X-ray (b) 12-month lateral orthostatic X-ray.
4. Discussion
This work examined the treatment of discogenic LBP in patients with degenerative disc disease treated with a discectomy and interbody fusion via lateral access. Isolated axial low back pain rapidly resolved after surgery and disability more gradually improved, as would be expected. Radiolographic analysis revealed improvements in segmental lordosis at treated levels and a high rate of solid fusion. Additionally, few complications occurred, as would be expected using a modern minimally invasive approach, and the patients were generally treated successfully through removal of the pathological intervertebral disc and by stabilizing and fusing the level.
This work represents a retrospective study on prospectively collected data in a small case series with midterm followup, so conclusions are limited to the study design drawbacks. The primary reason for a small sample size was the relative infrequency of surgical candidates for lumbar spine fusion surgery without radicular symptoms (only 10% of all cases in this series). This strengthens the results through sample homogeneity, but greatly limited the sample.
Intervertebral disc morphology continuously changes from birth to late stages of the human life [20]. Disc degeneration is a natural phenomenon, detectable in individuals as early as 11 to 16 years old. By the age of 50, approximately 10% of lumbar intervertebral discs would be classified as degenerated to some extent on MRI and severely degenerated in as many as 60% of 70-year-old discs [21, 22]. Macroscopical changes during this process have been described [23, 24]: the nucleus is the first to change and goes from exhibiting fluid-like to solid-like behavior; the annulus suffers a decrease in the number of layers, decrease in radial permeability, defects in the structure, and microfailure; subchondral bone/nucleus junction calcification, exhibition of focal defects and Modic changes culminate to display the ongoing inflammatory process.
Various phenomena are involved in lumbar disc disease. Genetics, trauma, nutrient pathways, cell death, and matrix synthesis can be primary degeneration inductors [24] and biomechanical matters also greatly contribute to the disease [25, 26]. Impaired neuromuscular control of the paraspinal and abdominal muscles (muscle hypo- or hyperfunctionality) and external forces (e.g., sustained and repetitive loading) can additionally cause disc damage [25, 26], to the point where only a narrow safe window remains between hypermobility (wear and tear) and underuse (immobilization).
Although in normal anatomy, intradiscal nerve terminations have a limited distribution (mostly on the posterolateral annulus), disc degeneration has been shown to have a massive ingrowth of nerves fibers [27–32]. These growths seem to penetrate from outside to inside the annulus, along the edges of annular fissures, dependent of the inflammation process and dependent upon specific markers like substance P and receptor to CGRP-ir nerve growth factor [29–31]. Nociceptive information is transmitted primarily by small neurons associated with inflammatory pain and some specific proinflammatory mediators (NGF; PGE2, IL-1, IL-6; IL-8) [29, 32, 33]. And importantly, these networks tend to resultantly function under peripheral and central sensitization [9, 29–31].
One of the most challenging factors of discogenic low back pain is an accurate differential diagnosis. Morphological and functional statuses of apophyseal joints, ligaments and musculature and spine biomechanics must be analyzed [9, 34–36]. Additionally, external forces and postural behavior also interfere in symptoms onset [25, 26]. Psychosocial factors such as depression, anxiety, and worker's compensation act an positive feedback in pain modulation and may be a drawback in diagnosis and treatment [11, 37–39].
Classically discs are innervated segmentally and discogenic pain pathways flow through the sinuvertebral nerve into the corresponding dorsal root ganglion and into the spinal cord, generating symptoms located at the index level [29, 40, 41]. More recently, an alternative pathway through the grey ramus communications has been described [41, 42]. The signal travels into the upper lumbar dorsal root ganglion (especially at the L2 level), when a L4-5 disc pathology may generate signals in an L2 dermatome, like a groin and anterior tight pain during a L4-5 provocative discography procedure [41, 42].
Identification of signs and symptoms of discogenic back pain includes continuous axial low back pain persistent in extended period deep in the central line of the spine, usually with no irradiation (few times with diffuse or inguinal irradiation), possible relief when lying, no significant worsening with movements, and worsened with axial load and long standing or sitting periods. In radiolographic analysis, low signal intensity of the disc on sagittal T2W, high-intensity zones, annular damages, and especially Modic changes corroborate clinical findings [9, 28, 36, 43].
Provocative discography is one of the possible tests to contribute in the diagnosis of discogenic pain, but a few studies have shown equivocal results for discography [44–46] and the procedure can also accelerate progression of degeneration changes in the lumbar disc [47]. False-positive rates were once reported to reach up to 40% [46], and the presence of many confounding factors can limit its potential: speed and pressure control; low/high pressure provocation; quiescent phase of the illness; somatization disorder; regular medications; abnormal psychometric scores; worker's compensation.
When a degenerated intervertebral disc is determined to be the primary pain generator, surgical removal must be considered. Nucleus replacement was one attempt to treat discogenic pain and maintain movement and function, but the ideal indication window is too narrow and several unwanted complications have occurred [48–52]. Lumbar fusion has been widely used for different pathological conditions resulting from idiopathic changes, degeneration, trauma, infection, or neoplasia. As reviewed elsewhere [53], lumbar fusion has more high-quality studies testifying favorable comparative outcomes [54–56] than with nonoperative care [57].
For a painful disc, discectomy and interbody fusion intend to remove the pathologic tissue, which presents itself as nonfunctional fibrotic structure, soaked with inflammatory mediators and nerve ingrowth, and to fuse the segment. Additionally, index motion is related to pain occurrence and can be treated with lumbar level stabilization, and the addition of interbody fusion has show the favorable results in lumbar fusion [56, 58, 59], especially for discogenic pain.
Lateral interbody fusion has been shown to significantly increase foramen and disc height [19], impact sagittal [60–62] and coronal plane reconstruction [15, 16, 18, 63, 64], and provide indirect decompression and relief of low back and irradiated symptoms [65, 66]. With true 90° lateral access, satisfactory results have also been shown in thoracic access for the treatment of tumor [67, 68], trauma [68], spondylolisthesis [61, 64], and disc herniation [69]. Moreover, artificial discs placed laterally have been an advance in lumbar arthroplasty due to anterior and posterior longitudinal ligament preservation [70].
If the affected lumbar level does not present with gross instability, a stand-alone interbody construction may be considered. In this instance, posterior muscle damage is prevented as well as posterior instrumentation complications. Biomechanical studies [71] have shown lateral interbody implants provide the largest reduction in range of motion in a stand-alone construct, with this stability increasing when moving from 18 mm cages (anteroposterior dimension), to wider ones (22 and 26 mm) [72].
Payment and reimbursement for lumbar fusion, especially for degenerative disc disease, are being rigorously reviewed by North American and worldwide institutions with the premise that it is ineffective. In this study, however, at 2 years postoperatively over 70% improvement in VAS and patient outcomes was demonstrated, much higher than previous studies on treatment for degenerative spine condition [55, 73–76]. This study, while somewhat limited, has shown that, in carefully selected patients, MIS lumbar fusion can be effective in treating isolated axial discogenic low back pain. The spine community must continue to debate the benefits and drawbacks of lumbar fusion for degenerative disc disease.
References
- 1.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. Journal of Bone and Joint Surgery A. 1990;72(3):403–408. [PubMed] [Google Scholar]
- 2.Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25(4):487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Manchikanti L, Datta S, Derby R, Wolfer LR, Benyamin RM, Hirsch JA. A critical review of the American pain society clinical practice guidelines for interventional techniques: part 1. Diagnostic interventions. Pain Physician. 2010;13(3):E141–E174. [PubMed] [Google Scholar]
- 4.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 17: bone growth stimulators and lumbar fusion. Journal of Neurosurgery. 2005;2(6):737–740. doi: 10.3171/spi.2005.2.6.0737. [DOI] [PubMed] [Google Scholar]
- 5.Gibson JNA, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane review. Spine. 2005;30(20):2312–2320. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim T, Tleyjeh IM, Gabbar O. Surgical versus non-surgical treatment of chronic low back pain: a meta-analysis of randomised trials. International Orthopaedics. 2008;32(1):107–113. doi: 10.1007/s00264-006-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreon LY, Glassman SD, Howard J. Fusion and nonsurgical treatment for symptomatic lumbar degenerative disease: a systematic review of oswestry disability index and MOS short form-36 outcomes. Spine Journal. 2008;8(5):747–755. doi: 10.1016/j.spinee.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Baisden J, Carragee EJ, Resnick DK, Shaffer WO, Loeser JD. Surgery for low back pain: a review of the evidence for an American pain society clinical practice guideline. Spine. 2009;34(10):1094–1109. doi: 10.1097/BRS.0b013e3181a105fc. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YG, Guo TM, Guo X, Wu SX. Clinical diagnosis for discogenic low back pain. International Journal of Biological Sciences. 2009;5(7):647–658. doi: 10.7150/ijbs.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Amaral V, Marchi L, Oliveira L, Pimenta L. Prevalence and relationship of emotional and clinical factors in patients with degenerative disc disease. Coluna/Columna. 2010;9(2):150–156. [Google Scholar]
- 12.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. The Spine Journal. 2006;6(4):435–443. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine. 2001;26(8):889–896. doi: 10.1097/00007632-200104150-00012. [DOI] [PubMed] [Google Scholar]
- 14.Voor MJ, Mehta S, Wang M, Zhang YM, Mahan J, Johnson JR. Biomechanical evaluation of posterior and anterior lumbar interbody fusion techniques. Journal of Spinal Disorders. 1998;11(4):328–334. [PubMed] [Google Scholar]
- 15.Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. Journal of Neurosurgery. 2011;15(1):92–96. doi: 10.3171/2011.3.SPINE10425. [DOI] [PubMed] [Google Scholar]
- 16.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurgical Focus. 2010;28(3):p. E8. doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine. 2010;35(26, supplement):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 18.Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine. 2010;35(26, supplement):S312–S321. doi: 10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010;35(26, supplement):S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 20.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 21.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo award in basic science. Spine. 2002;27(23):2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Miller JAA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173–178. [PubMed] [Google Scholar]
- 23.Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Spine. 2006;31(14):1522–1531. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 24.Urban JPG, Smith S, Fairbank JCT. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 25.Adams MA, McMillan DW, Green TP, Dolan P. Sustained loading generates stress concentrations in lumbar intervertebral discs. Spine. 1996;21(4):434–438. doi: 10.1097/00007632-199602150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Stokes IAF, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? Journal of Anatomy. 2010;217(1):1–15. doi: 10.1111/j.1469-7580.2010.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. Journal of Bone and Joint Surgery B. 2005;87(1):62–67. [PubMed] [Google Scholar]
- 29.Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine. 2004;29(10):1077–1081. doi: 10.1097/00007632-200405150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Aoki Y, Ohtori S, Ino H, et al. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine. 2004;29(23):2621–2626. doi: 10.1097/01.brs.0000146051.11574.b4. [DOI] [PubMed] [Google Scholar]
- 31.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. The Lancet. 1997;350(9072):178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa T, Ohtori S, Inoue G, Aoki Y, Moriya H, Takahashi K. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine. 2006;31(21):2418–2422. doi: 10.1097/01.brs.0000239159.74211.9c. [DOI] [PubMed] [Google Scholar]
- 33.Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. Journal of Bone and Joint Surgery B. 2002;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 34.Alqarni AM, Schneiders AG, Hendrick PA. Clinical tests to diagnose lumbar segmental instability: a systematic review. Journal of Orthopaedic and Sports Physical Therapy. 2011;41(3):130–140. doi: 10.2519/jospt.2011.3457. [DOI] [PubMed] [Google Scholar]
- 35.Leone A, Cassar-Pullicino VN, Guglielmi G, Bonomo L. Degenerative lumbar intervertebral instability: what is it and how does imaging contribute? Skeletal Radiology. 2009;38(6):529–533. doi: 10.1007/s00256-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 36.Wassenaar M, Rijn RM, Tulder MW, et al. Magnetic resonance imaging for diagnosing lumbar spinal pathology in adult patients with low back pain or sciatica: a diagnostic systematic review. European Spine Journal. 2011;21(2):220–227. doi: 10.1007/s00586-011-2019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBerard MS, LaCaille RA, Spielmans G, Colledge A, Parlin MA. Outcomes and presurgery correlates of lumbar discectomy in Utah Workers’ compensation patients. Spine Journal. 2009;9(3):193–203. doi: 10.1016/j.spinee.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Lurie JD, Berven SH, Gibson-Chambers J, et al. Patient preferences and expectations for care: determinants in patients with lumbar intervertebral disc herniation. Spine. 2008;33(24):2663–2668. doi: 10.1097/BRS.0b013e31818cb0db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore JE. Chronic low back pain and psychosocial issues. Physical Medicine and Rehabilitation Clinics of North America. 2010;21(4):801–815. doi: 10.1016/j.pmr.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Aoki Y, Takahashi Y, Takahashi K, et al. Sensory innervation of the portion of the lumbar intervertebral disc in rats. Spine Journal. 2004;4(3):275–280. doi: 10.1016/j.spinee.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Practice. 2008;8(1):18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 42.Sameda H, Takahashi Y, Takahashi K, et al. Dorsal root ganglion neurones with dichotomising afferent fibres to both the lumbar disc and the groin skin. A possible neuronal mechanism underlying referred groin pain in lower lumbar disc diseases. Journal of Bone and Joint Surgery. 2003;85(4):600–603. doi: 10.1302/0301-620x.85b4.13306. [DOI] [PubMed] [Google Scholar]
- 43.Rahme R, Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. American Journal of Neuroradiology. 2008;29(5):838–842. doi: 10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim C-H, Jee W-H, Byung C-S, Kim D-H, Ha K-Y, Park C-K. Discogenic lumbar pain: association with MR imaging and CT discography. European Journal of Radiology. 2005;54(3):431–437. doi: 10.1016/j.ejrad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Carragee EJ, Tanner CM, Khurana S, et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25(11):1373–1380. doi: 10.1097/00007632-200006010-00009. [DOI] [PubMed] [Google Scholar]
- 46.Carragee EJ, Alamin TF, Carragee JM. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine. 2006;31(5):505–509. doi: 10.1097/01.brs.0000201242.85984.76. [DOI] [PubMed] [Google Scholar]
- 47.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino J, Herzog R. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34(21):2338–2345. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 48.Lindley EM, Jaafar S, Noshchenko A, et al. Nucleus replacement device failure: a case report and biomechanical study. Spine. 2010;35(22):E1241–E1247. doi: 10.1097/BRS.0b013e3181dfbc78. [DOI] [PubMed] [Google Scholar]
- 49.Bertagnoli R, Schönmayr R. Surgical and clinical results with the PDN prosthetic disc-nucleus device. European Spine Journal. 2002;11(supplement 2)(2):S143–S148. doi: 10.1007/s00586-002-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klara P, Ray CD. Artificial nucleus replacement: clinical experience. Spine. 2002;27(17):p. 1949. doi: 10.1097/00007632-200206150-00022. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira L, Marchi L, Coutinho E, Pimenta L. A long-term clinical experience with three different nucleus replacement devices—lessons learned after 9-years follow-up. The Spine Journal. 2010;10(9, supplement):p. S135. [Google Scholar]
- 52.Pimenta L, Marchi L, Coutinho E, Oliveira L. Lessons learned after 9 years clinical experience with three different nucleus replacement devices. Seminars in Spine Surgery. 2012;24(1):43–47. [Google Scholar]
- 53.McGrory JE, Guyer RD. Lumbar fusion: a defensible option for discogenic low back pain? Seminars in Spine Surgery. 2011;23(4):227–234. [Google Scholar]
- 54.Fairbank J, Frost H, Wilson-MacDonald J, Yu LM, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. British Medical Journal. 2005;330(7502):1233–1239. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fritzell P, Hägg O, Wessberg P, Nordwall A. 2001 Volvo award winner in clinical studies: lumbar fusion versus nonsurgical treatment for chronic low back pain. A multicenter randomized controlled trial from the Swedish lumbar Spine study group. Spine. 2001;26(23):2521–2532. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- 56.Ohtori S, Kinoshita T, Yamashita M, et al. Results of surgery for discogenic low back pain: a randomized study using discography versus discoblock for diagnosis. Spine. 2009;34(13):1345–1348. doi: 10.1097/BRS.0b013e3181a401bf. [DOI] [PubMed] [Google Scholar]
- 57.Brox JI, Sørensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28(17):1913–1921. doi: 10.1097/01.BRS.0000083234.62751.7A. [DOI] [PubMed] [Google Scholar]
- 58.Weatherley CR, Prickett CF, O’Brien JP. Discogenic pain persisting despite solid posterior fusion. Journal of Bone and Joint Surgery B. 1986;68(1):142–143. doi: 10.1302/0301-620X.68B1.2934399. [DOI] [PubMed] [Google Scholar]
- 59.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25(7):853–857. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 60.Amaral R, Marchi L, Oliveira L, et al. Minimally invasive lateral alternative for thoracolumbar interbody fusion. Coluna/Columna. 2011;10(3):239–243. [Google Scholar]
- 61.Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. doi: 10.1100/2012/456346. The Scientific World Journal. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchi L, Oliveira L, Amaral R, et al. Anterior elongation as a minimally invasive alternative for sagittal imbalance—a case series. doi: 10.1007/s11420-011-9226-z. HSS Journal. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine. 2010;35(26, supplement):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 64.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine. 2011;36(1):26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 65.Billinghurst J, Akbarnia BA. Extreme lateral interbody fusion—XLIF. Current Orthopaedic Practice. 2009;20(3):238–251. [Google Scholar]
- 66.Oliveira L, Marchi L, Coutinho E, Abdala N, Pimenta L. The use of rh-BMP2 in standalone eXtreme lateral interbody fusion (XLIF): clinical and radiological results after 24 months follow-up. World Spinal Column Journal. 2010;1(1):19–25. [Google Scholar]
- 67.Uribe JS, Dakwar E, Le TV, Christian G, Serrano S, Smith WD. Minimally invasive surgery treatment for thoracic spine tumor removal: a mini-open, lateral approach. Spine. 2010;35(26, supplement):S347–S354. doi: 10.1097/BRS.0b013e3182022d0f. [DOI] [PubMed] [Google Scholar]
- 68.Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe JS. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine. 2010;35(26, supplement):S338–S346. doi: 10.1097/BRS.0b013e3182023113. [DOI] [PubMed] [Google Scholar]
- 69.Uribe J, Smith W, Pimenta L. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multi-center clinical experience. Journal of Neurosurgery. 2012;16(3):264–279. doi: 10.3171/2011.10.SPINE11291. [DOI] [PubMed] [Google Scholar]
- 70.Ozgur BM, Agarwal V, Nail E, Pimenta L. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS Journal. 2010;4(2):41–46. doi: 10.1016/j.esas.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cappuccino A, Cornwall GB, Turner AWL, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine. 2010;35(26, supplement):S361–S367. doi: 10.1097/BRS.0b013e318202308b. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira L, Marchi L, Coutinho E, Pimenta L. The subsidence rate in XLIF osteoporotic patients in standalone procedures. The Spine Journal. 2010;10, supplement(9):S51–S52. [Google Scholar]
- 73.Fairbank J, Frost H, Wilson-MacDonald J, Yu LM, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. British Medical Journal. 2005;330(7502):1233–1239. doi: 10.1136/bmj.38441.620417.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. Journal of Spinal Disorders and Techniques. 2003;16(2):113–122. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Kasis AG, Marshman LAG, Krishna M, Bhatia CK. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine. 2009;34(6):572–577. doi: 10.1097/BRS.0b013e3181973e35. [DOI] [PubMed] [Google Scholar]
- 76.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. Journal of Spinal Disorders and Techniques. 2005;18(1, supplement):S1–S6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]


