Abstract
Acetylcholinesterase inhibitors (AChEIs), such as donepezil, have been shown to improve cognition in mild to moderate Alzheimer’s disease (AD) patients. In this paper, our goal is to determine the relationship between altered cerebral blood flow (CBF) and intrinsic functional network connectivity changes in mild AD patients before and after 12-week donepezil treatment. An integrative neuroimaging approach was employed by combining pseudocontinuous arterial spin labeling (pCASL) MRI and resting-state functional MRI (R-fMRI) methods to determine the changes in CBF and functional connectivity (FC) in the cholinergic pathway. Linear regression analyses determined the correlations of the regional CBF alterations and functional connectivity changes with cognitive responses. These were measured with the Mini-Mental Status Examination (MMSE) scores and Alzheimer’s disease Assessment Scale-Cognitive subscale (ADAS-cog) scores. Our results show that the regional CBF in mild AD subjects after donepezil treatment was significantly increased in the middle cingulate cortex (MCC) and posterior cingulate cortex (PCC), which are the neural substrates of the medial cholinergic pathway. In both brain regions, the baseline CBF and its changes after treatment were significantly correlated with the behavioral changes in ADAS-cog scores. The intrinsic FC was significantly enhanced in the medial cholinergic pathway network in the brain areas of the parahippocampal, temporal, parietal and prefrontal cortices. Finally, the FC changes in the medial prefrontal areas demonstrated an association with the CBF level in the MCC and the PCC, and also were correlated with ADAS-cog score changes. These findings indicate that regional CBF and FC network changes in the medial cholinergic pathway were associated with cognitive performance. It also is suggested that the combined pCASL-MRI and R-fMRI methods could be used to detect regional CBF and FC changes when using drug treatments in mild AD subjects.
Keywords: acetylcholinesterase inhibitors, donepezil, cerebral blood flow, resting-state functional connectivity, Alzheimer’s disease
Introduction
Clinically, acetylcholinesterase inhibitors (AChEIs), 1 such as donepezil, have been approved to treat Alzheimer’s disease (AD) patients with the goal of improving cognitive benefits. According to the “cholinergic hypothesis,” disruption of the basal forebrain cholinergic pathways in the central nervous system significantly contributes to cognitive decline in advanced aging and AD (Bartus et al., 1982). The loss of cholinergic input to the cerebral neocortex and hippocampus results in a decreased acetylcholine (ACh) level, which is one of the principle neuromodulators of synaptic plasticity, including learning and memory (Buccafusco and Terry, 2000; Terry and Buccafusco, 2003). Hence, treatment of AD patients with donepezil could prevent the breakdown of ACh and enhance synaptic cholinergic transmission (Bartus, 2000). Clinical trials have shown that donepezil has modest effects on memory and cognitive improvement in responders (Black et al., 2007; Hansen et al., 2008; Pepeu and Giovannini, 2010; Rogers et al., 1998). However, the physiological mechanisms by which cholinergic treatments affect brain functions in specific regions are not fully understood. This is especially true of the action mechanism relative to donepezil at the brain system level.
Previous studies showed that heightened cerebral metabolism and cerebral blood flow (CBF) are essential in maintaining increased cholinergic activity (Terry and Buccafusco, 2003). Functional neuroimaging techniques, such as single-photon emission computed tomography (SPECT), positron emission tomography (PET) and arterial spin labeling (ASL) MRI, have been employed in AD research to measure treatment-mediated changes in regional CBF and cerebral metabolism in several distributed brain regions in the anterior cingulated cortex (ACC), posterior cingulate cortex (PCC), hippocampus, inferior parietal cortex (IPC) and frontal regions (Bohnen et al., 2005; Dai et al., 2009; Hanyu et al., 2010; Minoshima et al., 1997; Nakano et al., 2001; Staff et al., 2000; Tateno et al., 2008; Ushuijima et al., 2006; Yoshida et al., 2007). In particular, the CBF levels in the frontal brain regions are shown to be associated with AD treatment responses (Hanyu et al., 2003; Hongo et al., 2008). However, it is not fully understood why a similar donepezil treatment regime in different neuroimaging studies activated different brain regions. It is not known how these brain regions activated by donepezil treatment were interrelated.
In order to investigate interactions or interrelations between brain regions that responded to donepezil treatment, the resting-state functional connectivity MRI (R-fMRI) method was employed (Biswal et al., 1995). This method measures the temporal correlation between the spontaneous blood oxygenation level-dependent (BOLD) fluctuations in spatially separated, but functionally related, brain regions at resting-state, as extensively reviewed (Fox and Raichle, 2007). R-fMRI has been widely applied in investigating the functional network changes in normal and diseased human brains (Fox and Greicius, 2010), and it specifically has been applied to AD studies (Agosta et al., 2010; Buckner et al., 2009; Buckner et al., 2005; Chen et al., 2011a; Li et al., 2002a; Wang et al., 2006). In AD and other neuropsychiatric disorders, this modality also has been used to examine the relationship between neural network function changes and treatment outcomes (Goveas et al., 2011; Kozel et al., 2011; Lui et al., 2010). These studies suggest that R-fMRI is a valuable tool in examining changes in the intrinsic functional networks in targeted brain regions and the behavioral significance associated with pharmacotherapy.
In this study, our goal is to identify the impact of donepezil treatment on specific neural systems, thereby demonstrating cognitive benefit in patients with mild AD (Mini-Mental Status Examination score is larger than 23). To realize this goal, we employed an integrative neuroimaging approach by combining pseudocontinuous ASL (pCASL) MRI and the R-fMRI methods to determine the relationship between the altered CBF and the changes in the intrinsic functional network connectivity in the cholinergic pathway in mild AD before and after the 12-week donepezil treatment. We hypothesize that: 1) treatment of mild AD patients with donepezil will enhance the CBF level and functional connectivity in the cholinergic pathways; 2) the changes in the CBF level and functional connectivity strength in the cholinergic pathways are associated with changes in cognitive performance in subjects with mild AD. Our results demonstrate that treatment of mild AD subjects with donepezil primarily improves regional CBF and functional connectivity in the medial cholinergic pathway, which is associated with cognitive performance.
Materials and Methods
Participants
Fourteen patients with newly diagnosed mild AD (nine males and five females of age 77.6±7.1 years), who had never received any AD treatment medications, were recruited through Froedtert Hospital and the Medical College of Wisconsin Memory Disorders Clinic (Milwaukee, Wis., USA). The study was conducted with the approval of the Medical College of Wisconsin Institutional Review Board (IRB) and in compliance with the Health Insurance Portability and Accountability Act (HIPAA regulations). Written informed consent was obtained from all participants or caregivers. All patients underwent physical, psychiatric and neurological examinations before and after treatment. Participants with mild AD received the starting regimen of donepezil 5 mg/day for the first four weeks, and the dose was increased to 10 mg/day after four weeks. The baseline clinical visits occurred 14 ± 10 days prior to the baseline MRI scans for all participants. Each subject with mild AD received a baseline MRI scan on day 0 (ADB) and another scan within five days of the last day of 12-week donepezil treatment (ADT).
Inclusion and Exclusion Criteria
During the baseline clinical visit, mild AD was diagnosed in patients according to National Institute of Neurological Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al., 1984). Patients were examined by two experienced neurologists with expertise in dementia. They received a full clinical assessment, which included a neurological examination, Mini-Mental State Examination (MMSE), Alzheimer’s disease Assessment Scale-cognitive subscale (ADAS-cog), the Lawton Instrumental Activities of Daily Living Scale (IADL), and Neuropsychiatric Inventory (NPI) (Cummings et al., 1994; Folstein et al., 1975; Lawton and Brody, 1969; Rosen et al., 1984). None of the subjects had previously taken any medications that could affect the CBF. Exclusion criteria included history of claustrophobia, Hachinski Ischemia Scale score > 4, MMSE score ≤ 23, major psychiatric diagnoses, including primary psychotic and affective disorders, and other types of neurodegenerative or secondary dementias. Further criteria for exclusion included cardiovascular disease, a history of stroke, the presence of two or more lacunar infarcts of 5 mm each and the use of anticoagulants or other known vasoactive medications. No hypertension was observed in any subject at the time of the MRI scans. Two AD patients were excluded from the data analysis, because of incomplete scans. All behavioral data were analyzed with SPSS 16.0 software (http://www.spss.com/. Chicago, Ill.).
pCASL and R-fMRI Data Acquisition
All MRI scans were performed on a GE 3T Signa LX scanner (Waukesha, Wis.) with a standard quadrature transmit-receive head coil. Study subjects were asked to lie still with their eyes closed while in the scanner. First, high-resolution anatomical images were acquired using a 3D spoiled gradient-echo (SPGR) sequence with 144 continuous axial slices. The scan parameters were as follows: TE/TR/TI of 4/10/450 ms, flip angle of 12°, slice thickness of a 1 mm, matrix size of 256×192 and a field of view of 24×24 cm2. The functional perfusion MRI scans lasted 6 minutes. During this time, 12 axial slices were acquired. Each had 7-mm thickness for each slice and 1-mm gap by pseudocontinuous ASL (pCASL) MR pulse sequence (Dai et al., 2008). A single-shot EPI pulse sequence was employed for image acquisition with the following parameters: TR of 4 s, TE of 25 ms, labeling duration/post inversion delay of 1.5/1.75 s and a matrix size of 64×64. The localization of the imaging area was selected manually in order to cover the bottom margin of the inferior temporal lobe to the inferior parietal lobe. The tagging slice was placed 32.5 mm below the inferior edge of the imaging slab. Sagittal R-fMRI datasets of the whole brain were obtained in 6 minutes with a single-shot gradient echo-planar imaging pulse sequence with the following parameters: TR of 2 s, TE of 25 ms, flip angle of 90°; slice thickness of 4 mm, and a matrix size of 64×64; 36 slices were obtained without gap between slices.
Measurement of Absolute CBF
The pCASL image of each subject was analyzed using Analysis of Functional NeuroImages (AFNI) software (http://afni.nimh.nih.gov/afn/) and Matlab Version 10 (The Math Works, Inc., Natick, Mass.). First, subject motion was corrected by performing a volume registration on the pCASL data set. Separation of the tagged and nontagged images was then performed by concatenating odd and even scans, respectively. The mean difference of the pCASL images (ΔM) for each subject was calculated as the difference between the tagged and nontagged images. Furthermore, the absolute CBF was quantified, as previously described (Buckner et al., 2009; Wang et al., 2005). The formula for the calculation of absolute CBF is provided below:
where T1a (0.61s) is the longitudinal relaxation time of blood, M0 is the average image intensity of the control image, α is the labeling efficiency (85%) (Wu et al., 2007), τ and ω are the duration of the labeling pulse and postlabeling delay time, respectively. λ is the blood/tissue water partition coefficient (0.9 ml/g). Finally, the absolute CBF images for each subject were transformed to Talairach space through transformation matrix of SPGR high-resolution images using AFNI program (adwarp), and smoothed with a 10-mm Gaussian kernel for statistical comparisons. The differences in absolute CBF between ADB and ADT groups were measured by a paired t-test (p < 0.05, corrected cluster size > 1028 mm3). The associations between cognitive changes, such as the ΔADAS-cog, with the baseline CBF and the ΔCBF, after 12 weeks of donepezil treatment, were studied, using linear regression, after controlling any confounding effects of age and gender.
Functional Connectivity Analysis
Resting-state functional connectivity (FC) was calculated as the correlation between the spontaneous low-frequency fluctuations of the seed region of interest (ROI) and the whole brain (Fox et al., 2007). The data preprocessing procedure was carried out according to a previous study (Chen et al., 2011a). In brief, the spikes in raw R-fMRI data were removed, followed by the removal of the temporal signal trend and motion correlation. Furthermore, the potential confounding signals from the white matter (WM), cerebral spinal fluid (CSF), whole brain and motion were regressed out, using a general linear model. The cardiac aliasing (Glover et al., 2000) and the respiratory volume variation (Birn et al., 2006) were minimized, based on the cardiac pulse oxymeter signal and the respiratory belt signal using AFNI programs (3dretroicor). A band-pass filter was applied to keep the low-frequency fluctuations within 0.015 Hz and 0.1 Hz. Finally, all preprocessed images were spatially transformed to the Talairach space for group level analysis.
The seed regions were selected as the brain areas with significantly altered CBF after treatment. The average time course in each of the seeds was then extracted from the preprocessed R-fMRI data and cross-correlated with the entire brain voxel by voxel to calculate the Pearson product-moment correlation coefficient. Fisher’s z-transform and spatial smoothing (6-mm Gaussian) then were applied to obtain the FC maps for each subject. Within-group FC network patterns and between-group FC network differences were calculated using a one-sample t-test (p < 0.01, corrected cluster size > 1672 mm3) and paired t-test (p < 0.05, corrected cluster size > 4048 mm3), respectively. Finally, linear regression analyses were performed to examine the relationships among changes in the FC (ΔFC), baseline CBF, ΔCBF and ΔADAS-cog, while controlling for the possible confounding effects of age and gender as covariates using a general linear model. All voxelwise multiple comparisons were corrected with 3dClusterSim program in AFNI software.
Results
Demographics and Clinical Evaluations
Twelve AD subjects completed the follow-up study and were included in the data analysis. Table 1 shows the demographic information and cognitive testing scores for ADB and ADT. There were no significant differences in age (p = 0.992) and gender (p = 0.48) between the two groups. After 12 weeks of donepezil treatment, the ADT group had significantly improved ADAS-cog scores compared to the baseline measures (p < 0.002). However, no significant differences were found in the MMSE, NPI, and IADL scores between the two groups.
Table 1.
Demographics and Clinical Evaluations
| Mild AD (N = 12) |
p-value | ||
|---|---|---|---|
| Baseline | Treatment | ||
| Gender | 4F/8M | 0.480 | |
| Age | 77.6±7.1 | 77.9±7.2 | 0.992 |
| MMSE | 26.8±1.4 | 26.5±2.7 | 0.852 |
| IADL | 13.1±3.8 | 12.7±3.7 | 0.941 |
| ADAS-cog | 12.0±4.6 | 9.78±4.8 | 0.002* |
| NPI | 9.2±10 | 5.9±9.5 | 0.140 |
Note. – F: female, M: male, MMSE: Mini-Mental State Examination, ADAS-cog: Alzheimer’s disease Assessment Scale-cognitive Subscale, NPI: Neuropsychiatric Inventory, IADL: Instrumental Activities of Daily Living,
Statistically significant with paired t-test.
Enhanced CBF and its cognitive significance
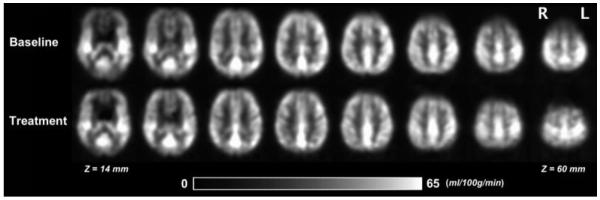
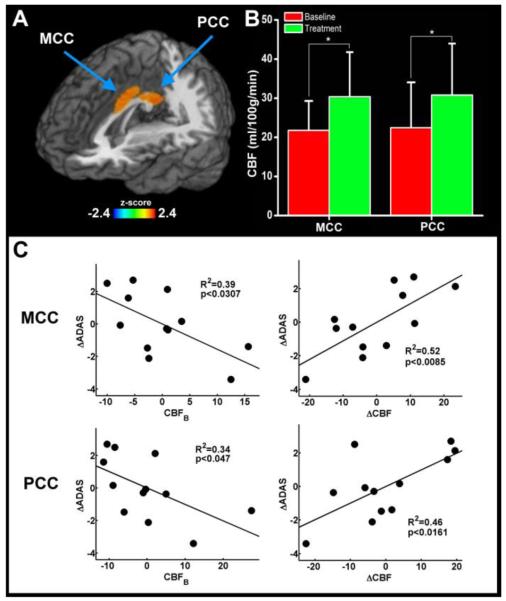
Figure 1 shows the quantitative group-average CBF images from the mild AD group at baseline (top) and 12 weeks after donepezil treatment (bottom). Figure 2A shows that the CBF was significantly increased in the bilateral middle cingulate cortex (MCC) and posterior cingulate cortex (PCC) (p < 0.05, corrected) in the ADT group, using a paired t-test between ADB and ADT groups. No other brain regions were found to have significant CBF changes. The MCC cluster center is located at the junction between BA 23 and 24 with the Talairach coordinates at x = -4 mm, y = -2 mm and z = 32 mm; the cluster size is 2064 mm3. The center of the PCC cluster is located at BA 23 with the Talairach coordinates at x = -2 mm, y = -44 mm and z = 25 mm; the cluster size is 1624 mm3. Figure 2B shows the significant changes in the CBF levels in the MCC and PCC regions between the ADB and ADT groups. Figure 2C illustrates the significant correlations between the ADAS-cog scores and CBF level in the MCC (R2 = 0.39, p < 0.03) and the PCC (R2 = 0.34, p < 0.05). The ΔCBF also was significantly correlated with the ΔADAS-cog in the MCC (R2 = 0.52, p < 0.009) and the PCC (R2 = 0.46, p < 0.02).
Figure 1.
Quantitative group-average CBF images from the mild AD group at baseline (top) and the same mild AD group 12 weeks after donepezil treatment (bottom).
Figure 2. Effects of 12-week donepezil treatment on CBF levels and cognitive performance in mild AD patients.
(A) The locations of MCC (BA 24) and PCC (BA 23) regions, as indicated by the arrows. (B) MCC and PCC showed increased CBF levels after donepezil treatment (p < 0.05). (C) The ΔADAS-cog scores and CBF at baseline, and ΔCBF in the MCC and PCC are significantly correlated after 12 weeks of donepezil treatment. The effects of individual subject age and gender variations were removed from the CBF and ADAS-cog measures.
MCC=middle cingulate cortex, PCC=posterior cingulate cortex, ADAS-cog=AD assessment scale-cognitive subscale, Δ=changes.
Enhanced FC in the MCC and PCC networks after treatment
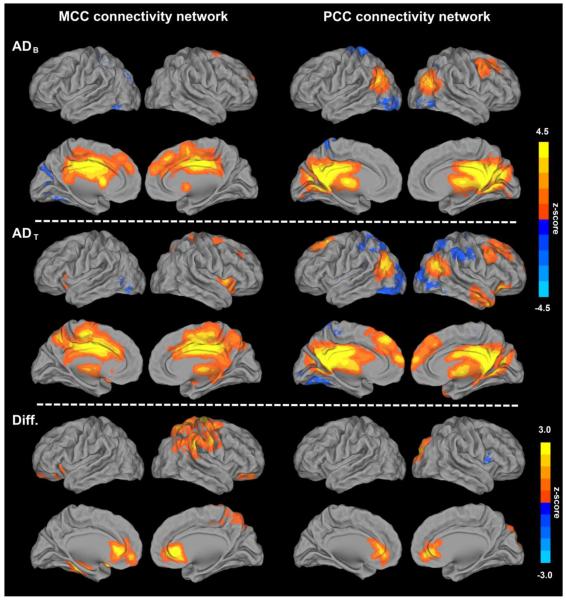
Figure 3 shows the FC network patterns of the MCC (left column) and the PCC (right column) for ADB (top row), ADT (middle row) and the FC enhancement after treatment (bottom row), obtained using a one-sample t-test (p < 0.01, corrected) and a paired t-test (p < 0.05, corrected), respectively. The MCC connectivity network showed connections with the subcortical, parietal, frontal and insula brain regions (Table 2). The brain regions with significantly increased MCC connectivity were found in the bilateral ventral medial prefrontal cortex (VMPFC), ventral anterior cingulate cortex (vACC), left parahippocampal gyrus, right precuneus, right precentral gyrus and right inferior parietal cortex (IPC) (Table 4). No significantly decreased MCC connectivity was found. The PCC connectivity showed connections in the prefrontal, temporal and partial brain regions after treatment (Table 3). After treatment, the brain regions with significantly increased PCC connectivity were found in the bilateral vACC and right cuneus (Table 4). A significantly decreased FC between the PCC and anterior insula was found in the right cerebral cortex.
Figure 3. Illustrations of functional connectivity network patterns of MCC and PCC before and after 12-week donepezil treatment in mild AD patients.
Compared to baseline connectivity patterns of the MCC and the PCC (top panel), donepezil treatment leads to enhanced functional connectivity in both regions (middle panel). Connectivity patterns before and after treatment were analyzed with one-sample t-test, and the details are shown in Tables 2 and 3, respectively. Bright colors indicate positive correlations, while blue colors indicate negative correlations. Brain regions that show significantly altered FC after donepezil treatment with paired t-test (bottom panel) are listed in Table 4.
MCC=middle cingulate cortex, PCC=posterior cingulate cortex.
Table 2.
Functional connectivity patterns of MCC and PCC networks before donepezil treatment in mild AD subjects
| Regions | BA | Cluster size (mm3) |
Talairach coordinates (LPI) |
Z values |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| MCC connectivity network | |||||||
| L | MCC (seed ROI) | 24 | 29200 | −4 | −2 | 32 | 5.18 |
| L | Caudate | −6 | 9 | 7 | 3.74 | ||
| R | SFG | 6 | 10 | 9 | 53 | 3.73 | |
| R | Caudate | 5 | 6 | 0 | 3.25 | ||
| L | MFG | 9 | 2552 | −3 | 46 | 25 | 3.41 |
| R | MFG | 9 | 7 | 50 | 22 | 2.89 | |
| L | Cuneus | 18, 19 | 9856 | −13 | −77 | 29 | −3.39 |
| PCC connectivity network | |||||||
| L | PCC (seed ROI) | 23 | 65200 | −2 | −44 | 25 | 5.76 |
| L | Thalamus | −3 | −17 | 5 | 4.43 | ||
| R | Thalamus | 6 | −15 | 7 | 4.29 | ||
| R | MTG | 39 | 6456 | 46 | −63 | 21 | 3.56 |
| L | MTG | 39 | 6152 | −49 | −63 | 22 | 3.49 |
| R | MFG | 8 | 3864 | 22 | 25 | 42 | 3.29 |
| L | Fusiform | 18 | 13120 | −26 | −86 | −12 | −4.12 |
| R | Fusiform | 37 | 48 | −58 | −11 | −3.18 | |
| L | Postcentral gyrus | 3 | 1688 | −18 | −36 | 63 | −3.32 |
Note. – BA: brodmann area, MCC: middle cingulate cortex, MeFG: medial frontal gyrus, SFG: superior frontal gyrus, PCC: posterior cingulate cortex, MTG: middle temporal gyrus, MFG: middle frontal gyrus
Table 4.
Brain regions with significantly altered functional connectivity to MCC and PCC after donepezil treatment in mild AD subjects
| Regions | BA | Cluster size (mm3) |
Talairach coordinates (LPI) |
Z values |
|||
|---|---|---|---|---|---|---|---|
| Z | X | Y | |||||
| MCC connectivity network | |||||||
| R | vACC | 24, 32 | 11080 | 5 | 32 | 1 | 3.98 |
| L | VMPFC | 10,11 | −9 | 49 | −8 | 2.84 | |
| R | Precuneus | 7 | 7128 | 17 | −45 | 56 | 3.44 |
| R | Precentral gyrus | 4 | 4560 | 18 | −23 | 65 | 3.47 |
| R | IPC | 40 | 61 | −34 | 29 | 2.86 | |
| L | Parahippocampus | 35, 36 | 2040 | −30 | −26 | −16 | 3.11 |
| PCC connectivity network | |||||||
| R | vACC | 32 | 2440 | 9 | 43 | 0 | 2.92 |
| R | Cuneus | 19 | 2400 | 21 | −82 | 32 | 3.25 |
| R | Insula | 13 | 1816 | 43 | 11 | 7 | −3.20 |
Note. – BA: brodmann area, vACC: ventral anterior cingulate cortex, VMPFC: ventral media prefrontal cortex, IPC: inferior parietal cortex
Table 3.
Functional connectivity patterns of MCC and PCC networks after donepezil treatment in mild AD subjects
| Regions | BA | Cluster size (mm3) |
Talairach coordinates (LPI) |
Z values |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| MCC connectivity network | |||||||
| L | MCC (seed ROI) | 24 | 40816 | −4 | −2 | 32 | 4.96 |
| L | vACC | 32 | −7 | 27 | 24 | 3.95 | |
| L | Caudate | −12 | 10 | 8 | 3.06 | ||
| L | Precuneus | 7 | −9 | −52 | 45 | 4.57 | |
| R | ACC | 32 | 8 | 28 | 24 | 3.31 | |
| R | Precuneus | 7 | 9 | −63 | 37 | 3.57 | |
| L | Putamen | 10888 | −19 | 6 | 10 | 4.35 | |
| R | Putamen | 4880 | 26 | 6 | −2 | 3.41 | |
| R | Insula | 13 | 37 | 13 | 2 | 3.07 | |
| L | Fusiform | 19 | 5496 | −37 | −70 | −11 | −3.22 |
| PCC connectivity network | |||||||
| L | PCC (seed ROI) | 23 | 50704 | −2 | −44 | 25 | 5.31 |
| L | Thalamus | −4 | −21 | 9 | 4.38 | ||
| R | Thalamus | 6 | −15 | 8 | 3.15 | ||
| L | MFG | 8 | 15992 | −2 | 38 | 41 | 4.88 |
| L | VMPFC | 10 | −2 | 56 | 14 | 3.93 | |
| L | vACC | 32 | −8 | 37 | 24 | 2.92 | |
| R | MFG | 8 | 28 | 19 | 38 | 4.44 | |
| R | VMPFC | 10 | 4 | 61 | 12 | 3.49 | |
| L | MTG | 39 | 8152 | −44 | −64 | 18 | 3.73 |
| R | MTG | 39 | 6576 | 44 | −64 | 25 | 4.39 |
| R | STG | 21, 38 | 3656 | 46 | 11 | −29 | 3.49 |
| L | Cuneus | 17 | 12064 | −16 | −86 | 10 | −3.58 |
| R | Cuneus | 17 | 20 | −81 | 11 | −2.78 | |
| R | SPC | 7 | 9728 | 25 | −60 | 47 | −3.21 |
| R | Postcentral gyrus | 3 | 55 | −23 | 39 | −3.32 | |
| L | SPC | 7 | 4928 | −26 | −58 | 47 | −3.66 |
Note. – BA: brodmann area, MCC: middle cingulate cortex, vACC: ventral anterior cingulate cortex, PCC: posterior cingulate cortex, MTG: middle temporal gyrus, MeFG: medial frontal gyrus, VMPFC: ventral medial prefrontal cortex, SPC: superior parietal cortex, MTG: middle temporal gyrus, STG: superior temporal gyrus, MFG: middle frontal gyrus.
Correlations between changes in ADAS-cog, CBF and the changes in FC strength
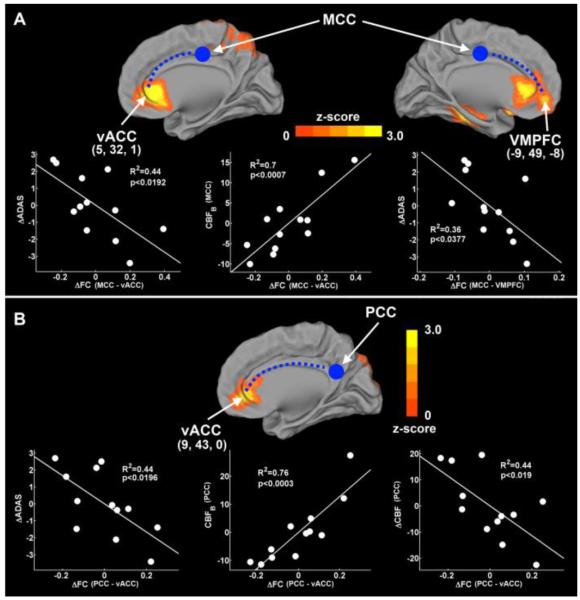
In Figure 3, the ΔADAS-cog score was significantly correlated with the ΔFC between the MCC and vACC (R2 = 0.44, p < 0.0192), and between the MCC and the VMPFC (R2 = 0.36, p < 0.0377) (Figure 4, top). The ΔFC between the MCC and the vACC was also significantly correlated with the baseline CBF level in the MCC (R2 = 0.7, p < 0.0007). In the PCC connectivity network (Figure 4, bottom), the ΔFC between the PCC and the vACC was significantly correlated with the ΔADAS-cog scores (R2 = 0.44, p < 0.0196), baseline CBF (R2 = 0.76, p < 0.0003) and the ΔCBF (R2 = 0.44, p < 0.019) in the PCC.
Figure 4. Behavioral significance of CBF, ΔCBF, and ΔFC in the MCC and PCC connectivity networks.
In the MCC connectivity network (A), the ΔFC in the vACC and the VMPFC was significantly correlated with the ΔADAS-cog scores. The ΔFC in the vACC was also significantly correlated with the baseline CBF in the MCC. In the PCC connectivity network (B), the ΔFC in vACC was significantly correlated with the ΔADAS-cog scores, baseline CBF, and ΔCBF in PCC. The blue solid circles and dotted lines represent the seed regions and the functional connections, respectively.
vACC=ventral anterior cingulate cortex, VMPFC=ventral prefrontal cortex, MCC=middle cingulate cortex, PCC=posterior cingulate cortex, ADAS-cog=AD assessment scale-cognitive subscale, Δ=changes.
Discussion
The results from this integrated pCASL-MRI and R-fMRI study demonstrate that cognitive performance in mild AD after 12-week donepezil treatment is associated with the alterations in the regional CBF and intrinsic functional networks. This conclusion is supported by three major experimental findings. First, regional CBF is significantly increased in the MCC and the PCC in the ADT group when compared to the ADB group. Second, the FC networks involving the MCC and the PCC are significantly enhanced in the ADT group when compared to the ADB group. Third, changes in the regional CBF and the FC networks are associated with the changes of cognitive performance as measured by the ADAS-cog scores.
Our results show increased regional CBF in the MCC and the PCC (Figure 2) after donepezil treatment. The previous study reports that the CBF of the posterior cingulate regions was significantly decreased in AD subjects compared with cognitively normal subjects (Bohnen et al., 2005; Dai et al., 2009; Hanyu et al., 2010; Minoshima et al., 1997; Nakano et al., 2001; Staff et al., 2000; Tateno et al., 2008; Ushuijima et al., 2006; Yoshida et al., 2007). Our findings suggest that an increased CBF in the PCC is reflective of an improvement of the cerebral blood flow values toward normalcy after donepezil treatment. These results also are consistent with the “cholinergic hypothesis” (Bartus, 2000). It is conceivable that treatment of AD with AChEIs should restore, or partially restore, synaptic activities within the cholinergic pathways, and the CBF increase reflects elevated brain metabolism and neural activity among brain regions within these cholinergic pathways. Intriguingly, the MCC and the PCC are the two most important regions located in the medial cholinergic pathway (MCP), a major pathway in human cholinergic networks (Selden et al., 1998). Our significant finding is that in using the R-fMRI technique most of the isolated brain regions that showed increased CBF in previous perfusion studies (Nakano et al., 2001; Staff et al., 2000; Tateno et al., 2008; Ushuijima et al., 2006; Yoshida et al., 2007) were functionally interconnected to the MCC and the PCC in the MCP pathway. These brain regions include the PCC, vACC, parietal cortices, MTG and VMPFC (Tables 2 and 3). We found that the FC of the parahippocampal gyrus, vACC, IPC and VMPFC connected to the MCC and the PCC were partially recovered after treatment in patients with mild AD (Table 4). More importantly, the changes in the FC were significantly correlated with the cognitive performance and the regional CBF in the MCC and the PCC (Figure 4). Furthermore, as a previous study reported, the connectivity increase of the posterior cingulate regions was significantly decreased in AD subjects compared with cognitively normal subjects (Buckner et al., 2009; Greicius et al., 2004). To address the question of PCC and MCC connectivity in control subjects, we employed and analyzed R-fMRI datasets previously acquired from age-matched cognitively healthy controls (12 subjects). The PCC and MCC connectivity in the control subjects was significantly higher than that in AD subjects. Therefore, the increased connectivity after treatment, as shown in Figure 3, could be interpreted as the improvement toward normalcy.
The MCP, in addition to the lateral cholinergic pathway, is a primary white matter pathway in the human cholinergic network. The MCP trajectory originates at the nucleus basalis of Meynert of the basal forebrain and extends medially through the cingullum and corpus callosum, primarily innervating the neocortex (Selden et al., 1998). Although the function of the MCP is not completely understood, it has been suggested that the posterior parts of the cingulate cortices, such as the MCC and the PCC, perform evaluative functions for spatial orientation and memory (Vogt et al., 1992). In animal studies, the septocingulate pathway, in which cholinergic neurons of the basal forebrain innervate the cingulate cortex, is critically involved in the working/episodic memory of rats (Dougherty et al., 1998). Recently, evidence from structural studies which used diffusion tensor imaging illustrates that the MCC and the PCC are an integral part of the core structures in the human cerebral cortex (Hagmann et al., 2008). These brain regions, which include the MCC, PCC, medial prefrontal lobe, middle temporal lobe and IPC, are hubs of the “structural highways” in the brain that interconnect functionally specialized networks (Buckner et al., 2009; Gong et al., 2009). Several studies indicate that disruption of networks that originate from these hub brain regions is associated with memory deficits and cognitive decline in dementia (Seeley et al., 2009; Xie et al., 2011). Thus, our findings of increased regional CBF and the enhancement of the underlying FC networks of the MCC and the PCC support the notion that donepezil treatment mediates positive synaptic changes in the cholinergic pathways in the AD brain. In turn, this will likely increase the corticocortical connection between the functionally interconnected brain regions. As a result, this enhanced connectivity in the neurocognitive networks may be responsible for the cognitive responses in patients following treatment.
Our results highlight the value and importance of examining donepezil-mediated effects on neurocognitive networks encompassing multiple interconnected brain regions in AD. It has been increasingly recognized that cognitive outputs are not likely to be mediated by individual cortical or subcortical brain regions. Rather, cognitive outputs are dependent on regions, which are interconnected and organized in ways that can facilitate synchronous neural activation, thereby forming the neurocognitive networks (Mesulam, 2009). Therefore, depending on the brain regions and the extent of their involvement in the neurocognitive networks, varying types and degrees of dysfunction can exist in the diseased brain. Recent studies have linked the different categories of neurodegenerative diseases to dysfunctions in the distinct and specific brain functional networks (Seeley et al., 2009). A number of studies also have shown that cognitive changes in patients with neurodegenerative diseases are more likely the result of changes in the functional neural networks containing multiple brain regions rather than isolated regions (Kozel et al., 2011; Xie et al., 2011). Hence, cognitive improvement following donepezil treatment suggests that the drug targets and affects functional systems or networks involving multiple brain regions. It enhances the cognitive outcomes by increasing the connectivity between these anatomically separated regions. These findings suggest that the multimodal MRI techniques utilized in the current study are powerful tools for studying the efficacy of drug treatment in the regional and system levels in mild AD.
Several limitations of the current study should be considered when interpreting the results. First, the American Academy of Neurology views AChEI as the standard of clinical care. As a result, the IRB did not allow a placebo-controlled arm for this study. We were unable to evaluate any possible placebo effect of donepezil. However, previous studies have demonstrated that there has been no significant placebo benefit found in several donepezil trials in respect to regional CBF changes (Rogers et al., 1998; Yoshida et al., 2007) and cognitive changes (Black et al., 2007). Therefore, the likelihood that the observed CBF changes are because of a placebo effect is minimal. Second, the pCASL-MRI employed in this study acquired axial images from the upper part of the inferior parietal lobe to the inferior part of the temporal lobe, but not the whole brain. Third, the susceptibility artifacts from thicker imaging slices (Li et al., 2002b) rendered a low signal-to-noise ratio. We were unable to detect CBF changes in the orbitofrontal cortex where previous SPECT studies showed positive findings (Hanyu et al., 2003; Hongo et al., 2008). In future studies, fast-imaging acquisition methods, such as the multislice excitation method (Feinberg et al., 2010; Jesmanowicz et al., 2011) and the latest parallel imaging techniques, will be used to acquire thinner slices with full-brain coverage in order to provide complete characterization of whole-brain CBF. Finally, the present study represents a small cohort for a treatment study, although recent studies have demonstrated that pCASL is highly reproducible with a relatively small sample size of 10 subjects (Wang et al., 2011), 12 subjects (Chen et al., 2011b), and 22 subjects (Xu et al., 2010). It will be necessary to include more subjects in future treatment studies to achieve a more adequate statistical power.
In summary, the pCASL-MRI and R-fMRI techniques revealed that the 12-week donepezil therapy enhances regional CBF in the MCC and the PCC, and improves the underlying functional connectivity to the whole brain in mild AD. Linear regression analysis further demonstrated that the treatment-induced cognitive changes are associated with the changes in regional CBF and the underlying functional connectivity in these patients. This combined method is able to detect the regional CBF changes in the isolated brain regions. In addition, it provides clues as to how these segregated brain regions are interconnected as a whole to form integrated functional brain networks. Hence, the current strategy may provide a new approach in studying treatment efficacy during the course of clinical treatment.
Highlights.
We study the treatment effects of donepezil in mild Alzheimer’s disease.
We use integrative MRI approach to examine functional changes after treatment.
Treatment improves regional cerebral blood flow in the cingulate cortices.
Treatment improves functional connectivity in the medial cholinergic pathways.
Cognitive performances are correlated with changes in blood flow and connectivity.
Acknowledgements
The authors thank Carrie M. O’Connor, MA, for editorial assistance, Douglas Ward, MS, for statistical analysis help, and Judi Zaferos-Pylant, BMS, and Yu Liu, MS, for MRI technical support. This study was partially funded by Eisai Inc. and Pfizer Inc. This study was also supported, in part, by NIH-NIA R01 grant AD 20279.
Footnotes
AChEI: acetylcholinesterase inhibitors, AD: Alzheimer’s disease, CBF: cerebral blood flow, ASL: arterial spin labeling, pCASL: pseudocontinuous arterial spin labeling, R-fMRI: resting-state functional MRI, FC: functional connectivity, MMSE: Mini-Mental Status Examination, ADAS-cog: Alzheimer’s disease Assessment Scale-Cognitive subscale, NPI: Neuropsychiatric Inventory, IADL: Instrumental Activities of Daily Living, MCC: middle cingulate cortex, PCC: posterior cingulate cortex, ACC: anterior cingulate cortex, IPC: inferior parietal cortex, vACC: ventral anterior cingulate cortex, VMPFC: ventral prefrontal cortex.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors have made substantial intellectual contributions to this manuscript in one or more of the following areas: design or conceptualization of the study, analysis or interpretation of the data, or drafting and revising the manuscript. All authors have given final approval of this manuscript. None of the authors of this paper have any possible conflicts of interest, financial or otherwise, related directly or indirectly to this work.
References
- Agosta F, Rocca MA, Pagani E, Absinta M, Magnani G, Marcone A, Falautano M, Comi G, Gorno-Tempini ML, Filippi M. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31:515–525. doi: 10.1002/hbm.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, Sun Y, Perdomo CA, Richardson S. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology. 2007;69:459–469. doi: 10.1212/01.wnl.0000266627.96040.5a. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Koeppe RA, Meltzer CC, Constantine G, Davis JG, Mathis CA, Dekosky ST, Moore RY. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV., Jr. Multiple central nervous system targets for eliciting beneficial effects on memory and cognition. J Pharmacol Exp Ther. 2000;295:438–446. [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ward BD, Xie C, Li W, Wu Z, Jones JL, Franczak M, Antuono P, Li SJ. Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology. 2011a;259:213–221. doi: 10.1148/radiol.10100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011b;33:940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–866. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KD, Turchin PI, Walsh TJ. Septocingulate and septohippocampal cholinergic pathways: involvement in working/episodic memory. Brain Res. 1998;810:59–71. doi: 10.1016/s0006-8993(98)00870-1. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas J, Xie C, Ward BD, Wu Z, Li W, Franczak M, Jones J, Antuono PG, Li S-J. Recovery of Hippocampal Network Connectivity Correlates with Cognitive Improvement in Mild Alzheimer’s Disease Patients Treated with Donepezil Assessed by Resting-State fMRI. JMRI. 2011 doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3:211–225. [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Hirao K, Kanetaka H, Iwamoto T, Koizumi K. The progression of cognitive deterioration and regional cerebral blood flow patterns in Alzheimer’s disease: a longitudinal SPECT study. J Neurol Sci. 2010;290:96–101. doi: 10.1016/j.jns.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Shimizu T, Tanaka Y, Takasaki M, Koizumi K, Abe K. Regional cerebral blood flow patterns and response to donepezil treatment in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;15:177–182. doi: 10.1159/000068785. [DOI] [PubMed] [Google Scholar]
- Hongo J, Nakaaki S, Shinagawa Y, Murata Y, Sato J, Tatsumi H, Tohyama J, Soma T, Iidaka T, Fukui T, Mimura M, Furukawa TA. SPECT-identified neuroanatomical predictor of the cognitive effects of donepezil treatment in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;26:556–566. doi: 10.1159/000181148. [DOI] [PubMed] [Google Scholar]
- Jesmanowicz A, Nencka AS, Li S-J, Hyde JS. Two-Axis Acceleration of Functional Connectivity Magnetic Resonance Imaging by Parallel Excitation of Phase-Tagged Slices and Half k-Space Acceleration. Brain Connectivity. 2011;1:81–90. doi: 10.1089/brain.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, Rao U, Lu H, Nakonezny PA, Grannemann B, McGregor T, Croarkin PE, Mapes KS, Tamminga CA, Trivedi MH. Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Front Psychiatry. 2011;2:7. doi: 10.3389/fpsyt.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Li S-J, Li Z, Wu G, Zhang M-J, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002a;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Li Z, Wu G, Zhao X, Luo F, Li SJ. Multiecho segmented EPI with z-shimmed background gradient compensation (MESBAC) pulse sequence for fMRI. Magn Reson Med. 2002b;48:312–321. doi: 10.1002/mrm.10219. [DOI] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Defining neurocognitive networks in the BOLD new world of computed connectivity. Neuron. 2009;62:1–3. doi: 10.1016/j.neuron.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Nakano S, Asada T, Matsuda H, Uno M, Takasaki M. Donepezil hydrochloride preserves regional cerebral blood flow in patients with Alzheimer’s disease. J Nucl Med. 2001;42:1441–1445. [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and memory. Chem Biol Interact. 2010;187:403–408. doi: 10.1016/j.cbi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 12):2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Staff RT, Gemmell HG, Shanks MF, Murray AD, Venneri A. Changes in the rCBF images of patients with Alzheimer’s disease receiving Donepezil therapy. Nucl Med Commun. 2000;21:37–41. doi: 10.1097/00006231-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Tateno M, Kobayashi S, Utsumi K, Morii H, Fujii K. Quantitative analysis of the effects of donepezil on regional cerebral blood flow in Alzheimer’s disease by using an automated program, 3DSRT. Neuroradiology. 2008;50:723–727. doi: 10.1007/s00234-008-0401-y. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Ushuijima Y, Okuyama C, Mori S, Kubota T, Nakai T, Nishimura T. Regional cerebral blood flow in Alzheimer’s disease: comparison between short and long-term donepezil therapy. Ann Nucl Med. 2006;20:425–429. doi: 10.1007/BF03027378. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3. T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD. Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage. 2011;54:1188–1195. doi: 10.1016/j.neuroimage.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-C, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- Xie C, Goveas J, Wu Z, Li W, Chen G, Franczak M, Antuono PG, Jones JL, Zhang Z, Li SJ. Neural basis of the association between depressive symptoms and memory deficits in nondemented subjects: resting-state fMRI study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M, Christian BT, Oakes TR, Johnson SC. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23:286–293. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ha-Kawa S, Yoshimura M, Nobuhara K, Kinoshita T, Sawada S. Effectiveness of treatment with donepezil hydrochloride and changes in regional cerebral blood flow in patients with Alzheimer’s disease. Ann Nucl Med. 2007;21:257–265. doi: 10.1007/s12149-007-0022-2. [DOI] [PubMed] [Google Scholar]