Abstract
As the trend continues toward the decreased invasiveness of medical procedures, image-guided percutaneous ablation has begun to supplant surgery for the local control of small tumors in the liver, kidney, and lung. New ablation technologies, and refinements of existing technologies, will enable treatment of larger and more complex tumors in these and other organs. At the same time, improvements in intraprocedural imaging promise to improve treatment accuracy and reduce complications. In this review, the latest advancements in clinical and experimental ablation technologies will be summarized, and new applications of image-guided tumor ablation will be discussed.
Keywords: Ablation, future, percutaneous, image-guided
In the last two decades, the development and refinement of needle-based ablation techniques using freezing or thermal energy have allowed local therapy of tumors in a minimally invasive manner. The benefits of the percutaneous approach include shortened hospital stay, lower morbidity, and decreased healthcare costs, as well as the ability to treat tumors in nonsurgical candidates.1,2
Multiple studies have demonstrated the safety and efficacy of percutaneous ablation for tumors in the liver,3,4 kidney,5,6 and lung,7,8 and in some situations ablation appears equally effective to traditional surgical resection.9,10 However, percutaneous ablation does face several significant shortcomings, which restrict its application in many patients. First and foremost is the limitation of tumor size. Studies in the liver, kidney, and lung demonstrate complete ablation in over 90% of tumors <3 cm in size.11,12,13 In larger lesions, however, residual disease and local tumor recurrence become increasingly common. Second is the issue of applicability; percutaneous ablation is currently only in routine clinical use for tumors in the liver, kidney, and lung. Finally, intraprocedural imaging is often suboptimal, either due to image degradation of the target lesion or poor delineation of the ablation zone. This review article summarizes the recent advances in the field of percutaneous ablation, as new technologies and refinements of existing technologies aim to overcome some of the aforementioned limitations. Future directions in the field will also be presented.
NEW TECHNOLOGIES AND REFINEMENTS OF EXISTING TECHNOLOGIES
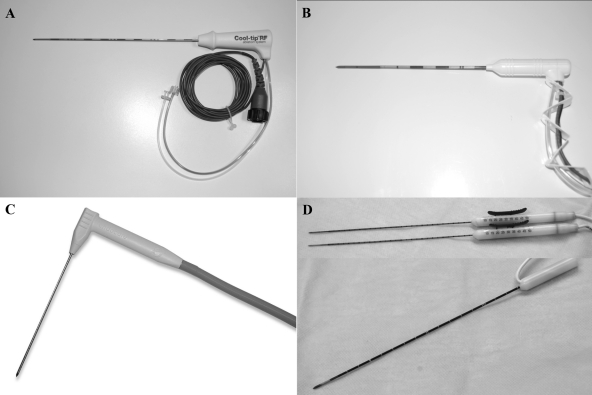
Representative examples of the needle-based ablation modalities are depicted in Fig. 1.
Figure 1.
Representative percutaneous ablation devices. (A) Cool-tip internally cooled radiofrequency electrode (Covidien, Mansfield, MA). (B) Monopolar (top) and bipolar (bottom) irreversible electroporation probes. (C) Evident internally cooled percutaneous microwave antenna (Covidien, Mansfield, MA). (D) Perc-24 cryoprobe (Endocare, Irvine, CA).
Radiofrequency Ablation
Radiofrequency ablation (RFA) uses rapidly alternating radiofrequency current to induce frictional heat around probes placed in tissue, producing cell death by coagulation necrosis. First-generation monopolar electrodes generated a cylindrical ablation with a diameter of only 1.6 cm.14 Refined electrodes using umbrella-shaped expandable tines or internal cooling increase conductive heat delivery, and are the most commonly used RFA devices in contemporary practice. However, the ablation zone produced by these devices is still limited to ~4 cm diameter.2 Depending on the ablation margin desired, this can be sufficient for treatment of lesions up to 2 to 3 cm, but in larger lesions, the number of overlapping ablations needed to produce complete ablation increases exponentially. Multiple needle repositionings are tedious and increase the potential for error and resultant incomplete ablation, especially with the image degradation that can occur with treatment.2
RFA has several other drawbacks, including dependence on thermal conduction, which can be limited by tissue desiccation and charring, and susceptibility to “heat-sink” effect, which may result in sublethal temperatures adjacent to vessels >3 mm in size.15,16 Also, tissue heterogeneity can result in islands of viable tumor within the ablation zone.17 Due to electromagnetic interference, only one RFA probe can be activated at a time, and grounding pads are required, which can be a site of skin burns. Attempts to overcome these limitations have led to technical advancements in the field of RFA over the last few years.
Commercially available RF generators produce ~200 W of power and 2000 mA of current. Higher power (1000 W), higher current generators (3000 mA) have been examined in vivo in healthy pig livers, and were found to enlarge the coagulation zone from a single 3-cm-active-length internally cooled electrode from 2.6 to 3.6 cm. When a 4-cm active length cluster electrode was used (not yet in clinical use), an ablation diameter of 5.0 cm could be achieved.18 The use of these high power generators is not without risk, however. At the highest current setting (4000 mA), burns to the bowel were observed in several animals, and one intraprocedural death occurred. Additional studies are required to evaluate the safety of high power generators in vivo.
The potential for injected saline to increase the ablation size was realized soon after the seminal articles on RFA. Injection of normal or hypertonic saline into the tissue adjacent to the RF electrode increases electrical conductance and thermal conductivity, thereby improving energy delivery and enlarging the ablation zone.19,20 However, the distribution of the injected liquid is unpredictable, which can result in an irregular ablation zone,21 and the diffusion of hot saline can cause burn injury to surrounding organs.22 An expandable electrode using saline injection and multistep deployment of tines to a maximum diameter of 7 cm is commercially available. Ablation of 31 hepatocellular carcinoma (HCC) lesions measuring 3 to 7.5 cm using this device yielded a complete ablation rate of 74%, with an average of 1.6 electrode insertions.23 The complication rate was 48%, but all complications were self-limited.23
Bipolar RF ablation utilizes a parallel-placed needle, or a second electrode within the same ablation needle, to serve as an electrical ground.24 Because the current flows directly from one electrode to the other, the dependence on thermal conduction is reduced, ablation times are faster, and energy loss from heat sink decreases.25 Grounding pads are not needed. However, early “dry” bipolar probes were plagued by tissue charring, which decreased current flow between the electrodes and often resulted in a flattened or discontinuous ablation zone.22,26 The combination of saline injection with bipolar probes improves current penetration and decreases charring. One “wet bipolar” device incorporates both electrodes and four saline exit sites into a single 1.8-mm-diameter applicator, and produces a homogeneous ablation zone with short axis diameter of ~3 cm in a live swine model.27
An alternative method of reducing charring with the bipolar electrode is to use internal cooling. A single water-cooled bipolar applicator examined in ex vivo bovine livers achieved a short axis ablation diameter of 3.2 cm.28 A recent study examined the use of carbon dioxide gas as a more powerful cooling agent (cryo RFA); incorporated into a single 1.8-mm-diameter bipolar applicator, short axis diameters of 4.4 cm could be achieved in ex-vivo bovine liver.29 These electrodes have not yet been studied in vivo.
A logical extension of the bipolar technique is the use of multipolar electrode arrays. Three internally cooled bipolar electrodes are placed in a triangular array, and a switching controller passes RF energy between all possible electrode pairings until target impedance is reached. This prevents tissue charring and creates a uniform ablation zone. Depending on the power applied, the spacing of the electrodes, and the duration of ablation, coagulation diameters of 4.2 to 8.4 cm have been achieved in ex vivo bovine liver.30 In a clinical study, this technology achieved an 81% complete ablation rate in hepatocellular carcinoma (HCC) measuring 5.0 to 8.5 cm, without major complications and usually in a single treatment session.31
A different tact to expand ablation zones with RFA is the use of complementary liposomal chemotherapy. The chemotherapy sensitizes the tumor to heat, increasing tumor destruction in the sizable rim of tissue around the ablation zone which is exposed to mildly elevated but otherwise sublethal temperature.32,33 Packaging of the chemotherapy within biologically inert liposomes prolongs circulation time and reduces systemic toxicity.34 An early trial comparing RFA alone with combined RFA and liposomal doxorubicin in 10 patients with liver tumors found a 25 to 30% larger ablation volume in the liposomal RFA group at 2 to 4 weeks after treatment, as well as more complete internal tumor destruction.35 Thermosensitive liposomes have also been developed, which promise to further increase the release of the chemotherapeutic drug in regions of tissue heating. A phase III clinical trial (HEAT study) examining thermosensitive liposomal RFA in 600 patients with HCC is currently underway.
Microwave Ablation
Microwave ablation employs a needle antenna to create a localized electromagnetic field. The field causes water molecules around the antenna to oscillate, producing heat. Since the mid-1990s, microwave ablation has slowly gained recognition as an alternative tumor ablation method, especially in Asia, where it is commonly used in liver applications.
Microwave ablation promises several theoretical advantages over RFA. Microwave ablation actively heats the ablation zone, rather than the passive conduction of heat relied upon by RFA. Thus, microwave ablation is not limited by tissue desiccation and charring, is less affected by heat sink, and can rapidly reach high temperatures, resulting in faster ablation times and a more uniform ablation zone.36,37 For larger lesions, multiple microwave antennae can be operated simultaneously to rapidly produce a large ablation area.4,38 Also, ultrasound visualization of the ablation zone of microwave ablation may be better than RFA, due to the lack of artifacts caused by desiccated and charred tissue.37,39
Early percutaneous microwave ablation devices showed satisfactory outcomes in treating small liver lesions,38,40 but ablation zones were small and the antennae were prone to overheating. Cooled-shaft microwave ablation antennae allow more power delivery without skin burns. Using cooled-shaft antennae, a 92% complete ablation rate was achieved in liver lesions up to 8 cm, and others have ablated tumors >10 cm in size.41 A cooled-shaft 915 MHz microwave ablation system has recently been approved for use in the United States, which yields a larger ablation zone compared with the 2,450 MHz cooled-shaft antennae used in Asia.42 The most extensive study of this device was intraoperative, where liver tumors up to 6 cm were treated, with a 95% complete ablation rate and only 2% local recurrence rate.43
One of the most promising advancements in percutaneous microwave ablation is the triaxial antenna. This device consists of a coaxial monopole antenna placed through an introducer needle, and is tunable to optimize its use in different tissues. The device maximizes energy transfer to the tissue while minimizing back heating, and allows for antenna diameter as small as 17 or even 18 gauge.44 A prototype 17-gauge cooled triaxial microwave antenna has been shown to produce 25% larger and more spherical ablations than a comparable 17-gauge internally cooled RF electrode in a porcine lung model.45
Multiple microwave antennae can be activated simultaneously to achieve synergistically larger zones of ablation. In vivo liver experiments show that simultaneous three probe microwave ablation lesions are 3 times larger in volume than sequential three probe lesions. The simultaneous application also resulted in more uniform coagulation, even near blood vessels, and better sphericity.46 However, clefting of the ablation zone can occur when the antennae are spaced by more than 1.7 cm. With the use of three 17-gauge triaxial microwave antennae, the improved input power and efficiency allows antenna spacing up to 3 cm, and in vivo experiments yield ablation zones averaging 5.3 × 7.6 cm. Vessels as large as 1.1 cm were completely coagulated, demonstrating the ability of this technique to overcome the heat-sink effect.47
Cryoablation
Cryoablation utilizes rapid freezing (as low as -196°C) and thawing cycles to induce tissue necrosis by ice crystal formation, cell dehydration, and impairment of tumoral microvasculature. Cryoablation has several theoretical advantages over RFA. The ice ball that forms is well seen with computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound, and can be tailored by controlling individual probe temperatures. Multiple probes can be simultaneously applied for a large ablation zone (>8 cm).48 Also, cryoablation is a relatively painless procedure that can often be controlled with nonnarcotic medications.49 However, the zone of complete lethality lies a variable distance inside the edge of the ice ball, 4 to 10 mm or more, meaning the ice ball must extend well beyond the tumor to ensure a satisfactory treatment margin.50 Cryoablation also can suffer a “cold-sink” effect from adjacent vessels,15 and rare but serious complications of cryoshock and parenchymal crack can occur, especially in liver applications.51
Historical cryoprobes used liquid nitrogen as a cryogen and were designed for intraoperative applications. The switch to argon gas as the cryogen allowed downsizing of probes to 2.4 mm (13-gauge), and enabled safe percutaneous use. MRI-compatible 1.5-mm (17-gauge) cryoprobes became available earlier this decade, and produce an ice ball essentially the same size as the 2.4-mm probes, despite a 38% smaller probe diameter (manufacturer's data).
High-Intensity Focused Ultrasound
The ability of focused ultrasound energy to noninvasively heat tissue has been recognized for decades. Using a transducer that delivers sonic energy at intensities ~10,000 times that used in diagnostic ultrasound, high-intensity focused ultrasound (HIFU) can produce almost instantaneous cell death in a precisely targeted volume. The main advantage of HIFU compared with other ablative technologies is its noninvasiveness. As the skin is not breached and the tumor is not punctured, there is no risk of needle-related hemorrhage or tumor seeding.52
HIFU ablates tissue primarily via the thermal effects of molecular friction and the mechanical effects of acoustic cavitation.53 The thermal effects are induced by ultrasound absorption and are relatively easy to control. The acoustic cavitation effects are less predictable, but have potential to enlarge the ablation area size and thereby reduce procedure time.54 A single application of a typical 1.5 MHz HIFU transducer produces a thermal lesion measuring ~2 mm in width and 1.5 to 2.0 cm in length.55 These lesions are “stacked” by movement or steering of the transducer to produce an overlapping ablation zone of the desired size.
At the time of the writing of this article, HIFU is faced with several limitations. Procedure times are prohibitively long for ablation of medium and large lesions, with some treatments lasting 6 hours or more. Organ movement can result in incomplete target ablation, or worse, ablation of nontarget structures. Reflection of the high-energy ultrasound waves by bone or gas-containing bowel can cause soft tissue damage, and skin burns can occur if there is poor acoustic coupling at the skin–transducer interface.53 Some of these drawbacks are being addressed by new technologies, such as real-time adaptive treatment under MRI guidance,56 which may improve the speed and accuracy of HIFU, and expand its capability in moving organs.
The injection of intravenous microbubbles during HIFU application may be able to enlarge the resultant ablation zones. The microbubbles act as “seeds” for cavitation, decreasing the threshold and increasing the activity of acoustic cavitation.55 This in turn leads to increased tissue temperatures with less sonication time as demonstrated in animal models,57,58 and may allow more rapid treatment compared with conventional techniques.
New geometries of HIFU transducers also promise to enlarge the coagulated volume and decrease treatment time. A toric transducer, composed of eight ultrasound transducers with focal zones distributed over a 10-mm cone, has been described. Using sequential 5-second applications of each HIFU transducer in healthy pig liver, a coagulative lesion with an almost 2-cm diameter can be produced in only 40 seconds.59
Irreversible Electroporation
Irreversible electroporation (IRE) refers to the process of permanent cell membrane permeabilization by application of a high external electrical field. The transmembrane potential from the external electrical field is thought to cause the formation of innumerable nano-sized pores in the membrane and subsequent disruption of intracellular homeostasis. If the applied electrical field is below a certain threshold, this event is only temporary and the cell membrane returns to normal when the external electrical field is removed (reversible electroporation). If the applied electric field exceeds the threshold, it will result in permanent disruption of cell membrane structures and intracellular homeostasis, leading to cell death.60 This latter effect, which was initially considered undesirable by researchers, has been recently seized upon as a novel technique for minimally invasive ablation.
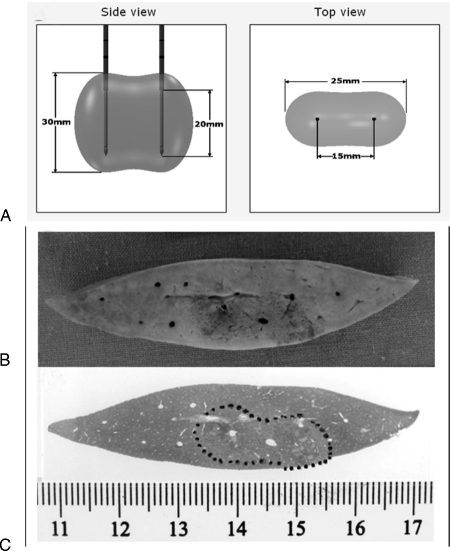
There are two main types of IRE probes: monopolar and bipolar. The monopolar system requires placement of two probes (19 gauge, maximum depth of 15 cm) into or bracketing the target. Applying 2500 V across two probes spaced 1.5 cm apart results in an ablation zone measuring ~2 × 3 × 3 cm61 (Fig. 2). To achieve larger ablation areas, up to six monopolar probes can be placed simultaneously. The bipolar system consists of a single probe (16 gauge, maximum depth of 18 cm). It contains two poles within its distal portion. Applying 2500 V across the two poles will create an ablation zone of ~2 × 2 × 3 cm.61
Figure 2.
Irreversible electroporation (IRE). (A) Pre-reatment planning estimation of IRE treatment zone (2000 V/cm with 1.5-cm distance using two monopolar probes). (B) and (C) Gross and hematoxylin and eosin stained IRE-treated liver specimens demonstrating clear demarcation between the treated and nontreated tissue.
IRE demonstrates several unique advantages over conventional thermal ablation methods. IRE has an ultrashort ablation time, less than one minute, to create an ablation area of ~3 cm in diameter.60 This short ablation time is expected to shorten anesthesia time, reduce complications, and empower treatment of more lesions in a single setting. Real-time ultrasound can be used for treatment monitoring, and accurately delineates the treatment zone, correlating well with the zone of necrosis seen on immunohistopathology.60 Visualization is not hindered by the presence of echogenic microbubbles that obscure the ablation zone with heat-based ablation. Because IRE is nonthermal, there is no “heat-sink” effect, and complete cell death surrounding vessels can be produced.60,62,63,64 Although IRE ablates the living cells, it preserves the cellular matrix and pericellular structures, so large vessels and bile ducts remain structurally and functionally intact.63,64 Finally, collateral damage to nearby structures when IRE is applied at the edge or dome of the liver appears less likely to occur compared with thermal modalities.61
IRE does have some disadvantages. Delivery of a high electrical field close to the heart during IRE of lung lesions or high left lobe liver tumors has caused arrhythmias including ventricular fibrillation in preclinical studies and in two patients in the preliminary clinical study. Electrocardiogram gating of IRE application can be used to avoid the application of electrical fields during systole, which is the phase of highest susceptibility, and may overcome this disadvantage (unpublished data). Nevertheless, additional caution is required in patients with known cardiac comorbidity. Also, the high voltage used by IRE results in significant muscle spasm. Muscle relaxants such as cisatracurium are therefore used when performing IRE ablation to prevent electric pulse-induced muscle contractions.
In vivo and clinical data using IRE are limited at the time of this review. A VX2 liver tumor ablation study in rabbits and a phase I clinical feasibility study in humans have been completed and are being prepared for publication.
NEW APPLICATIONS
Breast Cancer
Emerging research seeks to expand the use of percutaneous ablation to early-stage breast cancer. The current standard of care for small breast carcinomas is local excision with adjuvant radiation therapy. This carries a risk of bleeding and infection, and can result in serious breast asymmetry.65 Because the breast is a superficial organ, well visualized with ultrasound, and easily immobilized, it is amenable to percutaneous ablation. Minimally invasive treatment would be expected to reduce complications and improve cosmesis.
RFA is the most extensively studied modality in the breast. Ten feasibility studies of RFA followed by definitive resection in early-stage breast cancers have demonstrated an overall complete ablation rate of 87%66,67,68,69,70,71,72,73,74,75 and a low complication rate. The most recent study used a dedicated breast cool-tip RF electrode to treat primary breast cancers averaging 1.9 cm in diameter in 34 women. Total procedure time averaged 27 minutes, and a complete ablation rate of 97% was achieved. The ablation margins seen on postprocedure MRI were spherical and correlated well with histologic specimens. Cosmesis was excellent.70
The dielectric properties of microwave ablation, which allow preferential heating of water-rich tumors compared with adipose tissues, suggest its potential suitability for breast cancer treatment. Only feasibility studies have been performed thus far. The technique achieved tumor necrosis, but complete ablation was rare. Occasional skin burns occurred.76,77
Interest is increasing in cryoablation for breast masses, driven by the lower sedation requirements and good imaging visualization of the ablation zone. Ultrasound-guided cryoablation of fibroadenomas has already proven safe and durable.78 Feasibility studies of breast cancer cryoablation achieved complete ablation rates of 52 to 100% in T1 to T3 tumors.79,80,81 Recently, 11 patients with 21 breast cancer foci averaging 1.7 cm in diameter were treated under ultrasound and CT guidance, without follow-up excision. The mean ice ball diameter was 5.1 cm, using an average of 3.3 probes. No major complications were recorded, and no local recurrences were seen after 18 months. Patient satisfaction was very high.82
The excellent acoustic window and easy immobilization of the breast make it amenable to HIFU treatment. Thus far, studies of MRI-guided HIFU in the breast have been mostly limited to treatment and resection feasibility studies. These studies have shown that HIFU can ablate most of the tumor volume (mean percentage necrosis of 88 to 97%),83,84 but usually leaves some tumor behind at the treatment margins, with complete ablation rates of only 0 to 50%.83,84,85,86 One group using ultrasound-guided HIFU in a “treat-and-resect” protocol was able to achieve 100% necrosis in all 21 patients treated, likely related to the large margins (1.5–2.0 cm) sought during treatment.87 In all studies, HIFU was performed under moderate intravenous sedation, was well tolerated, and had no major complications.
IRE has not yet been applied to breast cancers in vivo, but in vitro study demonstrates lethality of electroporation against an aggressive breast cancer cell line, and the nonthermal nature is expected to reduce scarring and minimize cosmetic effects.88
Although percutaneous ablation has great potential benefit in breast cancer patients, several obstacles remain. Besides the treatment-specific limitations described above, patients with large tumors and extensive ductal carcinoma in situ or invasive lobular carcinoma are not ideal candidates. Also, significant scar tissue may be created in the ablation zone, which can reduce the accuracy of ultrasound or mammographic follow-up. There are no long-term data for breast cancer ablation without subsequent excision, and until these data are available, this treatment will likely be limited to nonsurgical candidates.
Prostate Cancer
Early-stage prostate cancer is exceedingly common, and conventional treatments (radical prostatectomy and radiation) incur significant morbidity. The alternative, watchful waiting, risks spread or invasion of the index tumor. Thus, the prostate is a suitable target for minimally invasive local therapy.
Cryotherapy of whole gland prostate cancer has already been established as a safe and effective alternative to conventional treatments, confirmed with long-term follow-up data.89,90 The idea of focal cryoablation, freezing the tumor while sparing the uninvolved prostate, is a logical next step. Transrectal ultrasound is used to guide placement of a cryoablation probe through the perineum and into the tumor. Because the neurovascular bundles can often be spared, the rates of impotence and incontinence may be lower than surgery or radiotherapy.91 In a study of 120 men with localized prostate cancer who had focal cryoablation over 12 years, 93% had no evidence of cancer at follow-up, despite the majority being labeled medium to high risk of recurrence. Eighty-five percent retained sexual function and all who had not had prior prostate surgery remained continent.92 Another study has shown comparable results.93
The prostate is well-suited for HIFU due to its good visualization with ultrasound, lack of intervening structures, and minimal respiratory movement. Transrectal ultrasound guidance is used. A recent study compiled long-term follow-up in 517 patients treated with HIFU for localized prostate cancer, and found that 72% remained biochemically disease-free at 5 years. No patients died due to prostate cancer. Some complications were encountered, including a 29% risk of erectile dysfunction and 17% incidence of urethral stricture, and prolonged catheterization was needed in 13% of patients due to prostate swelling.94 Other studies have confirmed 5-year disease-free survival rates of 68 to 78% for early-stage localized prostate cancer.95,96,97
In vivo experiments in dogs suggest that IRE of the prostate may offer several advantages over cryoablation and HIFU. The IRE lesions resolve rapidly, making urethral dysfunction from prostate swelling unlikely, and possibly facilitating imaging follow-up. Even when intentional IRE of the neurovascular bundle was performed, the nerve and vessel remained intact, suggesting that tumors involving this structure can be ablated with low risk of impotence. The urethra and rectum also seemed unaffected by IRE.98 Human trials have not yet been performed.
Uterine Fibroids
Uterine fibroids are exceedingly common and frequently symptomatic. Minimally invasive therapy using uterine artery embolization (UAE) is safe and effective, but the procedure is often painful, risks ovarian failure, and is not indicated in patients who wish to preserve fertility.99,100 Percutaneous ablation deserves attention as a treatment alternative.
Percutaneous radiofrequency ablation and cryoablation have been applied to uterine fibroids in several small case series. Ten women with 11 fibroids measuring up to 8 cm in size underwent RFA using an expandable electrode under ultrasound guidance. Operative time averaged 20 minutes, and technical success was 100%. Mean volume reduction was 91% at 12 months, and symptom severity scores significantly decreased in all but one patient. No complications were encountered.101 In the case of cryoablation, six patients with seven fibroids averaging 7.1 cm in diameter were treated in one study, using MR guidance and 2.9 cryoprobes per patient. Volume reduction was 79% at 12 months, and subjective symptoms improved in almost all patients.102 Two other small series of cryoablation also showed success.103,104
The use of HIFU for uterine fibroids has been well studied. MRI guidance is almost exclusively used. Although exclusion criteria are many (morbid obesity, lack of a clear pathway to the lesion, necrotic or degenerated fibroids), the treatment is feasible and effective in selected patients. Volume reduction of 36.5% at 6 months has been achieved,105 and symptom severity scores decrease significantly and durably after HIFU treatment. Re-intervention rates for HIFU were found to be 14% at 2 years,105 compared with 3.2% for myomectomy99 and 6.9 to 23.5% for UAE.99,100
Regardless of the technique used, percutaneous ablation for uterine fibroids is limited in patients with large (>8 cm diameter) and/or multiple (more than three) tumors, and in patients whose tumors cannot be accessed transabdominally. Nevertheless, an increasing role for percutaneous ablation in select patients is easily envisioned.
Intraprocedural Imaging
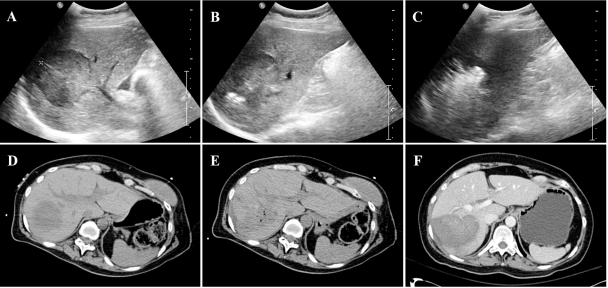
Percutaneous ablation is most commonly performed under ultrasound or noncontrast CT guidance. These modalities are sufficient for lesion detection and probe placement in most cases, but neither can depict the exact ablation margins during treatment (Fig. 3). MRI with temperature sensitive imaging is an alternative, but is expensive, not widely available, and requires the use of specialized MRI-compatible equipment.
Figure 3.
Computed tomography (CT) and ultrasound imaging of radiofrequency ablation (RFA). (A) Liver ultrasound demonstrates a 5-cm hypoechoic hepatocellular carcinoma. (B) An internally cooled cluster electrode has been placed, and echogenicity at the electrode tips marks the beginning of ablation. (C) Five minutes later, echogenic microbubbles obscure the lesion and the ablation zone. (D) Preprocedure noncontrast CT depicts the same lesion. (E) After multiple ablations and repositionings, the ablation zone and the lesion are poorly defined and indistinguishable. (F) Postprocedure contrast-enhanced CT clearly defines the ablation zone.
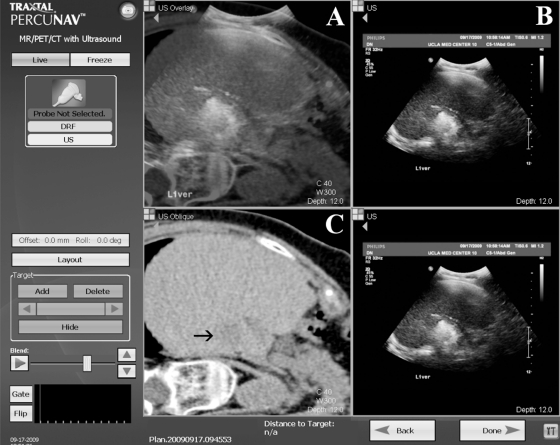
One potential solution is the use of real-time CT–ultrasound fusion imaging, which matches a preprocedural volumetric CT (with contrast, if desired) to real-time ultrasound images. An electromagnetic tracking system mounted on the ultrasound probe provides the position and orientation of the probe, and permits representation of the corresponding multiplanar reformatted CT image in the same plane and position. A recent feasibility study showed high and consistent levels of matching accuracy between preprocedure CT and real-time ultrasound imaging in an in vitro liver model, with mean registration error of only 3 mm.106 A clinical trial of a similar system used needles with internalized tip sensors, enabling virtual display of needle position within a previously obtained CT, to perform biopsies and ablations in 20 patients. Additional set-up time was ~5 minutes, and tracking error was ~6 mm.107 At our institution, the ability to provide real-time ultrasound guidance for lesions that are only visible on CT and the ability to enable accurate needle repositioning after the ultrasound picture is degraded by ablation treatment have proven to be useful applications of CT–ultrasound fusion technology (Fig. 4).
Figure 4.
Screen capture from the Traxtal PercuNav workstation (Philips, Andover, MA) during radiofrequency ablation. Blended computed tomography- (CT-) ultrasound image (A) superimposes the real-time ultrasound image (B) on the virtual noncontrast preprocedure CT image (C) in the corresponding plane and position. Although echogenic microbubbles obscure the target lesion on ultrasound, visualization of the lesion is maintained on the virtual CT image (arrow).
An extension of fusion imaging is the addition of computerized treatment planning. A semiautomated computer program can be used to analyze preprocedural CT, segment the tumor volume to be treated, and output the desired needle approaches for the necessary number of overlapping ablation spheres. These data can then be used with the fusion imaging technology to provide real-time guidance during needle placement. This has been studied for RFA of lung nodules in swine, and was feasible, though misregistration from respiratory movement led to an average error of needle placement of ~1 cm.108 Programs that improve registration by adding computerized modeling of organ motion over the breathing cycle have been developed, and promise accuracy to the millimeter level, but have not yet been applied to needle ablation.109 CT-integrated robots that partially or fully automate the needle placement process based on a user-defined treatment plan have been designed, and may eventually empower “point and click” tumor ablation.110
Recent advancements in ultrasound technology are also aiding guidance during percutaneous ablation. Contrast-enhanced flow imaging (four-dimensional ultrasonography) allows the operator to display the accurate positioning of the ablation needle in the target of hypervascular tumors such as HCC, and to better evaluate the completeness of ablation posttherapy, with good correlation to contrast-enhanced CT.111,112 Real-time temperature estimation using ultrasound appears feasible in tissue phantoms, but has been limited clinically by motion artifacts. New algorithms promise to improve clinical utility.113 Ultrasound-based elastography is being investigated as an alternative modality for treatment monitoring, taking advantage of the increased hardness of thermally ablated tissue compared with normal tissue.114,115
CONCLUSIONS
Percutaneous tumor ablation has made great strides over the last decade. Refinements of thermal ablation techniques have allowed larger ablation sizes with smaller instruments, and new technologies have enabled noninvasive ablation and nonthermal ablation with potential advantages in patient safety and treatment efficacy. Advances in intraprocedural imaging have improved treatment planning and produced a more accurate assessment of the ablation zone during treatment. As these technologies mature, the indications for percutaneous ablation continue to expand, and ablation promises to increasingly supplant surgery for local tumor therapy.
References
- Dupuy D E, Goldberg S N. Image-guided radiofrequency tumor ablation: challenges and opportunities—part II. J Vasc Interv Radiol. 2001;12(10):1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- Dodd G D, III, Soulen M C, Kane R A, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20(1):9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- Dong B, Liang P, Yu X, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180(6):1547–1555. doi: 10.2214/ajr.180.6.1801547. [DOI] [PubMed] [Google Scholar]
- Gill I S, Remer E M, Hasan W A, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173(6):1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- McDougal W S, Gervais D A, McGovern F J, Mueller P R. Long-term followup of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol. 2005;174(1):61–63. doi: 10.1097/01.ju.0000162046.45024.2b. [DOI] [PubMed] [Google Scholar]
- Simon C J, Dupuy D E, DiPetrillo T A, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- Chen M S, Li J Q, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J M, Svatek R, Park S, et al. Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumours. BJU Int. 2007;100(2):287–290. doi: 10.1111/j.1464-410X.2007.06937.x. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Goldberg S N, Lazzaroni S, Meloni F, Solbiati L, Gazelle G S. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210(3):655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226(2):417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- Lee J M, Jin G Y, Goldberg S N, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004;230(1):125–134. doi: 10.1148/radiol.2301020934. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Gazelle G S, Dawson S L, Rittman W J, Mueller P R, Rosenthal D I. Tissue ablation with radiofrequency using multiprobe arrays. Acad Radiol. 1995;2(8):670–674. [PubMed] [Google Scholar]
- Bhardwaj N, Strickland A D, Ahmad F, Atanesyan L, West K, Lloyd D M. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41(2):168–172. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- Lu D SK, Yu N C, Raman S S, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Gazelle G S, Compton C C, Mueller P R, Tanabe K K. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88(11):2452–2463. [PubMed] [Google Scholar]
- Solazzo S A, Ahmed M, Liu Z, Hines-Peralta A U, Goldberg S N. High-power generator for radiofrequency ablation: larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings. Radiology. 2007;242(3):743–750. doi: 10.1148/radiol.2423052039. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Ahmed M, Gazelle G S, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology. 2001;219(1):157–165. doi: 10.1148/radiology.219.1.r01ap27157. [DOI] [PubMed] [Google Scholar]
- Lee J M, Kim Y K, Lee Y H, Kim S W, Li C A, Kim C S. Percutaneous radiofrequency thermal ablation with hypertonic saline injection: in vivo study in a rabbit liver model. Korean J Radiol. 2003;4(1):27–34. doi: 10.3348/kjr.2003.4.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi T, Goldberg S N, Monti F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202(1):205–210. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- Burdío F, Güemes A, Burdío J M, et al. Large hepatic ablation with bipolar saline-enhanced radiofrequency: an experimental study in in vivo porcine liver with a novel approach. J Surg Res. 2003;110(1):193–201. doi: 10.1016/s0022-4804(02)00091-4. [DOI] [PubMed] [Google Scholar]
- Tarantino L SI, Sordelli I, Nocera V, et al. Ablation of large HCCs using a new saline-enhanced expandable radiofrequency device. J Ultrasound. 2009;12:69–74. doi: 10.1016/j.jus.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdío F, Güemes A, Burdío J M, et al. Hepatic lesion ablation with bipolar saline-enhanced radiofrequency in the audible spectrum. Acad Radiol. 1999;6(11):680–686. doi: 10.1016/S1076-6332(99)80117-2. [DOI] [PubMed] [Google Scholar]
- Haemmerich D, Wright A W, Mahvi D M, Lee F T, Jr, Webster J G. Hepatic bipolar radiofrequency ablation creates coagulation zones close to blood vessels: a finite element study. Med Biol Eng Comput. 2003;41(3):317–323. doi: 10.1007/BF02348437. [DOI] [PubMed] [Google Scholar]
- McGahan J P, Gu W Z, Brock J M, Tesluk H, Jones C D. Hepatic ablation using bipolar radiofrequency electrocautery. Acad Radiol. 1996;3(5):418–422. doi: 10.1016/s1076-6332(05)80677-4. [DOI] [PubMed] [Google Scholar]
- Burdío F, Navarro A, Sousa R, et al. Evolving technology in bipolar perfused radiofrequency ablation: assessment of efficacy, predictability and safety in a pig liver model. Eur Radiol. 2006;16(8):1826–1834. doi: 10.1007/s00330-005-0131-z. [DOI] [PubMed] [Google Scholar]
- Clasen S, Schmidt D, Dietz K, et al. Bipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: prediction of coagulation volume from applied energy. Invest Radiol. 2007;42(1):29–36. doi: 10.1097/01.rli.0000248973.95949.eb. [DOI] [PubMed] [Google Scholar]
- Rempp H, Voigtlander M, Clasen S, et al. Increased ablation zones using a cryo-based internally cooled bipolar RF applicator in ex vivo bovine liver. Invest Radiol. 2009;44(12):763–768. doi: 10.1097/RLI.0b013e3181b66d11. [DOI] [PubMed] [Google Scholar]
- Clasen S, Schmidt D, Boss A, et al. Multipolar radiofrequency ablation with internally cooled electrodes: experimental study in ex vivo bovine liver with mathematic modeling. Radiology. 2006;238(3):881–890. doi: 10.1148/radiol.2382050571. [DOI] [PubMed] [Google Scholar]
- Seror O, N'Kontchou G, Ibraheem M, et al. Large (>or = 5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes—initial experience in 26 patients. Radiology. 2008;248(1):288–296. doi: 10.1148/radiol.2481071101. [DOI] [PubMed] [Google Scholar]
- Monsky W L, Kruskal J B, Lukyanov A N, et al. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology. 2002;224(3):823–829. doi: 10.1148/radiol.2243011421. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Goldberg S N. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20(7):781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Shiota R, Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst. 1989;81(19):1484–1488. doi: 10.1093/jnci/81.19.1484. [DOI] [PubMed] [Google Scholar]
- Goldberg S N, Kamel I R, Kruskal J B, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol. 2002;179(1):93–101. doi: 10.2214/ajr.179.1.1790093. [DOI] [PubMed] [Google Scholar]
- Carrafiello G, Laganà D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6(Suppl 1):S65–S69. doi: 10.1016/j.ijsu.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Garrean S, Hering J, Saied A, et al. Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J Gastrointest Surg. 2009;13(2):334–340. doi: 10.1007/s11605-008-0715-4. [DOI] [PubMed] [Google Scholar]
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235(1):299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- Cha C H, Lee F T, Jr, Gurney J M, et al. CT versus sonography for monitoring radiofrequency ablation in a porcine liver. AJR Am J Roentgenol. 2000;175(3):705–711. doi: 10.2214/ajr.175.3.1750705. [DOI] [PubMed] [Google Scholar]
- Lu M D, Chen J W, Xie X Y, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology. 2001;221(1):167–172. doi: 10.1148/radiol.2211001783. [DOI] [PubMed] [Google Scholar]
- Kuang M, Lu M D, Xie X Y, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna—experimental and clinical studies. Radiology. 2007;242(3):914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Ni X, et al. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol. 2009;192(2):511–514. doi: 10.2214/AJR.07.3828. [DOI] [PubMed] [Google Scholar]
- Martin R C, Scoggins C R, McMasters K M. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17(1):171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- Brace C L, Laeseke P F, der Weide D W van, Lee F T. Microwave ablation with a triaxial antenna: results in ex vivo bovine liver. IEEE Trans Microw Theory Tech. 2005;53(1):215–220. doi: 10.1109/TMTT.2004.839308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace C L, Hinshaw J L, Laeseke P F, Sampson L A, Lee F T., Jr Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705–711. doi: 10.1148/radiol.2513081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A S, Lee F T, Jr, Mahvi D M. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10(3):275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- Brace C L, Laeseke P F, Sampson L A, Frey T M, der Weide D W van, Lee F T., Jr Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244(1):151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- Callstrom M R, Kurup A N. Percutaneous ablation for bone and soft tissue metastases—why cryoablation? Skeletal Radiol. 2009;38(9):835–839. doi: 10.1007/s00256-009-0736-4. [DOI] [PubMed] [Google Scholar]
- Bassignani M J, Moore Y, Watson L, Theodorescu D. Pilot experience with real-time ultrasound guided percutaneous renal mass cryoablation. J Urol. 2004;171(4):1620–1623. doi: 10.1097/01.ju.0000116536.39480.09. [DOI] [PubMed] [Google Scholar]
- Mala T, Samset E, Aurdal L, Gladhaug I, Edwin B, Søreide O. Magnetic resonance imaging-estimated three-dimensional temperature distribution in liver cryolesions: a study of cryolesion characteristics assumed necessary for tumor ablation. Cryobiology. 2001;43(3):268–275. doi: 10.1006/cryo.2001.2351. [DOI] [PubMed] [Google Scholar]
- Kariappa S M, Morris D L. Cryotherapy - a mature ablation technique. HPB (Oxford) 2006;8(3):179–181. doi: 10.1080/13651820500465469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marberger M. Ablation of renal tumours with extracorporeal high-intensity focused ultrasound. BJU Int. 2007;99(5 Pt B, 5 Pt B):1273–1276. doi: 10.1111/j.1464-410X.2007.06817.x. [DOI] [PubMed] [Google Scholar]
- Kim Y S, Rhim H, Choi M J, Lim H K, Choi D. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol. 2008;9(4):291–302. doi: 10.3348/kjr.2008.9.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement G T. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42(10):1087–1093. doi: 10.1016/j.ultras.2004.04.003. [DOI] [PubMed] [Google Scholar]
- ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93(1-3):111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- de Senneville B D, Mougenot C, Moonen C TW. Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound. Magn Reson Med. 2007;57(2):319–330. doi: 10.1002/mrm.21124. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Maruyama T, Takegami K, et al. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol. 2005;15(7):1415–1420. doi: 10.1007/s00330-005-2663-7. [DOI] [PubMed] [Google Scholar]
- Hanajiri K, Maruyama T, Kaneko Y, et al. Microbubble-induced increase in ablation of liver tumors by high-intensity focused ultrasound. Hepatol Res. 2006;36(4):308–314. doi: 10.1016/j.hepres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Melodelima D, N'Djin W A, Parmentier H, Chesnais S, Rivoire M, Chapelon J Y. Thermal ablation by high-intensity-focused ultrasound using a toroid transducer increases the coagulated volume. Results of animal experiments. Ultrasound Med Biol. 2009;35(3):425–435. doi: 10.1016/j.ultrasmedbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Lee E W, Loh C T, Kee S T. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6(4):287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- Lee E W, Prieto V, Chen C, et al. A novel hepatic ablation technique creating complete cell death: irreversible electroporation. Radiology. 2010 doi: 10.1148/radiol.10090337. In press. [DOI] [PubMed] [Google Scholar]
- Al-Sakere B, André F, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One. 2007;2(11):e1135. doi: 10.1371/journal.pone.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edd J F, Horowitz L, Davalos R V, Mir L M, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53(7):1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- Bajaj A K, Kon P S, Oberg K C, Miles D AG. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg. 2004;114(6):1442–1449. doi: 10.1097/01.prs.0000138813.64478.a7. [DOI] [PubMed] [Google Scholar]
- Burak W E, Jr, Agnese D M, Povoski S P, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer. 2003;98(7):1369–1376. doi: 10.1002/cncr.11642. [DOI] [PubMed] [Google Scholar]
- Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer. 2001;92(8):2036–2044. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Jeffrey S S, Birdwell R L, Ikeda D M, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999;134(10):1064–1068. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- Khatri V P, McGahan J P, Ramsamooj R, et al. A phase II trial of image-guided radiofrequency ablation of small invasive breast carcinomas: use of saline-cooled tip electrode. Ann Surg Oncol. 2007;14(5):1644–1652. doi: 10.1245/s10434-006-9315-2. [DOI] [PubMed] [Google Scholar]
- Manenti G, Bolacchi F, Perretta T, et al. Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology. 2009;251(2):339–346. doi: 10.1148/radiol.2512080905. [DOI] [PubMed] [Google Scholar]
- Medina-Franco H, Soto-Germes S, Ulloa-Gómez J L, et al. Radiofrequency ablation of invasive breast carcinomas: a phase II trial. Ann Surg Oncol. 2008;15(6):1689–1695. doi: 10.1245/s10434-008-9875-4. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Earashi M, Fujii H, Yokoyama K, Harada K, Tsuneyama K. Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol. 2006;93(2):120–128. doi: 10.1002/jso.20398. [DOI] [PubMed] [Google Scholar]
- Hayashi A H, Silver S F, der Westhuizen N G van, et al. Treatment of invasive breast carcinoma with ultrasound-guided radiofrequency ablation. Am J Surg. 2003;185(5):429–435. doi: 10.1016/s0002-9610(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Fornage B D, Sneige N, Ross M I, et al. Small (<or = 2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology. 2004;231(1):215–224. doi: 10.1148/radiol.2311030651. [DOI] [PubMed] [Google Scholar]
- Imoto S, Wada N, Sakemura N, Hasebe T, Murata Y. Feasibility study on radiofrequency ablation followed by partial mastectomy for stage I breast cancer patients. Breast. 2009;18(2):130–134. doi: 10.1016/j.breast.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Gardner R A, Vargas H I, Block J B, et al. Focused microwave phased array thermotherapy for primary breast cancer. Ann Surg Oncol. 2002;9(4):326–332. doi: 10.1007/BF02573866. [DOI] [PubMed] [Google Scholar]
- Vargas H I, Dooley W C, Gardner R A, et al. Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol. 2004;11(2):139–146. doi: 10.1245/aso.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Littrup P J, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234(1):63–72. doi: 10.1148/radiol.2341030931. [DOI] [PubMed] [Google Scholar]
- Sabel M S, Kaufman C S, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11(5):542–549. doi: 10.1245/ASO.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Pfleiderer S O, Marx C, Camara O, Gajda M, Kaiser W A. Ultrasound-guided, percutaneous cryotherapy of small (<or = 15 mm) breast cancers. Invest Radiol. 2005;40(7):472–477. doi: 10.1097/01.rli.0000166935.56971.ff. [DOI] [PubMed] [Google Scholar]
- Morin J, Traoré A, Dionne G, et al. Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg. 2004;47(5):347–351. [PMC free article] [PubMed] [Google Scholar]
- Littrup P J, Jallad B, Chandiwala-Mody P, D'Agostini M, Adam B A, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20(10):1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness— initial experience. Radiology. 2003;227(3):849–855. doi: 10.1148/radiol.2281012163. [DOI] [PubMed] [Google Scholar]
- Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203(1):54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Khiat A, Gianfelice D, Amara M, Boulanger Y. Influence of post-treatment delay on the evaluation of the response to focused ultrasound surgery of breast cancer by dynamic contrast enhanced MRI. Br J Radiol. 2006;79(940):308–314. doi: 10.1259/bjr/23046051. [DOI] [PubMed] [Google Scholar]
- Zippel D B, Papa M Z. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer. 2005;12(1):32–38. doi: 10.2325/jbcs.12.32. [DOI] [PubMed] [Google Scholar]
- Wu F, Wang Z B, Cao Y D, et al. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J Surg Oncol. 2007;96(2):130–136. doi: 10.1002/jso.20769. [DOI] [PubMed] [Google Scholar]
- Neal R E, Davalos R V. The feasibility of irreversible electroporation for the treatment of breast cancer and other heterogeneous systems. Ann Biomed Eng. 2009;37(12):2615–2625. doi: 10.1007/s10439-009-9796-9. [DOI] [PubMed] [Google Scholar]
- Bahn D K, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60(2, Suppl 1):3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- Donnelly B J, Saliken J C, Ernst D S, et al. Prospective trial of cryosurgical ablation of the prostate: five-year results. Urology. 2002;60(4):645–649. doi: 10.1016/s0090-4295(02)01839-3. [DOI] [PubMed] [Google Scholar]
- Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol. 2008;26(5):500–505. doi: 10.1016/j.urolonc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Onik G. Focal therapy for prostate cancer—120 patients with up to 12-year follow-up. San Diego, CA: Paper presented at: Society of Interventional Radiology 34th Annual Scientific Meeting; March 7–12, 2009.
- Lambert E H, Bolte K, Masson P, Katz A E. Focal cryosurgery: encouraging health outcomes for unifocal prostate cancer. Urology. 2007;69(6):1117–1120. doi: 10.1016/j.urology.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Uchida T, Shoji S, Nakano M, et al. Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: eight-year experience. Int J Urol. 2009;16(11):881–886. doi: 10.1111/j.1442-2042.2009.02389.x. [DOI] [PubMed] [Google Scholar]
- Poissonnier L, Chapelon J-Y, Rouvière O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol. 2007;51(2):381–387. doi: 10.1016/j.eururo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Blana A, Walter B, Rogenhofer S, Wieland W F. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology. 2004;63(2):297–300. doi: 10.1016/j.urology.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Uchida T, Ohkusa H, Yamashita H, et al. Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer. Int J Urol. 2006;13(3):228–233. doi: 10.1111/j.1442-2042.2006.01272.x. [DOI] [PubMed] [Google Scholar]
- Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6(4):295–300. doi: 10.1177/153303460700600405. [DOI] [PubMed] [Google Scholar]
- Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85. doi: 10.1007/s00270-007-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkers N A, Hehenkamp W JK, Smit P, Ankum W M, Reekers J A, Birnie E. Economic evaluation of uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids: results from the randomized EMMY trial. J Vasc Interv Radiol. 2008;19(7):1007–1016, quiz 1017. doi: 10.1016/j.jvir.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119. doi: 10.1007/s00270-009-9707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuhara Y, Shimizu T, Kodama Y, et al. Magnetic resonance-guided percutaneous cryoablation of uterine fibroids: early clinical experiences. Cardiovasc Intervent Radiol. 2006;29(4):552–558. doi: 10.1007/s00270-004-6163-y. [DOI] [PubMed] [Google Scholar]
- Cowan B D. Myomectomy and MRI-directed cryotherapy. Semin Reprod Med. 2004;22(2):143–148. doi: 10.1055/s-2004-828620. [DOI] [PubMed] [Google Scholar]
- Sewell P E, Arriola R M, Robinette L, Cowan B D. Real-time I-MR-imaging—guided cryoablation of uterine fibroids. J Vasc Interv Radiol. 2001;12(7):891–893. doi: 10.1016/s1051-0443(07)61517-6. [DOI] [PubMed] [Google Scholar]
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–589. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- Crocetti L, Lencioni R, Debeni S, See T C, Pina C D, Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43(1):33–39. doi: 10.1097/RLI.0b013e31815597dc. [DOI] [PubMed] [Google Scholar]
- Krücker J, Xu S, Glossop N, et al. Electromagnetic tracking for thermal ablation and biopsy guidance: clinical evaluation of spatial accuracy. J Vasc Interv Radiol. 2007;18(9):1141–1150. doi: 10.1016/j.jvir.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovac F, Cheng P, Campos-Nanez E, et al. Radiofrequency ablation of lung tumors in swine assisted by a navigation device with preprocedural volumetric planning. J Vasc Interv Radiol. 2010;21(1):122–129. doi: 10.1016/j.jvir.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lu W, Low D A, Deasy J O, Hope A J, El Naqa I. 4D-CT motion estimation using deformable image registration and 5D respiratory motion modeling. Med Phys. 2008;35(10):4577–4590. doi: 10.1118/1.2977828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B J, Locklin J K, Viswanathan A, et al. Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future. J Vasc Interv Radiol. 2007;18(1 Pt 1):9–24. doi: 10.1016/j.jvir.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Maeno T, Ayada M, et al. Four-dimensional ultrasonography for therapeutic radiofrequency ablation for hepatocellular carcinoma. Hepatogastroenterology. 2006;53(70):521–525. [PubMed] [Google Scholar]
- Gallotti A, D'Onofrio M, Ruzzenente A, et al. Contrast-enhanced ultrasonography (CEUS) immediately after percutaneous ablation of hepatocellular carcinoma. Radiol Med. 2009;114(7):1094–1105. doi: 10.1007/s11547-009-0436-0. [DOI] [PubMed] [Google Scholar]
- Liu D, Ebbini E S. Real-time two-dimensional temperature imaging using ultrasound. Conf Proc IEEE Eng Med Biol Soc. 2009;(2009):1971–1974. doi: 10.1109/IEMBS.2009.5333444. [DOI] [PubMed] [Google Scholar]
- Souchon R, Rouvière O, Gelet A, et al. Visualization of HIFU lesions using elastography of the human prostate in vivo: preliminary results. Ultrasound Med Biol. 2003;29(7):1007–1015. doi: 10.1016/s0301-5629(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Rivens I, Shaw A, Civale J, Morris H. Treatment monitoring and thermometry for therapeutic focused ultrasound. Int J Hyperthermia. 2007;23(2):121–139. doi: 10.1080/02656730701207842. [DOI] [PubMed] [Google Scholar]