Abstract
Although nephron-sparing surgery remains the gold standard treatment for small renal tumors, minimally invasive image-guided percutaneous ablation is becoming a viable alternative to operative resection. Percutaneous radiofrequency ablation (RFA) and cryoablation show high technical success rates, a relatively low incidence of residual or recurrent tumor, and competitive rates of patient survival. In this review, an overview of the current status of image-guided percutaneous ablation of renal tumors is presented, with a focus on procedure indications and patient selection, technical aspects of ablation procedures, and treatment outcomes and patient follow-up.
Keywords: Percutaneous, ablation, radiofrequency, cryoablation, kidney, renal, tumor
The incidence of renal tumors is rising in the United States, accounting for more than 50,000 new cases annually.1 This is mainly because of an increase in the diagnosis due to widespread use of cross-sectional imaging.2 Renal cell carcinoma constitutes 4% of all adult malignancies,3 and ~70 to 80% of patients present with localized low-stage disease.4,5 Nephron-sparing surgery continues to remain the gold standard treatment for small renal tumors. Although operative resection has been shown to be effective for treatment of small renal tumors and for preservation of renal function, it does have morbidity and mortality risks.6,7,8 Early laparoscopic renal cryoablation results have shown success and offer a less invasive technique for destroying small renal tumors.9 Image-guided percutaneous ablation of small renal tumors is less invasive, incurs less damage to uninvolved noncancerous renal tissue,10 and is becoming a viable alternative to nephron-sparing surgery.10 The purpose of this article is to present an overview of image-guided percutaneous ablation of renal tumors.
GENERAL CONCEPTS
Radiofrequency ablation (RFA) and cryoablation are the two most commonly used ablation modalities.11,12 Each has unique technical features, and neither of the two has clearly been proven to be superior to the other (Table 1).13 RFA destroys tumors by coagulation necrosis from temperatures above 60°C, and cryoablation causes tumor necrosis by intracellular dehydration and disruption of cell membranes from temperatures below -20°C. Both modalities are minimally invasive, as the thermal energy is emitted by placing one or more needle-like applicators into tumors. The ablation is performed under an imaging guidance modality such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI). The procedures can be performed in an outpatient setting. The choice of percutaneous ablation modality is based on the availability of equipment, the interventional radiologist's experience with a particular modality, and limitations based on anatomy and patient characteristics. The most important factors determining outcome of ablation are tumor size and location of tumor in the kidney. The best results are achieved in tumors <4 cm in diameter.14,15 The least challenging tumor location is posterior exophytic (Fig. 1) and the most challenging location is central hilar. Central tumors carry a higher risk for bleeding, damage to hilar structures such as collecting system and vessels, and have a higher recurrence rate due to thermal heat-sink effect. Depending on the location of an anterior tumor, it may be approachable percutaneously. However, treatment of these tumors may require a transrenal or transhepatic approach. Tumors that abut central vessels have a higher risk of recurrence because of “thermal heat-sink” phenomenon. This phenomenon occurs when the thermal energy required to achieve cytotoxicity during ablation is dissipated by the blood flow in the vessels abutting the tumor. All tumors less than 3 cm can be treated in one session and the need for more than one ablation session increases with tumor size >3 cm.14 Ablation of a 5 to 10 mm margin around the tumor is required to achieve the best results.
Table 1.
Comparison of Main Technical Features of Radiofrequency Ablation and Cryoablation in Percutaneous Image-Guided Ablation of Renal Tumors
| Radiofrequency Ablation | Cryoablation |
|---|---|
| Typical ablation: 20–30 minutes | Typical ablation: 30–40 minutes |
| Less bleeding | More bleeding |
| More pain (greater need for general anesthesia) | Less pain (moderate sedation, outpatient) |
| Ablation zone not visible during ablation | Ablation zone visible during ablation |
| • More tumor recurrence | • Less tumor recurrence |
| • Higher need for repeat ablation | • Lower need for repeat ablation |
| • Higher risk of nontarget ablation | • Lower risk of nontarget ablation |
| • Postablation intravenous contrast needed | • Postablation intravenous contrast not needed |
| Larger ablation zone per applicator (requires less applicators) | Smaller ablation zone per applicator (requires more applicators) |
| Grounding pads (risk of skin burn) | Cumbersome equipment |
| CT monitoring during ablation not possible (artifacts) | CT monitoring during ablation possible |
| Interferes with pacemakers | No interference with pacemakers |
| More likely to damage the collecting system | Less likely to damage the collecting system41 |
| Less control over individual applicators | More control over individual applicators |
CT, computed tomography.
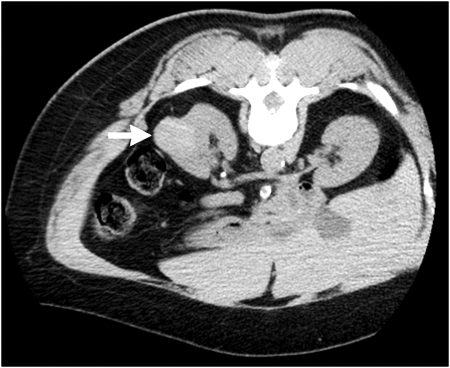
Figure 1.
A 65-year-old woman with left-sided renal mass. Axial noncontrast computed tomography (CT) image performed prior to percutaneous ablation reveals 3.6-cm-diameter high attenuation exophytic mass (arrow) arising from posterior lateral aspect of left kidney. CT-guided biopsy confirmed papillary renal cell carcinoma.
PROCEDURE INDICATIONS AND PATIENT SELECTION
Image-guided percutaneous ablations are especially ideal in patients who do not want to undergo surgery, elderly patients with significant medical comorbidities that preclude them from surgery, patients with renal insufficiency, solitary kidney, transplanted kidney, and multifocal tumors or patients with diseases such as von Hippel-Lindau, which predisposes them to develop multiple renal tumors. Documentation of renal cell carcinoma by needle biopsy is most often necessary, as up to 25% of renal masses <3 cm are benign. Ideally, biopsy should be performed in a session separate from ablation. Tumors should be isolated to kidney with no evidence of vascular invasion or metastases. The best results are anticipated from tumors <4 cm in diameter, although larger tumors have been successfully ablated percutaneously. The decision to perform percutaneous ablation is best made as a cooperative multidisciplinary agreement between an interventional radiologist and a urologist.
PREPROCEDURE EVALUATION AND PRECAUTIONS
Before the procedure, a physical exam should be performed, and the patient's medical history should be reviewed. Informed consent is required, and patients must understand that percutaneous ablations are relatively new procedures without proven long-term results. Allergies to contrast media, antibiotic medications, or anesthetic drugs should also be noted. Laboratory blood tests should include hematocrit, platelet count, prothrombin time, and international normalized ratio (INR), partial thromboplastin time (PTT), and creatinine with calculation of an estimated glomerular filtration rate (eGFR). A platelet count of more than 100,000 per mL, INR of less than 1.5, and normal PTT would ideally be met prior to the procedure.
Patients should not be acutely coagulopathic. Warfarin, aspirin, and clopidogrel should ideally be stopped at least 7 days before the procedure. The interventional radiologist should consult the referring physician prior to withholding anticoagulation medication. In patients with strict warfarin requirements, special arrangements can be made so ablation is performed using a “heparin window,” in which warfarin is held and patients are systemically anticoagulated with heparin until the time of procedure.
Patients are instructed to abstain from eating for 6 hours prior to ablation so they are able to receive intravenous moderate sedation or anesthesia. They may take other routine oral medications with small sips of water. Modification of the insulin regimen in diabetics should be considered during the food restriction period prior to the procedure. Nephrology consultation may be needed prior to ablation in patients with chronic renal insufficiency (estimated GFR <60 mL/min).
IMAGING GUIDANCE
Percutaneous image-guided ablation can be performed under ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) guidance. The tumor should be well visualized on the imaging modality planned for the procedure. The advantages of ultrasound are its “real-time” capability and lack of ionizing radiation. Ultrasound can be used for accurate placement of applicators before switching to CT or MRI for further monitoring. However, imaging with ultrasound is highly operator dependent and may be compromised in certain settings, such as patients with a large body habitus, the presence of abundant bowel gas, and when the tumor is near a focal loop of bowel that needs to be avoided. Another setback to ultrasound is the degradation of landmarks during ablation. In cryoablation, image degradation is caused by acoustic shadowing on the far side of the ice ball, and in RFA it is produced by microbubbles.
CT is not as operator dependent as ultrasound and is very accessible. Its wide field of view is excellent to cover the critical organs and structures that need to be avoided. CT scanning is much less sensitive to body habitus than is ultrasound and CT images are not affected by bowel gas. If CT is the chosen image guidance method, the target lesion should ideally be visible on a noncontrast examination. Percutaneous ablation can be performed using a conventional CT scanner or a CT scanner with real-time fluoroscopic capability.
MRI is the least commonly used imaging modality for percutaneous ablation. It provides excellent soft tissue resolution with multiplanar imaging capability and lacks ionizing radiation. MR fluoroscopic sequences can be used for real-time guidance. MR thermography can assess cytotoxic tissue temperatures noninvasively. Image-guided percutaneous ablation can be performed using a dedicated interventional magnet, a conventional solenoid magnet, or an open magnet. The ice ball is visualized as a zone of decreased signal intensity on T1- and T2-weighted images. MRI-compatible ablation equipment is available to perform both RFA and cryoablation under MRI guidance.16,17
ABLATION PROCEDURES
The patient should be placed in the most comfortable position (prone, supine, lateral decubitus, or oblique) that also facilitates the procedure. Intravenous midazolam and fentanyl are the most commonly used medications for moderate sedation. The drug dosage is titrated for patient comfort and is monitored with telemetry and pulse oximetry by a sedation nurse or anesthesia team. At my institution, all percutaneous ablations are performed under general anesthesia with endotracheal intubation. If desired, a biopsy of the lesion can be performed in the same session prior to the ablation. A challenge with same day biopsy is that bleeding associated with the biopsy may obscure the tumor.
The procedure plan should be outlined by the interventional radiologist before the procedure. The number of applicators used for a particular tumor depends on the equipment used and the size of tumor (Fig. 2). Placement of more than one applicator is often required to cover the entire tumor and the desired rim of surrounding parenchyma. The length of devices extending outside of the patient and their connecting cords, as well as patient positioning and the angle of applicator placement, all have major practical implications and should all be planned beforehand. Frequently, the injection of intravenous contrast medium during the procedure can help visualize relatively inconspicuous lesions. Smaller doses of iodinated contrast (~50 mL) are usually sufficient for CT visualization and can allow for a repeat bolus later in the procedure if necessary. Similarly, there are circumstances when sonographic or MRI contrast agents may be useful.
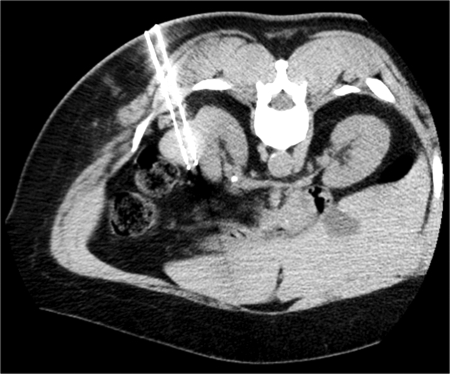
Figure 2.
Percutaneous cryoablation of mass shown in Fig. 1. Intraprocedural computed tomography (CT) image shows appropriate position of two applicators within tumor, spaced appropriately to gain complete tumor coverage during ablation.
Adjacent critical structures may be damaged when they are located within the target region. Occasionally, a second percutaneous needle can be inserted and carbon dioxide, water, air, or balloons may be placed to separate bowel, for instance, from the target tumor.15,18,19 It is important not to use saline for hydrodissection in RFA cases, as saline conducts current and may damage organs. Nonionic 5% dextrose in water should be used instead. Certain patient positioning maneuvers or the interventional radiologist's hands may also be used to move away bowel loops from the tumor. Retrograde ureteral stents can be used to circulate warm or cold fluid during the ablation period to provide some protection to the ureter.15,20 The applicator can also be used as a lever to move the kidney away from adjacent structures.21 If a transpleural approach is inevitable, it can be pursued and any pneumothorax that develops may be treated accordingly. Alternatively, an iatrogenic pneumothorax or hydrothorax can be created to avoid potential damage to the lung.
Unless a tined RFA applicator is used, most applicators have no tines and produce an oval zone of ablation. After adequate positioning of the applicator(s) is complete, the tumor is ablated once or more according to the suggested manufacturer protocol. If the patient's renal function allows, a contrast-enhanced CT or MRI scan may be performed after removing all applicators. This will allow for an immediate assessment of the adequacy of the ablation. MRI-guided ablations can also be immediately assessed by MR thermography. Certain areas with suboptimal ablation can then be immediately re-treated during the same session. At a minimum, it is usually helpful to do a noncontrast scan of the treatment area following applicator removal to assess for complications.
An advantage of cryoablation over heat-based ablation modalities is its relative painlessness.22 This may be of importance for patients who cannot undergo general anesthesia or receive deep moderate sedation due to medical comorbidities.
In contrast to heat-based ablation modalities, the ice ball can be clearly seen with CT23,24 (Fig. 3), MRI,25 and ultrasound imaging.26 This imaging characteristic allows the operator to ascertain coverage of the tumor more confidently and also to better protect certain nearby critical structures. Because of this feature, cryoablated lesions tend to have less residual tumor.27 Intravenous contrast is usually not needed immediately following cryoablation to verify proper coverage of tumor by the ablation zone. This is a significant advantage for patients with renal insufficiency. The outer edge of the ice ball that is visualized is not cytotoxic.
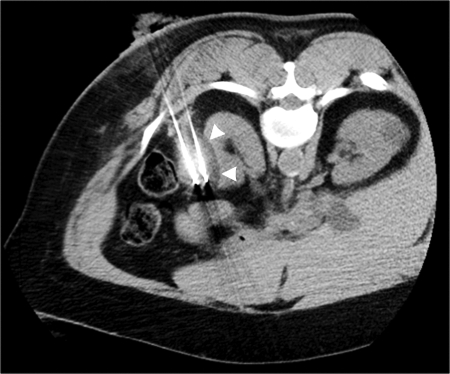
Figure 3.
During cryoablation of mass shown in Figs. 1 and 2, reduced attenuation ice ball (arrowheads) readily seen on noncontrast computed tomography (CT) after three 10-minute freeze cycles with interposed 5-minute thaw periods. At the discretion of the operator, no protective measures were taken to separate colon abutting lateral aspect of mass in this particular case.
Unlike the heat-based ablation modalities, freezing does not cauterize or coagulate vessels within the ablation zone. Hence, patients may be at slightly higher risk of bleeding during or immediately following cryoablation (Fig. 4). For this reason, patients with borderline coagulation status should have laboratory abnormalities more vigorously corrected. One relative limitation of current cryoablation applicators is the size of the ablation zone created by a single applicator as compared with the same size applicator from any of the heat-based ablation modalities. In general, each 2 mm applicator creates an ~2 cm diameter of necrosis.28 For this reason, insertion of multiple simultaneous applicators is commonly needed to cover the entire tumor and desired margin of surrounding parenchyma. A simple rule-of-thumb is to position the applicators 1 cm from the margin of the tumor and 1 to 2 cm apart from each other, giving priority to the periphery of the tumor.23 It is important to remember that all the available cryoablation applicators currently have an extension cord attached to them. This cord contains tubing for gases, and in certain versions, temperature sensor wiring as well. The weight of the cord produces torque during and after placement of the applicator. When planning for cryoablation, it is important to make sure the trajectory of each applicator has enough purchase inside the patient so they are not constantly pulled or deviated by the torque produced by the cord. Depending on the type of the applicator used, a significant portion of the applicator and cord may extend outside of the patient. This may cause significant technical problems when performing cryoablation under CT or MRI guidance. When positioning the patient for the procedure, it is critical to remember to provide enough space between the patient and gantry so that the equipment is not contaminated during the procedure. Additionally, the interventional radiologist will have far more freedom to make fine adjustments during the procedure without having to take the patient out of the gantry repeatedly.
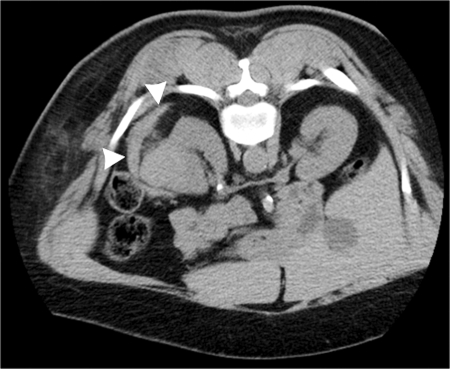
Figure 4.
Final postablation computed tomography (CT) image following cryoablation of mass shown in Figs. 1 to 3 demonstrates small perinephric hematoma (arrowheads), which caused no clinical sequela.
TREATMENT OUTCOMES
Technical success rates of 97 to 100% are reported with RFA and cryoablation.12,14,15 In a series of 616 patients, the incidence of residual or recurrent tumor was 13.4% with a single radiofrequency ablation and 3.9% with a single cryoablation.11 In this series, the overall incidence of residual or recurrent tumor after repeated ablation therapy in all patients was 4.2%.11 The overall 2-year survival rate was 82.5%, including patients who died of unrelated causes, and the metastasis-free survival rate was 97% at 2 years.11 Five-year local and distant tumor-free rate after RFA has been reported at 94%.29 Similar success rates are reported with cryoablation, but the mean follow-up time period is shorter at 1 to 2 years.23
OTHER ABLATION MODALITIES
Microwave is a heat-based modality which involves deploying microwave energy (300–3000 MHz) from a nontined applicator (antenna) into a tumor causing oscillation of ions, which creates heat resulting in coagulative necrosis. Advantages include faster ablation times and less susceptibility to thermal heat-sink effect.30
Laser is another heat-based ablation modality that deposits laser energy into the tumor via tiny fibers. Laser energy raises the tissue temperature and causes coagulative necrosis. Laser ablation has been successfully performed in few renal tumors,31 and can be done under CT or MRI guidance. Flexibility of the applicator outside of the patient is an advantage when working in a CT or MRI gantry. Relative small ablation zones mandate multiple applicator placement.
Irreversible electroporation (IRE) is a novel nonthermal ablation modality that causes apoptotic cell death by creating microscopic holes in cell membranes when cells are exposed to specific electrical fields. This modality is much faster than other minimally invasive ablation techniques. Another advantage of IRE is that it is not affected by thermal sink phenomenon.32 Preliminary animal and human experience is promising. The need for general anesthesia with paralytic agents is an issue, however.
High-intensity focused ultrasound (HIFU), is a noninvasive heat-based ablation modality. It causes coagulation necrosis by focusing a high-intensity ultrasonic beam onto a small volume of target lesion. Respiratory movements and overlying ribs are major problems with the use of HIFU in renal tumors.33,34
COMPLICATIONS
The rate of major complications from percutaneous ablation of renal tumors is ~2 to 6%.12,14,35 Complications include hemorrhage, pneumothorax, and bowel and nerve injury. In contrast to other heat-based ablation modalities, cryoablation does not cauterize blood vessels within the ablation zone. Therefore, hemorrhage at the ablation zone after removal of applicators appears to be slightly more common when compared with heat-based ablation modalities. Hemorrhage after cryoablation is most often self-limited and is managed with conservative management. Damage to the renal collecting system and the neighboring structures such as nerves and bowel are among other reported complications.
PATIENT FOLLOW-UP
If the patient's renal function allows, the ablated tumor should be evaluated by CT or MRI without and with intravenous contrast at 1, 3, 6, 12, 18, and 24 months after ablation. Early postablation studies show the ablation zone as a nonenhancing area surrounded by a thin smooth rim enhancement.36 This rim is considered a physiologic response to thermal ablation and disappears within 3 months.37 Ideally, the ablation zone should include the entire tumor and the expected 5 to 10 mm noncancerous rim. The ablation zone appears T1 hyperintense and T2 hypointense.38,39 The ablation zone continues to decrease in size over time.37 Complete disappearance of cryoablated tumors over time has been reported.40 A thin curvilinear hyperattenuating rim or halo on CT imaging, which is hypointense on T1-weighted images, is commonly seen parallel to the tumor extending to the perinephric fat. This halo may persist for several months after treatment.38,39 Nodular or irregular enhancement within the ablation zone and enlargement of ablation zone is considered suspicious for residual tumor or recurrence.39 These areas may be biopsied and retreated by another session of ablation.
CONCLUSION
Nephron-sparing surgery continues to remain the gold standard treatment for small renal tumors. Image-guided percutaneous ablation is a viable option for patients who cannot undergo surgery because of medical comorbidities. Early clinical experience with these ablation modalities is promising.
References
- Chow W H, Devesa S S, Warren J L, Fraumeni J F., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- Pantuck A J, Zisman A, Belldegrun A S. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- American Cancer Society Cancer Statistics 2008. Atlanta, GA: American Cancer Society; 2008.
- Luciani L G, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997) Urology. 2000;56:58–62. doi: 10.1016/s0090-4295(00)00534-3. [DOI] [PubMed] [Google Scholar]
- Janzen N K, Kim H L, Figlin R A, et al. Surveillance after radical or nephron sparing surgery for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- Fergany A F, Hafez K S, Novick A C. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year follow up. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- Gill I S, Kavoussi L R, Lane B R, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- Breda A, Finelli A, Janetschek G, Porpiglia F, Montorsi F. Complications of laparoscopic surgery for renal masses: prevention, management, and comparison with the open experience. Eur Urol. 2009;55:836–850. doi: 10.1016/j.eururo.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Spaliviero M, Moinzadeh A, Gill I S. Laparoscopic cryotherapy for renal tumors. Technol Cancer Res Treat. 2004;3:177–180. doi: 10.1177/153303460400300210. [DOI] [PubMed] [Google Scholar]
- Hui G C, Tuncali K, Tatli S, Morrison P R, Silverman S G. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19:1311–1320. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Gervais D A, McGovern F J, Wood B J, et al. Radiofrequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- Atwell T D, Farrell M A, Leibovich B C, et al. Percutaneous renal cryoablation: experience treating 115 tumors. J Urol. 2008;179:2136–2141. doi: 10.1016/j.juro.2008.01.144. [DOI] [PubMed] [Google Scholar]
- Maybody M, Solomon S B. Image-guided percutaneous cryoablation of renal tumors. Tech Vasc Interv Radiol. 2007;10:140–148. doi: 10.1053/j.tvir.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Boss A, Clasen S, Kuczyk M, et al. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol. 2005;40:583–590. doi: 10.1097/01.rli.0000174473.32130.28. [DOI] [PubMed] [Google Scholar]
- Miki K, Shimomura T, Yamada H, et al. Percutaneous cryoablation of renal cell carcinoma guided by horizontal open magnetic resonance imaging. Int J Urol. 2006;13:880–884. doi: 10.1111/j.1442-2042.2006.01432.x. [DOI] [PubMed] [Google Scholar]
- Farrell M A, Charboneau J W, Callstrom M R, et al. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181:1315–1317. doi: 10.2214/ajr.181.5.1811315. [DOI] [PubMed] [Google Scholar]
- Kam A W, Littrup P J, Walther M M, et al. Thermal protection during percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol. 2004;15:753–758. doi: 10.1097/01.rvi.0000133535.16753.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais D A, Arellano R S, McGovern F J, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma: part 2, lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:72–80. doi: 10.2214/ajr.185.1.01850072. [DOI] [PubMed] [Google Scholar]
- Cantwell C P, Wah T M, Gervais D A, et al. Protecting the ureter during radiofrequency ablation of renal cell cancer: a pilot study of retrograde pyeloperfusion with cooled dextrose 5% in water. J Vasc Interv Radiol. 2008;19:1034–1040. doi: 10.1016/j.jvir.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Park B K, Kim C K. Using an electrode as a lever to increase the distance between renal cell carcinoma and bowel during CT-guided radiofrequency ablation. Eur Radiol. 2008;18:743–746. doi: 10.1007/s00330-007-0816-6. [DOI] [PubMed] [Google Scholar]
- Allaf M E, Varkarakis I M, Bhayani S B, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation – initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- Littrup P J, Ahmed A, Aoun H D, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–392. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Solomon S B, Chan D Y, Jarrett T W. Percutaneous cryotherapy of kidney tumors. Am J Urol Rev. 2004;2:369–371. [Google Scholar]
- Silverman S G, Tuncali K, Sonnenberg E van, et al. Renal tumors MR imaging-guided percutaneous cryotherapy – initial experience in 23 patients. Radiology. 2005;236:716–724. doi: 10.1148/radiol.2362041107. [DOI] [PubMed] [Google Scholar]
- Gill I S, Novick A C, Meraney A M, et al. Laparoscopic renal cryoablation in 32 patients. Urology. 2000;56:748–753. doi: 10.1016/s0090-4295(00)00752-4. [DOI] [PubMed] [Google Scholar]
- Matin S F, Ahrar K, Cadeddu J A, et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol. 2006;176:1973–1977. doi: 10.1016/j.juro.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Permpongkosol S, Nicol T L, Link R E, et al. Differences in ablation size in porcine kidney, liver, and lung after cryoablation using the same ablation protocol. AJR Am J Roentgenol. 2007;188:1028–1032. doi: 10.2214/AJR.06.0810. [DOI] [PubMed] [Google Scholar]
- Gervais D A, McGovern F J, Arellano R S, McDougal W S, Mueller P R. Radiofrequency ablation of renal cell carcinoma. Part 1. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- McDougal W S, Gervais D A, McGovern F J, Mueller P R. Long-term follow-up of patients with renal cell carcinoma treated with radio frequency ablation with curative intent. J Urol. 2005;174:61–63. doi: 10.1097/01.ju.0000162046.45024.2b. [DOI] [PubMed] [Google Scholar]
- Simon C J, Dupuy D E, Mayo-Smith W W. Microwave ablation: principles and applications. Radiographics. 2005;25:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- Dick E A, Joarder R, De Jode M G, Wragg P, Vale J A, Gedroyc W M. Magnetic resonance imaging-guided laser thermal ablation of renal tumours. BJU Int. 2002;90(9):814–822. doi: 10.1046/j.1464-410x.2002.03026.x. [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality: clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- Marberger M, Schatzl G, Kranston D, Kennedy J E. Extracorporeal ablation of renal tumors with high intensity focused ultrasound. BJU Int. 2005;95:52–55. doi: 10.1111/j.1464-410X.2005.05200.x. [DOI] [PubMed] [Google Scholar]
- Klatte T, Marberger M. High-intensity focused ultrasound for the treatment of renal masses: current status and future potential. Curr Opin Urol. 2009;19:188–191. doi: 10.1097/MOU.0b013e328323f641. [DOI] [PubMed] [Google Scholar]
- Johnson D B, Solomon S B, Su L M, et al. Defining the complications of cryoablation and radiofrequency ablation of small renal tumors: a multi-institutional review. J Urol. 2004;172:874–877. doi: 10.1097/01.ju.0000135833.67906.ec. [DOI] [PubMed] [Google Scholar]
- Smith S, Gillams A. Imaging appearances following thermal ablation. Clin Radiol. 2008;63:1–11. doi: 10.1016/j.crad.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Merkle E M, Nour S G, Lewin J S. MR imaging follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: findings in 18 patients during first 6 months. Radiology. 2005;235:1065–1071. doi: 10.1148/radiol.2353040871. [DOI] [PubMed] [Google Scholar]
- Uppot R N, Silverman S G, Zagoria R J, Tuncali K, Childs D D, Gervais D A. Imaging-guided percutaneous ablation of renal cell carcinoma: a primer of how we do it. AJR Am J Roentgenol. 2009;192:1558–1570. doi: 10.2214/AJR.09.2582. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Solomon S B, Bluemke D A, Fishman E K. Computed tomography and magnetic resonance imaging appearance of renal neoplasms after radiofrequency ablation and cryoablation. Semin Ultrasound CT MR. 2009;30:67–77. doi: 10.1053/j.sult.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill I S, Remer E M, Hasan W A, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173:1903–1907. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- Janzen N K, Perry K T, Han K R, et al. The effects of intentional cryoablation and radiofrequency ablation of renal tissue involving the collecting system in a porcine model. J Urol. 2005;173:1368–1374. doi: 10.1097/01.ju.0000147014.69777.06. [DOI] [PubMed] [Google Scholar]