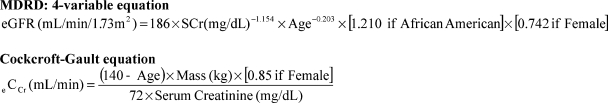Abstract
Contrast-induced nephropathy (CIN) is a widely recognized and clinically significant problem in patients undergoing an increasing number of minimally invasive procedures that require contrast administration. Contrast-induced nephropathy is the third most common cause of hospital-acquired renal failure and has significant prognostic implications on patient outcomes. Interventional practitioners are faced with challenging decisions regarding prophylaxis and patient management. The major risk factor for developing CIN is preexisting renal dysfunction, particularly in association with diabetes. Patients are considered to be at risk when estimated glomerular filtration rate (eGFR) or estimated creatinine clearance (eCCr) is less than 60. The cornerstone of prevention of CIN is appropriate risk stratification, intravenous hydration with normal saline or sodium bicarbonate, appropriate withholding of nephrotoxic medications, use of low or iso-osmolar contrast media, and various intraprocedural methods for iodinated contrast dose reduction. Although N-acetylcysteine administration is popular, it remains unproven. Practitioners must be familiar with prevention strategies and diagnosis of CIN to minimize its clinical impact.
Keywords: Contrast-induced nephropathy, CIN, renal failure, contrast media, prevention
With escalating use of radiographic contrast media in clinical practice and increasing incidence of chronic kidney disease in an aging population, contrast-induced nephropathy (CIN) is widely recognized as a significant source of morbidity and mortality. Contrast-induced nephropathy is the third most common cause of hospital-acquired renal failure and has significant prognostic implications on patient outcomes.1,2,3,4 Independent of renal failure, the development of even mild CIN is associated with increased rates of morbidity and mortality. Whether or not this association is causal is unclear, and the development of CIN may simply reflect a marker for poor overall outcome.5
It is difficult for practitioners performing interventional procedures to make informed decisions regarding risk stratification and prevention strategies for CIN because the data are unclear and often contradictory. What follows is meant to be a practical guide for prevention of CIN in the scope of interventional radiology practice based on current evidence and consensus recommendations.
DEFINING CONTRAST-INDUCED NEPHROPATHY
Contrast-induced nephropathy is broadly defined as acute kidney dysfunction occurring after exposure to intravascular contrast media that is not attributable to other causes. There is, however, no specific standard definition that is broadly agreed upon or utilized in the literature. This heterogeneity of definitions substantially limits the evaluation of data across the literature.
Ideally, direct measurement of glomerular filtration rate (GFR) before and after contrast administration would provide the most accurate measurement of renal function. Unfortunately, measurement of GFR by inulin clearance or other accurate measures is neither practical nor well tolerated in the clinical setting.
The most commonly used measurement of renal function is serum creatinine, an end product of muscle metabolism. Estimation of renal function based on serum creatinine is fraught with error. Creatinine levels are influenced by many factors including age, gender, body composition, and nutritional status. Additionally, normal serum creatinine levels are typically maintained until GFR is reduced by ∼50%.6 Therefore, a significant reduction in renal function may be missed by solely monitoring serum creatinine. Creatinine levels are also influenced by prevention strategies such as intravenous (IV) hydration and N-acetylcysteine (NAC) administration. Although limited as a marker, serum creatinine is easy to measure in the clinical setting, cost-effective, and most of the published literature regarding CIN is based on serum creatinine measurements.
A variety of definitions of CIN are present in the literature. They most commonly consist of either a relative (25–100%) or absolute (0.3–1.0 mg/dL) increase in serum creatinine above baseline levels. It has been suggested that a change of 0.3 mg/dL is not statistically significant in many laboratories.7 The most commonly used definition of CIN, therefore, is either a relative increase in serum creatinine from baseline value of 25% or an absolute increase of 0.5 mg/dL within 48 to 72 hours after contrast exposure. Additionally, the creatinine elevation must not be attributable to other causes and must persist for 2 to 5 days.7,8
Documented oliguria (urine output <0.5 mL/kg/h for >6 h) is another definition for CIN, although it is not clinically sensitive as patients often maintain urine output in the face of significant renal insult if they are adequately hydrated.9 If oliguria develops, it is also typically associated with a significant increase in serum creatinine, which will fall within the above definitions of CIN.
CONTROVERSY AND LIMITATIONS INHERENT IN THE LITERATURE
Given the inaccuracy of defining CIN based on serum creatinine, random variations in daily creatinine measurement, lack of an adequate control group or long term (>72 h) follow-up in most published studies, and heterogeneous definitions of CIN throughout the literature, the actual incidence and clinical significance of CIN has been called into question.5,10 Newhouse et al examined the random fluctuations of serum creatinine in 32,161 hospitalized patients who had 5 days of consecutive serum creatinine measurements without any intravascular administration of contrast in the preceding 10 days.11 Depending on the definition, 6 to 35% of those patients would have fit criteria for being diagnosed with CIN. These rates are not substantially different from the rates of CIN in the published literature. Indeed, recent publications have highlighted two earlier studies by Cramer et al and Heller et al that included matched controls who did not receive intravascular contrast.11,12,13,14 These studies demonstrated no substantial differences in CIN (defined as serum creatinine increase of >50%) between the contrast and noncontrast control groups. Acknowledging the limitations of these studies, these observations suggest that the incidence of CIN in the published literature has likely been overestimated. Despite these controversies, however, the vast majority of published literature, consensus panels, and published guidelines all agree that CIN is a clinically significant problem that does have a substantial impact on patient outcome.
PATHOPHYSIOLOGY OF CIN7,15
Although there are many complex pathways involved in the development of CIN, the end result is thought to be ischemic injury to the renal medulla. Under normal conditions, the renal medulla is poorly oxygenated and operates in a near hypoxic environment. After administration of contrast, renal blood flood temporarily increases, then decreases over a prolonged period. These changes are mediated by a complex interplay of many factors. Renal vasoconstriction plays a major role and is mediated by vasoactive substances such as endothelin, adenosine, nitric oxide, and prostaglandins. Direct cytotoxic and osmotic effects of contrast on renal tubules also play a role and may be partly mediated by free radical formation. Increased intratubular pressure, increased urine viscosity, and tubular obstruction further contribute to renal injury.
NATURAL HISTORY OF CIN
Most CIN is self-limited. Serum creatinine typically increases over 1 to 3 days, peaks at 4 to 5 days, and returns to baseline in 7 to 14 days.15,16 More severe CIN, however, may be associated with a delayed peak in serum creatinine and a slower return to steady state, which may remain above baseline values. These cases may be associated with oliguria. In a small subset of patients, temporary or permanent dialysis is required.
The median 2-year survival rate in patients who require dialysis is 19% and in-hospital mortality is as high as 36%.17 Even with the development of mild CIN that does not require intervention, there is increased morbidity and mortality independent of other risk factors. Whether this effect is causal is unclear, and the development of CIN may simply reflect a poor overall prognostic marker for outcome.5
TREATMENT7
Treatment of CIN begins with identification of the injury and is similar for other causes of acute renal failure. Patients should be monitored by a nephrologist in an inpatient setting. Management typically involves careful monitoring of electrolytes, adjusting nutrition, and strict monitoring of body weight and fluid balance. Metabolic disturbances such as hyperphosphatemia can be managed with phosphate binders (calcium carbonate); hyperkalemia with dietary restriction of potassium, potassium binding resins, or insulin and dextrose infusion; and metabolic acidosis may require oral sodium bicarbonate. In rare cases, patients may require temporary or permanent dialysis.
DEFINING AT-RISK POPULATIONS
Contrast-induced nephropathy occurs most commonly in patients with identifiable risk factors. Many risk factors have been reported in the literature (Table 1), although few have been proven to be independent factors.6,7,15,18,19,20 Given that the risk of CIN seems to rise exponentially with the number of risk factors present, it is prudent to evaluate all potential risks prior to contrast administration.
Table 1.
Reported Risks for Contrast-Induced Nephropathy
| Nonmodifiable risks |
| Primary |
| Preexisting renal disease |
| Diabetes associated with renal disease |
| Acute kidney injury (esp. acute tubular necrosis) |
| Hypotension/sepsis |
| Secondary |
| Cardiovascular disease (esp. congestive heart failure) |
| Advanced age (>70 years) |
| Cirrhosis |
| Nephrotic syndrome |
| Myeloma |
| Organ transplantation |
| Human immunodeficiency virus |
| Metabolic disorders |
| Hyperuricemia, hypercholesterolemia, hypercalcemia |
| Modifiable risks |
| Dehydration |
| Recent contrast administration (<72 hours) |
| Contrast volume |
| Contrast type (HOCM >LOCM/IOCM) |
| Contrast administration route (IA >IV) |
| Nephrotoxic medications |
| Nonsteroidal antiinflammatory drugs |
| Aminoglycosides, vancomycin, amphotericin B |
| Loop diuretics |
| Immunosuppressives: cyclosporine A |
Review of the patient history will identify most risk factors and should be the first step in patient evaluation. Scoring systems have been developed for risk stratification and are more often utilized in the interventional cardiology literature where patients are critically ill and need emergent percutaneous coronary intervention (PCI). For patients undergoing interventional radiologic or diagnostic computed tomographic (CT) procedures, risk stratification is typically performed based on estimation of baseline renal function, which is the single most important predictor for the development of CIN.
Preexisting renal insufficiency
The most important and predictive risk factor for developing CIN is preexisting renal insufficiency.6,7,15,18,19,20 Classic teaching is that the more severe the preexisting renal disease, the higher the subsequent risk of CIN, and the more likely that CIN will be clinically significant. Risk stratification is predominantly performed based on baseline renal function and will be discussed in further detail later in the text.
Diabetes
Prospective studies have demonstrated that diabetes without coexisting renal insufficiency is not a true independent risk factor for CIN.21 Diabetes with associated chronic renal insufficiency (CRI), however, does confer a higher risk of CIN than in a nondiabetic patient with equivalent renal function.15 Diabetic patients with CRI are also more likely to experience oliguric renal failure and require dialysis compared with nondiabetics with equivalent renal function.21
Other Risk Factors6,7,15,18,19,20
Patients with acute kidney injury, particularly acute tubular necrosis, are at particularly high risk for contrast administration. Sepsis, hypotension, cardiovascular disease (congestive heart failure in particular), and cirrhosis result in renal hypoperfusion and increased risk of contrast-induced kidney damage. Sepsis may also induce renal injury as a result of bacterial toxin damage to renal tubules. Advanced age is a risk factor due to reduction in renal mass, function, and perfusion, although exact age thresholds are undetermined. Myeloma was previously considered a risk factor for CIN, as it is associated with renal disease, although with adequate hydration rates of CIN are very low.
Nephrotoxic medications
Chronic use of nonsteroidal antiinflammatory drugs (NSAIDS), aminoglycosides, vancomycin, amphotericin B, immunosuppressive medications such as cyclosporine, and loop diuretics likely also increase the risk of CIN. These agents, however, have not been proven as independent risks for CIN.7 When clinically feasible, nephrotoxic medications should be discontinued for 24 to 48 hours before the procedure and held for 24 to 48 hours after the procedure to minimize additive nephrotoxic effects.
Contrast
The type, volume, and route of administration of iodinated contrast all play important roles in the risk of CIN. Higher doses, intraarterial (IA) compared with IV administration, and high-osmolar contrast media (HOCM) all increase the risk for development of CIN in at-risk populations. Additionally, the administration of multiple doses of contrast within 72 hours is a risk.7
Metformin6,19
Metformin does not predispose to CIN. If renal function decreases while a patient is on metformin, however, there is a risk of lactic acidosis, which may be fatal in a substantial number of cases. For this reason, it is recommended that metformin be held for 48 hours after the administration of contrast media until it has been established that renal function has not been significantly altered.
RISK STRATIFICATION
Although not without controversy, current trends suggest that risk stratification for preexisting renal disease should be performed with either estimated GFR (eGFR) or estimated creatinine clearance (eCCr).9,19 Both values can be calculated from the serum creatinine (SCr) based on equations validated in adult populations. Because creatinine clearance estimates the GFR, both measurements can be used interchangeably for the purpose of risk stratification. These equations account for differences in muscle mass in adult populations based on several variables (age, gender, race, weight) and are thought to result in more sensitive and specific measurements of renal function than serum creatinine alone. It is important to remember that these formulas were developed for assessment of patients with chronic renal disease, not acute renal dysfunction.
Estimated GFR is most commonly calculated with the four-variable Modification of Diet in Renal Disease (MDRD) formula and eCCr by using the Cockcroft-Gault formula (Fig. 1). Numerous online calculators are available. Both formulas have limitations in those with extremes of body mass (i.e., muscle wasting or extreme obesity). The serum creatinine used for risk stratification should be a baseline value, prior to hydration or administration of NAC, which may falsely decrease serum creatinine levels.
Figure 1.
Estimating estimated glomerular filtration rate (eGFR) or estimated creatinine clearance (eCCr) from serum creatinine (SCr) (mg/dL).
When utilizing eGFR for risk stratification, there is general agreement that patients with eGFR >60 are at low risk for CIN, and those with eGFR <30 are at very high risk for CIN, regardless of the route of contrast administration (IV or IA). Patients with eGFR <30 have a 30 to 40% risk of CIN and a 2 to 8% risk of dialysis compared with a general population risk of 2% for CIN.9,22 These risk categories correspond to the Kidney Disease Outcome Quality Initiative (KDOQI) stages of chronic kidney disease (Table 2).
Table 2.
Kidney Disease Outcome Quality Initiative Stages of Chronic Kidney Disease
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal/increased GFR | >90 |
| 2 | Kidney damage with mild decrease in GFR | 60–89 |
| 3 | Kidney damage with moderate decrease in GFR | 30–59 |
| 4 | Kidney damage with severe decrease in GFR | 15–29 |
| 5 | Kidney failure | <15 |
GFR, glomerular filtration rate.
There is greater uncertainty with substratification of risk when the GFR is between 30 and 60, and currently it is difficult to make confident evidence-based recommendations in this group.19 Currently, most define this category as “at risk.” The threshold of eGFR <60 for defining risk, however, is controversial and likely includes many patients with a mildly reduced GFR that are truly at relatively low risk.9
A publication by Thomsen et al of a pooled analysis of two studies of IV administration of contrast for CT, found that only 0.6% of patients (1/170) with eGFR >40 mL/min met the definition of CIN.23 Their recommendation was to take precautions in patients before IV contrast administration when eGFR is less than 40 mL/min, and before IA administration when eGFR is less than 60 mL/min. Analysis of the literature by Katzberg et al has suggested analogous conclusions for stratification of IV administration thresholds; these conclusions were based on subgroup analysis of multiple prospective studies of patients who underwent IV administration of contrast for CT.10 Higher presumed risks associated with IA administration of contrast media explain the differences in the threshold recommendations.20 Although not currently accepted by any consensus panel, these recommendations appear to be based on reasonable evidence and may help guide the interventionalist regarding substratification of this risk group. It is still prudent to take basic, well-accepted precautions such as IV hydration in those undergoing IV contrast administration with any eGFR <60 until evidence has been more solidified.
WHO TO SCREEN
Healthy adults without risk factors younger than 40 years old are at very low risk and do not need routine screening of serum creatinine. In the absence of any risk factor, it is exceedingly rare that serum creatinine will be elevated in this patient population.19 Patients greater than 40 years old, inpatients, or those with any risk factors should be screened.9 Screening of outpatients should include a SCr within 30 days, and inpatients should have a SCr within 24 to 48 hours. Indications for screening are given in Table 3.
Table 3.
Indications for Obtaining a Screening Serum Creatinine Level
| Any risk factor in Table 1 |
| Age >40 years old |
| Inpatient |
| History of “kidney disease” |
| Tumor |
| Transplant |
| History of renal surgery |
| Solitary kidney |
| Family history of kidney failure |
CONTRAST AGENTS USED IN INTERVENTIONAL RADIOLOGY
Iodinated contrast media15,24
The most widely utilized agent for intravascular administration in interventional radiology is iodinated contrast. Iodinated contrast agents came into widespread use in the 1950s with the advent of high-osmolar contrast media (HOCM). These agents are ionically bound to a sodium atom and dissociate in plasma creating two osmotic particles. They carry three iodine particles per two osmotic particles (1.5:1 ratio). Their decreased tolerability and higher risk of adverse reactions have largely been attributed to their high osmolality (1500–2200 mOsm) compared with plasma (300 mOsm).
Second-generation low-osmolar contrast media (LOCM) were developed in the 1970s and have largely supplanted HOCM in clinical use today. All are able to carry three iodine particles per one osmotic particle (3:1 ratio). Most have covalent bonding, and are thus termed nonionic. Because the bonds do not dissociate in plasma, there are fewer osmotically active particles leading to a lower overall osmolality (600–900 mOsm) compared with high-osmolar contrast agents. An exception is Ioxaglate (Hexabrix®, Covidien plc, Dublin, Ireland), which is an ionic dimer. This ionically bound LOCM carries six iodine particles per two osmotic particles and dissociates in plasma, also resulting in a ratio of three iodine particles to a single osmotic particle.
The most recent contrast agents developed in the 1980s are known as isoosmolar contrast media (IOCM). They are nonionic dimers that allow six iodine particles to be attached to one osmotic particle (6:1 ratio). This results in a contrast media that is isoosmolar (300 mOsm) to plasma. The only commercially available IOCM today is iodixanol (Visipaque®, Amersham Health, Princeton, NJ).
IODINATED CONTRAST SELECTION
Large clinical trials and meta-analyses have demonstrated that LOCM results in substantially less CIN than HOCM in patients with preexisting renal dysfunction.18,21,25,26 No benefit has been demonstrated in those without renal dysfunction. Although there is no proven decrease in CIN in normal populations, better tolerability and fewer side effects of LOCM have largely resulted in their supplanting HOCM in routine clinical practice.
The results of numerous clinical studies and meta-analyses comparing IOCM and LOCM, however, are broadly conflicting.25 Initial reports suggested the IOCM resulted in less CIN than LOCM, however, subsequent studies have failed to confirm these findings. Morcos et al performed a review of the literature in 2008 and found that only three studies and one meta-analysis had shown less toxicity of IOCM when compared with LOCM.25 A single study had, in fact, demonstrated a higher incidence of CIN with IOCM compared with LOCM.25 Although currently there is little compelling evidence to choose IOCM over LOCM with respect to CIN, many practitioners continue to choose to use IOCM in the highest risk patients for IA administration.
A more compelling argument for use of IOCM is improved patient tolerance with peripheral intrarterial (IA) administration.9 Several studies comparing the tolerability of the low-osmolar contrast iodixanol to various LOCM demonstrated that iodixanol is associated with less pain and heat discomfort.27,28,29,30 Less pain potentially results in less motion artifact during digital subtraction angiography (DSA) acquisition, better image quality, and potential reduction in overall contrast and radiation dose, particularly in patients who are ill and poorly compliant.
VOLUME OF IODINATED CONTRAST
It is generally accepted that the risk of developing CIN is related to the dose of contrast administered.6 This notion is based primarily on evidence derived from cohort studies. Various publications, particularly in the interventional cardiology literature, have attempted to identify threshold doses of contrast that predict CIN or dialysis.
A study by Cigarroa et al suggested that in patients undergoing percutaneous coronary intervention (PCI), exceeding a volume of contrast greater than 5 mL/kg body weight divided by serum creatinine (mg/dL) strongly predicts nephropathy that requires dialysis.31 These findings have been retrospectively validated in the PCI population by a larger study by Freeman et al reviewing 16,592 PCI procedures. In fact, Freeman et al found that exceeding the maximal dose calculated by this formula was the strongest independent predictor of nephropathy requiring dialysis.32 A recent study by Laskey et al demonstrated that patients undergoing PCI who received a ratio of contrast dose in mL to calculated creatinine clearance (mL/min) of less than 3.7 had a low rate of CIN compared with those exceeding that dose.33 These equations (Fig. 2) can be used to calculate a theoretical maximum threshold dose for a given procedure to minimize CIN. Although validated for patients undergoing PCI, these data can be extrapolated for use in patients undergoing other interventional procedures.
Figure 2.
“Threshold” dose for contrast to minimize contrast-induced nephropathy in percutaneous coronary intervention. (†Cigarroa RG et al. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med 1989;86(6 Pt 1):649–652. ‡Laskey WK, et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol 2007;50(7):584–590.)
These equations do have limitations, however, and often result in a moderately high dose of calculated maximal contrast, especially in those with preexisting renal disease. For instance, a 60-year-old Caucasian male who weighs 106 kg with a creatinine of 2.09 mg/dL has an eGFR of 33 mL/min/1.73m2. According to the “threshold” equations, the maximal contrast dose for this patient is 120 to 130 cc, an amount substantially greater than many interventional practitioners would feel comfortable giving a patient with this abnormal baseline renal function. In general, the less contrast administered to patients at risk for CIN, the better.
ADMINISTRATION ROUTE
Intraarterial administration of contrast is generally thought to result in greater incidence of CIN than IV administration, which may be due to several factors. Intrarenal concentration of contrast media is higher after IA administration at or above the renal arteries than during IV administration, where a dilution effect may be somewhat protective. Arterial procedures may also produce additional injury to the kidney such as atheroemboli.
Gadolinium-Based Contrast Agents
Historically, gadolinium agents were used in patients at higher risk for CIN to minimize the load of iodinated contrast. These strategies are now limited by the recognition of nephrogenic systemic fibrosis (NSF), a debilitating progressive condition involving fibrosis of the skin, joints, and internal organs that occurs in patients with severe renal insufficiency. Gadolinium can therefore no longer be recommended as a safe alternative to iodinated contrast agents for patients with renal insufficiency.
Carbon Dioxide (CO2)
CO2 is a nonnephrotoxic negative contrast agent. It can be substituted for iodinated contrast in certain angiographic procedures. Classic teaching is that CO2 should not be used in arteries above the diaphragm to prevent CO2 reflux into the cerebral vasculature or the spinal artery, which may result in seizures, stroke, or paralysis. When used properly, CO2 angiography and venography are effective methods to obtain diagnostic information without the risk of renal toxicity. Operators should be familiar with the technical aspects of CO2 administration to prevent complications.
STRATEGIES FOR RENAL PROTECTION
Despite extensive study of a variety of agents for renal protection, use of low or isoosmolar contrast agents and IV hydration with normal saline or sodium bicarbonate are the only strategies that have been shown to be effective in the reduction of CIN in those at risk. Although popular, use of NAC remains unproven.
Hydration
NORMAL SALINE
IV fluid hydration with normal saline is the mainstay of practice in the prevention of CIN. It is low-risk, carries few side effects, and is cost-effective. Randomized trials have found IV hydration with normal saline to be consistently effective in the prevention of CIN. Fluid hydration is usually administered to patients of all risk categories, but is considered a requirement in the management of patient with an estimated GFR <60 mL/min/1.73m2.9
Although the exact mechanism is unclear, it is theorized that the administration of IV fluids increases intravascular volume, promotes diuresis, dilutes the overall intravascular contrast load, induces vasodilation, suppresses the renin–angiotensin–aldosterone axis, and suppresses the release of antidiuretic hormone.20
The composition of IV fluid hydration has been studied in the prevention of CIN. Mueller et al performed a large prospective, randomized control study to investigate normal saline (NS) IV infusion and the subsequent rates of CIN following contrast administration. The researchers found that NS (0.9%) was superior to half-NS (0.45%).34 In particular, the researchers found that female patients, diabetic patients, patients receiving greater than 250 mL of contrast, and patients undergoing emergent interventions were the most likely to benefit from isotonic hydration.34 IV hydration has also been found to be more effective than oral hydration.
Despite universal agreement of hydration with NS as a preventative measure against CIN, a single regimen has not been established. Generally, starting earlier and continuing longer are considered best practice. It is recommended to administer at least 500 cc of fluid within the 3 hours prior to the procedure. Additionally, hydration should be continued at least 6 to 8 hours following the procedure.16 Clinical judgment should be used when hydrating patients with cardiac dysfunction or severe renal insufficiency where volume overload may be a consideration.
SODIUM BICARBONATE
Intravenous administration of sodium bicarbonate has also gained substantial popularity in the prevention of CIN, but recent publications have demonstrated mixed results and enthusiasm has become somewhat tempered. The theoretical benefit of sodium bicarbonate is decreased acidification of the urine and renal medullary environment, which may theoretically reduce free radical injury, although many of the effects of sodium bicarbonate administration are also likely due to simple IV hydration.
In 2004, Merten et al published the first single-center randomized control trial of 119 patients comparing IV hydration with sodium bicarbonate (154 mEq/L; 3 cc/kg/h for 1 hour preprocedure and 1 cc/kg/h for 6 hours postprocedure) compared with administration of a similar volume of NS.35 They demonstrated a significant decrease in CIN in the sodium bicarbonate group (1.7%) compared with the NS group (13.6%). Since this original study, numerous randomized trials have been published. Many studies favor a benefit of sodium bicarbonate over NS, although several recent publications have demonstrated no benefit compared with the administration of NS.36 Several meta-analyses have been performed; they also suggested a benefit to sodium bicarbonate over NS with respect to CIN, although there were no differences in hard clinical endpoints such as death or the need for dialysis.37,38 Many of the analyzed publications were small in size, nonblinded, single center, and somewhat heterogeneous in their populations. Additionally, there is concern regarding publication bias.
A retrospective review of 7977 patients by From et al actually suggested increased CIN following the administration of sodium bicarbonate.39 Despite many limitations of this retrospective study, with only a small proportion of patients (489/7977) receiving sodium bicarbonate, it highlights the need for a larger randomized, well-controlled study.
Sodium bicarbonate is most commonly administered at a concentration of 154 mEq/L, at a rate of 3 cc/kg/h for one hour prior to intervention, and 1 cc/kg/h for 6 hours after contrast. It has also been used at a rate of 1 cc/kg/h for 12 hours before and 12 hours after administration of contrast. Recent studies have not demonstrated a difference between the two administration schemes.38 Table 4 outlines common hydration regimens used for renal protection.
Table 4.
Sample Intravenous Hydration Regimens
| Inpatient |
| 0.9% NS 1 cc/kg/h for 12 hours pre, 1 cc/kg/h for 12 hours post |
| Outpatient/emergent |
| 0.9% NS 2 cc/kg/h for 2–3 hours pre, 1 cc/kg/h for 6 hours post |
| 0.9% NS or Na Bicarb (154 mEq/L): 3 cc/kg/h for 1 hour pre, 1 cc/kg/h for 6 hours post |
N-ACETYLCYSTEINE
NAC is an antioxidant and a derivative of the amino acid cysteine. It has been demonstrated in animal models to decrease renal injury from ischemic and nephrotoxic effects. Potential mechanisms of NAC for CIN prophylaxis include both antioxidant and vasodilatory effects.40 Despite poor oral bioavailability due to a large first pass effect, NAC may provide antioxidant benefit as a precursor for glutathione, another antioxidant.41
In 2000, Tepel et al reported the first human trial for NAC in the prevention of CIN.40 In this prospectively randomized trial of 83 patients undergoing CT with IV contrast, patients who received a standard IV hydration protocol with normal saline were randomized to placebo versus NAC administered at a dose of 600 mg orally twice a day for 2 days, starting the day prior to the procedure. Contrast-induced nephropathy (SCr increase of >0.5 mg/dL) occurred in 2% of the NAC group compared with 21% of the control group (p < 0.01). Since this initial trial, numerous studies have been published, some finding substantial benefit, and others reporting a lack of efficacy.42,43 A 2006 publication by Marenzi et al has also suggested a dose-dependent effect of NAC.44 In 354 patients with acute myocardial infarction undergoing coronary angiography and angioplasty, patients were randomized to placebo, standard dose (600 mg), and double-dose (1200 mg) NAC protocols. NAC was administered by IV bolus prior to the procedure and then orally twice daily for 2 days postprocedure. The investigators found that the risk for CIN was reduced by 54.5% in standard-dose NAC and 75.8% in double-dose NAC. This dose-related phenomenon had not been demonstrated in prior studies and has yet to been confirmed in larger prospective studies.41
Fishbane et al reviewed the medical literature in 2008 and found more than 20 published studies investigating the use of NAC to prevent CIN.41 In his analysis, he found that studies with negative results outnumbered those with positive results by a 2-to-1 margin, and most studies were felt to be underpowered. More than 10 meta-analyses have been published, most of which found a net benefit for NAC, although many concerns have been raised. Nearly all of the meta-analyses found substantial statistically significant heterogeneity among the studies included, which limited the ability to effectively pool the data.41 Publication bias is also suspected; Vaitkus et al reported that among abstracts presented at scientific meetings, far fewer negative studies of NAC resulted in publications.45
An additional question regarding the administration of NAC and its renoprotective effects relates to concerns that NAC may lower serum creatinine without affecting renal function. Hoffmann et al gave NAC to 50 healthy volunteers who did not receive contrast media, and demonstrated a statistically significant lowering of serum creatinine levels, which in turn increased eGFR.46 In contrast, cystatin C levels, felt to be a more accurate measure of GFR than serum creatinine, did not change. Admittedly, the magnitude of this change was quite small, with NAC only causing a mean decrease in serum creatinine at 4 hours from 0.85 ± 0.14 mg/dL to 0.82 ± 0.13 mg/dL (p < 0.05).41,46 Poletti et al randomized patients with renal insufficiency receiving contrast for CT to NAC versus placebo. When CIN was defined based on changes in serum creatinine, there was a statistically significant decrease in CIN in the NAC group; however, when CIN was defined using cystatin C levels, there was no significant difference.47
Independent of effects on CIN, NAC is felt to be a cardioprotective agent. These findings are also somewhat controversial and are predominantly based on studies in the interventional cardiology literature. Due to a relatively low-risk profile, high patient tolerability, low-cost, and potential cardioprotective effects, NAC remains a commonly employed agent, albeit without a solid basis in scientific evidence.
NAC is most commonly administered as a dose of 600 to 1200 mg by mouth twice daily on the day before the procedure and the day of the procedure. IV regimens are utilized as well (150 mg/kg in 500 mL NS over 30 minutes prior to contrast administration and 50 mg/kg in 500 mL NS over 4 hours after contrast) although they are associated with a low incidence of anaphylactoid reaction.41,48 Given the lack of compelling evidence of efficacy, contrast studies should not be delayed to administer NAC.
DISCONTINUATION OF NEPHROTOXIC MEDICATIONS
In the case of elective procedures, all nephrotoxic medications should be discontinued prior to administration of contrast media. Specifically, medications such as NSAIDS and loop diuretics should be stopped 24 to 48 hours before a contrast study to reduce the risk of CIN. Unfortunately, this is not always possible in clinical practice. In patients with severe pulmonary edema or in emergent interventional procedures, it may not be possible to stop medications or delay intervention long enough to avoid nephrotoxicity from the combined effects of contrast and drugs. However, when possible these medications should be stopped at least one day before administration of contrast and should not be restarted until at least 48 hours later (or as deemed appropriate by clinical judgment with supporting laboratory data).9
PROPHYLACTIC HEMODIALYSIS
Despite removing a large portion of contrast media from the blood circulation, hemodialysis performed immediately after contrast administration has been demonstrated to be ineffective at reducing the incidence of CIN and has no role in its management.7,19,49 This is likely due to the fact that contrast injury occurs rapidly after the administration of contrast, that hemodialysis reduces circulating volume, and that hemodialysis may itself be nephrotoxic by activation of inflammatory pathways.
HEMOFILTRATION
Hemofiltration, unlike hemodialysis, is a form of continuous renal replacement therapy where there is no significant change in intravascular volume. Marenzi et al have studied hemofiltration to reduce the incidence of CIN.50 In this study, 114 patients were randomized to IV hydration with NS and hemofiltration for 4 to 8 hours preprocedure, nothing during the procedure, and hemofiltration 18 to 24 hours postprocedure. The incidence of CIN was reduced from 50% to 5%. In hospital mortality was reduced from 14% to 2%, and 12-month mortality was reduced from 30% to 10%. A confounding factor is the administration of heparin for hemofiltration. Despite very encouraging results, hemofiltration is costly, must be performed in the ICU setting, and carries its own inherent risks. Given limited evidence and high-cost, hemofiltration has not gained widespread acceptance although it may eventually be utilized in patients at very high risk.
PATIENTS ON HEMODIALYSIS
For patients with end-stage renal failure who are currently on chronic hemodialysis, no prophylaxis prior to administration is indicated. Volume expansion and increased serum osmolarity from contrast administration may require dialysis earlier than usual, however.
This clinical setting should be differentiated from the case of those on acute, intermittent, or temporary dialysis who may be at the highest risk of contrast-induced kidney injury.
Procedural Techniques for Minimizing Iodinated Contrast
Techniques for minimizing contrast dose during radiologic procedures are often not discussed in the literature although they remain critically important to reduction of contrast dose during procedures. Because contrast volume is related to the risk for contrast nephropathy, any method for decreasing iodinated contrast load may be potentially beneficial.
Dilution is often the easiest and most effective method for contrast dose reduction. With today's high-quality DSA imaging equipment and a compliant patient, contrast can be diluted with NS to substantially decrease contrast dose. In extremities, dilution of 1:3 to 1:5 (contrast:saline) will often result in diagnostic images. In the abdomen, dilution of 1:2 can be adequate if the patient is able to hold respiration adequately and if they are of the correct body habitus.
Use of digital software enhancements such as image stacking software can be utilized to further improve visualization, particularly in the extremities. Bolus chasing techniques can also be used to gather the most information from a single injection.
Catheter position also has a dramatic impact on the contrast load required to visualize a target. The closer a catheter is to the target, the less contrast will be needed for adequate visualization. For instance, if staged runoff arteriography is performed from the pelvis to the lower extremity, subsequent movement of the catheter into the most distal position allowable will dramatically decrease contrast dose due to less dilution with circulating blood volume.
Other procedural techniques to reduce dose include use of CO2 angiography, use of biplane angiography to obtain multiple views with a single injection, and staging procedures to minimize maximal contrast load given at a single intervention. Utilizing adjunctive imaging such as rotational CT or intravascular ultrasound may also help guide interventions and minimize contrast use.
SUMMARY OF RECOMMENDATIONS
The first and most important step in renal protection is risk stratification and determination of the necessity of contrast administration. Stratification based on eGFR/eCCr is currently recommended, although practitioners should not ignore other risk factors, most notably acute renal injury, diabetes associated with CRI, acute hypotension/sepsis, and multiple doses of contrast administered within 72 hours, which cause additive risk.
Avoidance of the use of contrast through utilization of other imaging modalities (noncontrast CT/magnetic resonance imaging [MRI], ultrasound, nuclear medicine, noninvasive vascular laboratories) remains the only method to completely eliminate the risk of CIN.
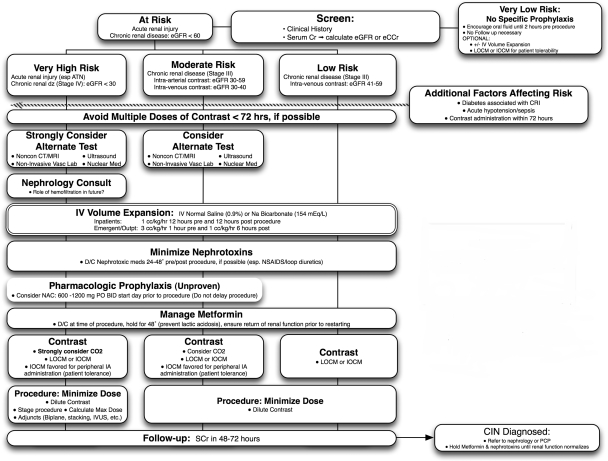
If contrast use is justified, most standard precautions are taken in those with eGFR/eCCr of <60. These precautions include minimizing effects of nephrotoxic medications, IV hydration with NS or sodium bicarbonate, and use of low or isoosmolar contrast media. Substratification of risk based on administration route and eGFR has been discussed and is included in Fig. 3, although this is not broadly accepted. Isoosmolar contrast may be preferred during peripheral IA administration of contrast, predominantly based on patient tolerance and decreased pain with administration. Although NAC administration is popular, it remains unproven. Metformin should be held at the time of procedure for 48 hours until it has been established that renal function has normalized.
Figure 3.
Recommendations for risk stratification and prophylaxis of contrast-induced nephropathy. (Adapted from Goldfarb S et al. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc 2009;84(2):170–179.)
Patients at the highest risk (eGFR/eCCr of <30) should have a nephrology consult prior to the procedure. Every effort should be made to reduce contrast dose including use of CO2 and dilution of iodinated contrast whenever possible. Despite compelling evidence, many practitioners will choose isoosmolar contrast in the highest-risk individuals based on very limited evidence. Limited evidence also suggests that hemofiltration may be beneficial in the highest-risk populations, although further validation is needed prior to widespread adoption of this practice, which carries its own inherent risks as well as high cost and utilization of hospital resources.
All patients with eGFR/eCCr <60 should have follow-up measurement of serum creatinine to screen for CIN, and demonstration of CIN should prompt referral to a nephrologist or the patient's primary care provider.
CONCLUSION
Contrast-induced nephropathy is a widely recognized and clinically significant problem in patients undergoing an increasing number of minimally invasive procedures that require contrast administration. The major risk factor for developing CIN is preexisting renal dysfunction, particularly in association with diabetes. Patients are considered to be at risk when eGFR/eCCr is less than 60.
The cornerstone of prevention is appropriate risk stratification, IV hydration with NS or sodium bicarbonate, appropriate withholding of nephrotoxic medications, use of low or isoosmolar contrast, and various intraprocedural methods for iodinated contrast dose reduction. Although NAC administration is popular, it remains unproven. Practitioners must be familiar with prevention strategies and diagnosis of CIN to minimize its clinical impact.
References
- Hou S H, Bushinsky D A, Wish J B, Cohen J J, Harrington J T. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- Rihal C S, Textor S C, Grill D E, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- Levy E M, Viscoli C M, Horwitz R I. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275(19):1489–1494. [PubMed] [Google Scholar]
- Ellis J H, Cohan R H. Reducing the risk of contrast-induced nephropathy: a perspective on the controversies. AJR Am J Roentgenol. 2009;192(6):1544–1549. doi: 10.2214/AJR.09.2368. [DOI] [PubMed] [Google Scholar]
- Segal A J, Ellis J H, et al. ACR, Manual on Contrast Media: Version 6. 2008. Available at: http://www.acr.org/contrast-manual. Accessed November 1, 2009. Available at: http://www.acr.org/contrast-manual
- Gleeson T G, Bulugahapitiya S. Contrast-induced nephropathy. AJR Am J Roentgenol. 2004;183(6):1673–1689. doi: 10.2214/ajr.183.6.01831673. [DOI] [PubMed] [Google Scholar]
- Kolonko A, Kokot F, Wiecek A. Contrast-associated nephropathy—old clinical problem and new therapeutic perspectives. Nephrol Dial Transplant. 1998;13(3):803–806. doi: 10.1093/ndt/13.3.803. [DOI] [PubMed] [Google Scholar]
- Goldfarb S, McCullough P A, McDermott J, Gay S B. Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc. 2009;84(2):170–179. doi: 10.4065/84.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzberg R W, Lamba R. Contrast-induced nephropathy after intravenous administration: fact or fiction? Radiol Clin North Am. 2009;47(5):789–800, v. doi: 10.1016/j.rcl.2009.06.002. v. [DOI] [PubMed] [Google Scholar]
- Newhouse J H, Kho D, Rao Q A, Starren J. Frequency of serum creatinine changes in the absence of iodinated contrast material: implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376–382. doi: 10.2214/AJR.07.3280. [DOI] [PubMed] [Google Scholar]
- Cramer B C, Parfrey P S, Hutchinson T A, et al. Renal function following infusion of radiologic contrast material. A prospective controlled study. Arch Intern Med. 1985;145(1):87–89. [PubMed] [Google Scholar]
- Heller C A, Knapp J, Halliday J, O'Connell D, Heller R F. Failure to demonstrate contrast nephrotoxicity. Med J Aust. 1991;155(5):329–332. doi: 10.5694/j.1326-5377.1991.tb142293.x. [DOI] [PubMed] [Google Scholar]
- Rao Q A, Newhouse J H. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology. 2006;239(2):392–397. doi: 10.1148/radiol.2392050413. [DOI] [PubMed] [Google Scholar]
- Rudnick M R, Kesselheim A, Goldfarb S. Contrast-induced nephropathy: how it develops, how to prevent it. Cleve Clin J Med. 2006;73(1):75–80. 83–87. doi: 10.3949/ccjm.73.1.75. [DOI] [PubMed] [Google Scholar]
- Schweiger M J, Chambers C E, Davidson C J, et al. Prevention of contrast induced nephropathy: recommendations for the high risk patient undergoing cardiovascular procedures. Catheter Cardiovasc Interv. 2007;69(1):135–140. doi: 10.1002/ccd.20964. [DOI] [PubMed] [Google Scholar]
- McCullough P A, Wolyn R, Rocher L L, Levin R N, O'Neill W W. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Barrett B J, Parfrey P S. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- Benko A, Fraser-Hill M, Magner P, et al. Canadian Association of Radiologists Canadian Association of Radiologists: consensus guidelines for the prevention of contrast-induced nephropathy. Can Assoc Radiol J. 2007;58(2):79–87. [PubMed] [Google Scholar]
- Ellis J H, Cohan R H. Prevention of contrast-induced nephropathy: an overview. Radiol Clin North Am. 2009;47(5):801–811, v. doi: 10.1016/j.rcl.2009.06.003. v. [DOI] [PubMed] [Google Scholar]
- Rudnick M R, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47(1):254–261. doi: 10.1038/ki.1995.32. [DOI] [PubMed] [Google Scholar]
- McCullough P A, Sandberg K R. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4(Suppl 5):S3–S9. [PubMed] [Google Scholar]
- Thomsen H S, Morcos S K. Risk of contrast-medium-induced nephropathy in high-risk patients undergoing MDCT—a pooled analysis of two randomized trials. Eur Radiol. 2009;19(4):891–897. doi: 10.1007/s00330-008-1206-4. [DOI] [PubMed] [Google Scholar]
- Thomsen H S, Morcos S K. Radiographic contrast media. BJU Int. 2000;86(Suppl 1):1–10. doi: 10.1046/j.1464-410x.2000.00586.x. [DOI] [PubMed] [Google Scholar]
- Morcos S K. Contrast-induced nephropathy: are there differences between low osmolar and iso-osmolar iodinated contrast media? Clin Radiol. 2009;64(5):468–472. doi: 10.1016/j.crad.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Taliercio C P, Vlietstra R E, Ilstrup D M, et al. A randomized comparison of the nephrotoxicity of iopamidol and diatrizoate in high risk patients undergoing cardiac angiography. J Am Coll Cardiol. 1991;17(2):384–390. doi: 10.1016/s0735-1097(10)80103-2. [DOI] [PubMed] [Google Scholar]
- Manke C, Marcus C, Page A, Puey J, Batakis O, Fog A. Pain in femoral arteriography. A double-blind, randomized, clinical study comparing safety and efficacy of the iso-osmolar iodixanol 270 mgI/ml and the low-osmolar iomeprol 300 mgI/ml in 9 European centers. Acta Radiol. 2003;44(6):590–596. doi: 10.1080/02841850312331287709. [DOI] [PubMed] [Google Scholar]
- Justesen P, Downes M, Grynne B H, Lang H, Rasch W, Seim E. Injection-associated pain in femoral arteriography: a European multicenter study comparing safety, tolerability, and efficacy of iodixanol and iopromide. Cardiovasc Intervent Radiol. 1997;20(4):251–256. doi: 10.1007/s002709900147. [DOI] [PubMed] [Google Scholar]
- Pugh N D, Sissons G R, Ruttley M S, Berg K J, Nossen J O, Eide H. Iodixanol in femoral arteriography (phase III): a comparative double-blind parallel trial between iodixanol and iopromide. Clin Radiol. 1993;47(2):96–99. doi: 10.1016/s0009-9260(05)81180-8. [DOI] [PubMed] [Google Scholar]
- Verow P, Nossen J O, Sheppick A, Kjaersgaard P. A comparison of iodixanol with iopamidol in aorto-femoral angiography. Br J Radiol. 1995;68(813):973–978. doi: 10.1259/0007-1285-68-813-973. [DOI] [PubMed] [Google Scholar]
- Cigarroa R G, Lange R A, Williams R H, Hillis L D. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86(6 Pt 1):649–652. doi: 10.1016/0002-9343(89)90437-3. [DOI] [PubMed] [Google Scholar]
- Freeman R V, O'Donnell M, Share D, et al. Blue Cross-Blue Shield of Michigan Cardiovascular Consortium (BMC2) Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90(10):1068–1073. doi: 10.1016/s0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]
- Laskey W K, Jenkins C, Selzer F, et al. NHLBI Dynamic Registry Investigators Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(7):584–590. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Mueller C, Buerkle G, Buettner H J, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162(3):329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- Merten G J, Burgess W P, Gray L V, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291(19):2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- Brar S S, Shen A Y, Jorgensen M B, et al. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300(9):1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- Hogan S E, L'Allier P, Chetcuti S, et al. Current role of sodium bicarbonate-based preprocedural hydration for the prevention of contrast-induced acute kidney injury: a meta-analysis. Am Heart J. 2008;156(3):414–421. doi: 10.1016/j.ahj.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Navaneethan S D, Singh S, Appasamy S, Wing R E, Sehgal A R. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(4):617–627. doi: 10.1053/j.ajkd.2008.08.033. [DOI] [PubMed] [Google Scholar]
- From A M, Bartholmai B J, Williams A W, Cha S S, Pflueger A, McDonald F S. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol. 2008;3(1):10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepel M, der Giet M van, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- Fishbane S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. 2008;3(1):281–287. doi: 10.2215/CJN.02590607. [DOI] [PubMed] [Google Scholar]
- Amini M, Salarifar M, Amirbaigloo A, Masoudkabir F, Esfahani F. N-acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials. 2009;10:45. doi: 10.1186/1745-6215-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D A, Norsworthy K J, Kern S J, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32. doi: 10.1186/1741-7015-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354(26):2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- Vaitkus P T, Brar C. N-acetylcysteine in the prevention of contrast-induced nephropathy: publication bias perpetuated by meta-analyses. Am Heart J. 2007;153(2):275–280. doi: 10.1016/j.ahj.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Fischereder M, Krüger B, Drobnik W, Krämer B K. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15(2):407–410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- Poletti P A, Saudan P, Platon A, et al. I.v. N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. AJR Am J Roentgenol. 2007;189(3):687–692. doi: 10.2214/AJR.07.2356. [DOI] [PubMed] [Google Scholar]
- Baker C S, Wragg A, Kumar S, De Palma R, Baker L R, Knight C J. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41(12):2114–2118. doi: 10.1016/s0735-1097(03)00487-x. [DOI] [PubMed] [Google Scholar]
- Vogt B, Ferrari P, Schönholzer C, et al. Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am J Med. 2001;111(9):692–698. doi: 10.1016/s0002-9343(01)00983-4. [DOI] [PubMed] [Google Scholar]
- Marenzi G, Marana I, Lauri G, et al. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N Engl J Med. 2003;349(14):1333–1340. doi: 10.1056/NEJMoa023204. [DOI] [PubMed] [Google Scholar]