Abstract
This phase 1/2 study is the first prospective evaluation of lenalidomide-bortezomib-dexamethasone in front-line myeloma. Patients (N = 66) received 3-week cycles (n = 8) of bortezomib 1.0 or 1.3 mg/m2 (days 1, 4, 8, 11), lenalidomide 15 to 25 mg (days 1-14), and dexamethasone 40 or 20 mg (days 1, 2, 4, 5, 8, 9, 11, 12). Responding patients proceeded to maintenance or transplantation. Phase 2 dosing was determined to be bortezomib 1.3 mg/m2, lenalidomide 25 mg, and dexamethasone 20 mg. Most common toxicities included sensory neuropathy (80%) and fatigue (64%), with only 27%/2% and 32%/3% grade 2/3, respectively. In addition, 32% reported neuropathic pain (11%/3%, grade 2/3). Grade 3/4 hematologic toxicities included lymphopenia (14%), neutropenia (9%), and thrombocytopenia (6%). Thrombosis was rare (6% overall), and no treatment-related mortality was observed. Rate of partial response was 100% in both the phase 2 population and overall, with 74% and 67% each achieving very good partial response or better. Twenty-eight patients (42%) proceeded to undergo transplantation. With median follow-up of 21 months, estimated 18-month progression-free and overall survival for the combination treatment with/without transplantation were 75% and 97%, respectively. Lenalidomide-bortezomib-dexamethasone demonstrates favorable tolerability and is highly effective in the treatment of newly diagnosed myeloma. This study is registered at http://clinicaltrials.gov as NCT00378105.
Introduction
The aim of front-line therapy for multiple myeloma (MM) is to substantially decrease tumor burden, either in preparation for consolidation with high-dose melphalan therapy with autologous stem cell transplantation (ASCT) or as a means in itself to provide long-term disease control. The degree of disease reduction is associated with improved outcome, including prolonged progression-free survival (PFS) and overall survival (OS),1–3 both after preparation for or after consolidation with ASCT,3 and in patients not proceeding to ASCT.2 The introduction of the proteasome inhibitor bortezomib and the immunomodulatory drugs thalidomide and lenalidomide has been associated with improved survival.4,5 Combinations of bortezomib or lenalidomide with conventional anti-MM drugs have demonstrated very high overall response rates and quality of response in the front-line setting, as reviewed recently.6 On the basis of phase 3 studies, bortezomib7,8 is approved for the treatment of newly diagnosed and relapsed MM, and lenalidomide plus dexamethasone9,10 is approved for patients after at least 1 previous therapy.
Bortezomib and lenalidomide have different but overlapping mechanisms of anti-MM activity in preclinical studies.11–13 Bortezomib-induced tumor cell death has been associated with activation of both the mitochondrial, caspase-9–mediated and Fas/caspase-8–mediated apoptotic pathways, as well as the induction of endoplasmic reticulum stress and inhibition of nuclear factor κ-B signaling.11,12 Lenalidomide primarily triggers the caspase-8–mediated apoptotic pathway and also down-regulates nuclear factor κ-B activity via a mechanism distinct from that of bortezomib.13 Both bortezomib11 and the immunomodulatory drugs13 enhance the activity of dexamethasone, and synergy has been demonstrated between bortezomib and lenalidomide.13 These preclinical findings have translated into clinical efficacy; bortezomib plus dexamethasone14,15 and lenalidomide plus dexamethasone16–18 have shown substantial activity in the front-line treatment of MM. A phase 1 study of lenalidomide plus bortezomib in patients with relapsed or relapsed, refractory MM demonstrated favorable toxicity and promising response and survival; as well as the addition of dexamethasone producing an increased response rate.19 A phase 2 study in the relapsed setting also has demonstrated efficacy with lenalidomide, bortezomib, and dexamethasone.20
The phase 1/2 study reported herein is the first prospective evaluation of the combination of lenalidomide, bortezomib, and dexamethasone as treatment for newly diagnosed MM. The aims were to determine the maximum tolerated dose (MTD) of the combination in the front-line setting and to evaluate safety and activity.
Methods
Patients
Patients aged 18 years or older with newly diagnosed, symptomatic MM who had received no previous systemic anti-MM therapy (except corticosteroids for hypercalcemia or spinal cord compression, not exceeding 160 mg of dexamethasone or equivalent in a 2-week period in advance of enrollment) and had a Karnofsky Performance Status (KPS) of at least 60% were eligible. Previous local radiotherapy must have been completed at least 2 weeks before study entry. Patients were excluded if they had grade 2 or greater peripheral neuropathy, serum creatinine greater than 2.5 mg/dL, platelets less than 50 000/μL, absolute neutrophil count less than 1000/μL, hemoglobin less than 8.0 g/dL, transaminases elevated 2 or more times the upper limit of normal, or other specific significant and active comorbidities or infections. Review boards at all participating institutions approved the study, which was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients provided written informed consent.
Study design and treatment
This open-label phase 1/2 (http://www.ClinicalTrials.gov: NCT00378105) study was conducted at 6 centers in the United States, with enrollment between September 13, 2006, and February 18, 2008. The primary end points were to determine the MTD of bortezomib, lenalidomide, and dexamethasone (phase 1) and to evaluate the response rate (partial response [PR] or better) to the combination (phase 2). Secondary end points included complete plus near-complete response (CR + nCR) rate, duration of response (DOR), PFS, OS, and toxicity. The rate of very good partial response (VGPR) or better also was determined, reflecting the recent introduction of the International Uniform Response Criteria.21
Eight 3-week treatment cycles of intravenous bortezomib on days 1, 4, 8, and 11; oral lenalidomide on days 1 to 14; and oral dexamethasone on days 1, 2, 4, 5, 8, 9, 11, and 12 were given. Patients who achieved at least a PR could elect to proceed to ASCT after a minimum of 4 cycles. Responding patients could receive maintenance therapy comprising 3-week cycles of bortezomib on days 1 and 8 and lenalidomide on days 1 to 14 at the dose levels tolerated at the end of cycle 8, plus dexamethasone 10 mg on days 1, 2, 8, and 9. All patients received, unless contraindicated, aspirin prophylaxis (81 or 325 mg daily) or alternative anticoagulation for prevention of deep-vein thrombosis, antiviral therapy (eg, acyclovir) for herpes zoster prevention, and bisphosphonates.
Dose escalation and determination of phase 2 dosing
Four dose levels (1-4) were initially studied (Table 1), with bortezomib doses of 1.0, 1.3, 1.3, and 1.3 mg/m2 and lenalidomide doses of 15, 15, 20, and 25 mg, respectively, plus a dexamethasone dose of 40 mg (cycles 1-4) then 20 mg (cycles 5-8). Because of dose-limiting toxicity (DLT) specifically associated with dexamethasone at dose level 4, an additional dose level with reduced dexamethasone dosing was included (4M; bortezomib 1.3 mg/m2, lenalidomide 25 mg, dexamethasone 20 mg [cycles 1-4] then 10 mg [cycles 5-8]).
Table 1.
Patient disposition by dose level in the phase 1 and phase 2 portions and numbers of patients experiencing DLT
| Dose level | Bortezomib dose, mg/m2 | Lenalidomide dose, mg | Dexamethasone dose, mg (cycles 1-4/5-8) | No. enrolled/treated | DLT, n |
|---|---|---|---|---|---|
| Phase 1 dose escalation | 22/21* | 2 | |||
| Dose level 1 | 1.0 | 15 | 40/20 | 3/3 | |
| Dose level 2 | 1.3 | 15 | 40/20 | 3/3 | |
| Dose level 3 | 1.3 | 20 | 40/20 | 4/3* | |
| Dose level 4 | 1.3 | 25 | 40/20 | 6/6 | 2 |
| Dose level 4M | 1.3 | 25 | 20/10 | 6/6 | |
| Phase 1 expanded cohort | 11/10† | ||||
| Dose level 4M | 1.3 | 25 | 20/10 | 11/10† | |
| Phase 2 | 35/35 | NA | |||
| Dose level 4M | 1.3 | 25 | 20/10 | 35/35 | NA |
DLT indicates dose-limiting toxicity; and NA, not applicable.
One patient did not receive treatment because of elevated creatinine and was not included in determination of maximum tolerated dose; a replacement patient was enrolled to dose level 3.
One patient was enrolled but did not receive study treatment because of acute renal failure, and was replaced.
Cohorts of 3 patients were sequentially enrolled, starting at dose level 1. If one of the first 3 patients experienced DLT, another cohort was enrolled at that same dose level. If none of the first 3 or only 1 of 6 patients experienced DLT, the subsequent cohort was enrolled to the next highest dose level. If 2 or more patients experienced DLT, dose escalation was halted and either an alternative dose level was investigated or the previous dose level was to be declared the MTD. A cohort of 6 patients was to be enrolled for the investigation of dose level 4M. After completion of the dose-escalation component, 10 additional patients were enrolled within phase 1 at the MTD/maximum planned dose (MPD; no further dose escalation of bortezomib or lenalidomide) to provide additional data on subsequent cycle toxicities. In the phase 2 portion, an additional 35 patients were enrolled at this dose level.
DLT was defined as grade 3 or greater nonhematologic toxicity attributable to 1 or more of the study drugs, or grade 4 hematologic toxicity (thrombocytopenia with platelets < 10 000/μL on more than one occasion despite transfusion support; neutropenia occurring for > 5 days and/or resulting in neutropenic fever with temperature ≥101°F, confirmed on 2 occasions) occurring during cycle 1, or inability to receive therapy on day 1, cycle 2, because of persisting drug-related toxicity from cycle 1.
Dose modifications were not permitted during cycle 1 of the phase 1 portion, except for the occurrence of DLT. Dose reductions for bortezomib (1.3→1.0→0.7 mg/m2), lenalidomide (5-mg decrements), and dexamethasone (40→20→10 mg), and subsequently discontinuation, were required for specific study drug–related toxicities as attributed by the investigators. Patients with bortezomib-related peripheral neuropathy or neuropathic pain were managed according to established guidelines.22 Patients could be withdrawn from study because of unacceptable toxicity, a significant treatment delay, or progressive disease at any time.
Assessments
Response was assessed according to European Group for Blood and Marrow Transplant criteria,23 modified to include nCR24 and, from the International Uniform Response Criteria,21 VGPR. Confirmation for all response categories required 2 assessments at least 6 weeks apart, per European Group for Blood and Marrow Transplant criteria. Blood and urine samples were collected for M-protein quantification and immunofixation at every cycle for response evaluation. Full response assessment, including extramedullary plasmacytoma assessment, skeletal survey, and bone marrow aspirate/biopsy, was conducted at the end of cycle 8 and after cycle 4 for patients proceeding to ASCT. Bone marrow aspirate/biopsy also was performed at baseline for cytogenetic assessment and as required to confirm CR. Soft-tissue plasmacytomas were assessed every cycle. Safety was evaluated throughout the study and during long-term follow-up. Adverse events (AEs) were graded by use of the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0
Statistical analysis
A 1-stage design was used for the phase 2 part of the study, which required at least 22 of 35 patients to achieve a PR or better to consider the treatment promising. The design was selected to have high probability (0.86) of concluding the treatment to be effective when it is (defined as true response rate of 70%) and low probability (0.09) when not (true response rate of 50%).
Exact binomial 90% confidence intervals (CIs) were reported for response rates. The Kaplan-Meier method was used to estimate distributions of DOR (time of first response to progression or death, censoring at the date patients were last known to be alive and disease-free for patients who had not progressed or died), PFS (time of treatment initiation to progression or death, censoring as for DOR), and OS (time of treatment initiation to death, censoring at the date patients were last known to be alive for those who had not died). Patients also were censored at the date of initiation of nonprotocol therapy, excluding bisphosphonates and erythropoietin. Per protocol, patients could undergo ASCT after 4 cycles. To account for the impact of informative censoring, which was evaluated by sensitivity analyses,25 patients were not censored at the time of ASCT in time-to-event analyses but were followed until progression or death, or censored as described previously. PFS and OS estimates therefore correspond to the overall treatment path, denoted as lenalidomide-bortezomib-dexamethasone with/without ASCT.
Post-hoc analyses were performed to evaluate rates of VGPR or better and PFS by disease stage, baseline β2-microglobulin and albumin, and baseline cytogenetics by fluorescence in situ hybridization. A post-hoc 1-year landmark analysis was performed to estimate PFS by ASCT status. The data cutoff was July 28 2009; analyses were performed with SAS software.
Results
Patients
Sixty-eight patients with symptomatic MM were enrolled. Two patients did not receive study treatment, 1 in dose level 3 (because of elevated creatinine as a result of rapidly progressing disease and myeloma kidney) and 1 in dose level 4M (also because of acute renal failure secondary to rapid disease progression), leaving a treated population of 66 patients: 21 in the phase 1 dose-escalation population, 10 in the phase 1 expanded cohort, and 35 in the phase 2 cohort (Table 1). Baseline demographic and disease characteristics are summarized in Table 2.
Table 2.
Baseline demographics and disease characteristics
| Characteristic | All treated patients, N = 66 | Phase 2 population, n = 35 |
|---|---|---|
| Median age, y (range) | 58 (22-86) | 59 (22-86) |
| Male sex, n (%) | 36 (55) | 19 (54) |
| Race, n (%) | ||
| White | 53 (80) | 27 (77) |
| Black | 11 (17) | 7 (20) |
| Other | 2 (3) | 1 (3) |
| ECOG performance status, n (%) | ||
| 0 | 43 (65) | 17 (49) |
| 1 | 17 (26) | 13 (37) |
| 2 | 6 (9) | 5 (14) |
| ISS stage at treatment initiation, n (%) | ||
| I | 29 (44) | 12 (34) |
| II | 31 (47) | 19 (54) |
| III | 6 (9) | 4 (11) |
| Durie-Salmon stage at diagnosis, n (%)* | ||
| I | 22 (33) | 9 (26) |
| II | 21 (32) | 13 (37) |
| III | 23 (35) | 13 (37) |
| MM subtype, n (%) | ||
| IgG | 45 (68) | 20 (57) |
| IgA | 15 (23) | 11 (31) |
| Light chain | 6 (9) | 4 (11) |
| β2-microglobulin level, n (%) | ||
| < 3.5 mg/L | 44 (67) | 19 (54) |
| 3.5-5.5 mg/L | 17 (26) | 13 (37) |
| > 5.5 mg/L | 5 (8) | 3 (9) |
| Albumin level, n (%) | ||
| < 3.5 g/dL | 24 (36) | 17 (49) |
| ≥ 3.5 g/dL | 42 (64) | 18 (51) |
| Elevated LDH, n (%) | 6 (9) | 4 (11) |
| Lytic lesions, n (%)† | ||
| None | 16 (24) | 5 (14) |
| 1-3 bones | 15 (23) | 10 (29) |
| > 3 bones | 34 (52) | 19 (54) |
| Abnormal metaphase cytogenetics, n (%) | 6 (9) | 2 (6) |
| FISH abnormalities,‡n (%) | ||
| Del 13q | 24 (47) | 16 (53) |
| Del 17p | 5 (10) | 2 (7) |
| t(4;14) | 2 (5) | 2 (9) |
| t(11;14) | 11 (22) | 7 (23) |
| Del 17p and/or t(4;14) | 6 (12) | 3 (8) |
ECOG indicates Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; IgA, immunoglobulin A; IgG, immunoglobulin G; ISS, International Staging System; LDH, lactate dehydrogenase; and MM, multiple myeloma.
All patients were symptomatic at enrollment/treatment initiation.
Data missing for 1 patient in the phase 2 population.
Numbers of patients with results for del 13q, del 17p, t(4;14), and t(11;14) status were 51, 50, 41, and 51, respectively, for the overall population, and 30, 30, 22, and 30 for the phase 2 population. N values for analyses of “Del 17p and/or t(4;14)” were 50 for the overall population and 36 for the phase 2 population. A patient was determined to have abnormal cytogenetics if, when FISH was used, the percentage of nuclei consistent with these cytogenetic abnormalities exceeded the normal ranges established for the respective probes in the cytogenetics laboratory of each participating institution.
Determination of maximum tolerated dose
Two patients in dose level 4 experienced DLT, specifically grade 3 hyperglycemia and grade 3 alanine transaminase elevation, both attributable to dexamethasone by the investigator. An additional dose level 4M was investigated, enrolling 6 patients per protocol; no further DLT was reported in these initial 6 patients enrolled in phase 1 nor in the subsequent expanded phase 1 cohort, and 4M was established as the MPD.
Treatment exposure and safety
The median number of cycles received of any study drug, including fully completed cycles during maintenance therapy, was 10 (range, 2-34), with medians of 8 (range, 2-33), 10 (range, 2-34), and 8 (range, 2-33) cycles of bortezomib, lenalidomide, and dexamethasone, respectively. Overall, 39 (59%) patients received at least 8 cycles of all 3 agents. At least 1 dose modification was required in 48 (73%) patients, including 29 (44%), 23 (35%), and 32 (48%) patients who required bortezomib, lenalidomide, and dexamethasone dose modifications, respectively. The most common reasons for which bortezomib dose modifications were required were neuropathic pain (n = 10; 15%), sensory neuropathy (n = 9; 14%), and fatigue (n = 5; 8%). The most common reasons for which lenalidomide dose modifications were required were fatigue (n = 4; 6%), neuropathic pain (n = 3; 5%), sensory neuropathy (n = 3; 5%), and rash (n = 3; 5%). The most common reasons for which dexamethasone dose modifications were required were per protocol (n = 5; 8%), lower-extremity edema (n = 4; 6%), mental status (n = 3; 5%), and tremor (n = 3; 5%). In total, 39%, 42%, and 35% of patients received all doses of bortezomib, lenalidomide, and dexamethasone, respectively, at the planned full dose (ie, with no doses reduced, held, or not given).
Fourteen (21%) patients remained on study treatment at data cutoff. Twenty-eight (42%) patients had discontinued treatment with lenalidomide-bortezomib-dexamethasone by the end of cycle 8 because of proceeding to ASCT (n = 13), completion of protocol therapy (n = 6), patient/physician choice (n = 4), unacceptable toxicity (n = 3; elevated creatinine, neuropathic pain, thrombosis), death as a result of unrelated cardiac ischemia (n = 1, in a patient with a history of cardiac complications, attributed by the investigator to the patient's cardiac history), and receipt of nonprotocol therapy (n = 1). An additional 24 (36%) patients discontinued treatment with lenalidomide-bortezomib-dexamethasone during the maintenance phase (beyond cycle 8) because of completion of protocol-directed therapy (n = 10), disease progression (n = 6), withdrawal of consent (n = 3), proceeding to ASCT (n = 2), patient/physician choice (n = 1), unacceptable toxicity (n = 1; rash), and increase in lenalidomide dose (n = 1).
The most common toxicities, and all grade 3/4 AEs, are shown in Table 3. Sensory neuropathy, motor neuropathy, and neuropathic pain were reported in 53 (80%), 12 (18%), and 21 (32%) patients, respectively, including at grade 2 in 18 (27%), 2 (3%), and 7 (11%), and at grade 3 in 1 (2%), 1 (2%), and 2 (3%) patients, respectively. There was no grade 4 neuropathy. Grade 3 or 4 toxicities reported in at least 5% of patients included lymphopenia (14%), neutropenia (9%), thrombocytopenia (6%), hypokalemia (5%), and hypophosphatemia (5%). The overall rate of thrombosis, including pulmonary embolism, was 6% (n = 4). There was no treatment-related mortality.
Table 3.
Adverse events reported in at least 15% of patients in the treated population (N = 66) plus all other events reported at grade 3 or 4 severity
| Event | Total, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|
| Neuropathy, sensory | 53 (80)† | 1 (2) | 0 |
| Fatigue | 42 (64) | 2 (3) | 0 |
| Constipation | 40 (61) | 0 | 0 |
| Edema limb | 30 (45) | 0 | 0 |
| Muscle pain | 29 (44) | 1 (2) | 0 |
| Rash/desquamation | 24 (36) | 1 (2) | 0 |
| Diarrhea | 23 (35) | 0 | 0 |
| Nausea | 21 (32) | 0 | 0 |
| Neuropathic pain | 21 (32) | 2 (3) | 0 |
| Extremity, limb pain | 20 (30) | 2 (3) | 0 |
| Insomnia | 20 (30) | 1 (2) | 0 |
| Hyperglycemia | 18 (27) | 1 (2)‡ | 0 |
| Dizziness | 17 (26) | 2 (3) | 0 |
| Constitutional, other | 12 (18) | 0 | 0 |
| Dyspnea | 12 (18) | 0 | 0 |
| Neuropathy, motor | 12 (18) | 1 (2) | 0 |
| Platelets | 12 (18) | 1 (2) | 3 (5) |
| Pruritis/itching | 12 (18) | 0 | 0 |
| Neutrophils | 10 (15) | 5 (8) | 1 (2) |
| Anxiety | 9 (14) | 1 (2) | 0 |
| Dry skin | 9 (14) | 1 (2) | 0 |
| Lymphopenia | 9(14) | 7 (11) | 2 (3) |
| Vision, blurred | 9 (14) | 1 (2) | 0 |
| Alanine transaminase | 8 (12) | 2 (3)‡ | 0 |
| Hypokalemia | 7 (11) | 3 (5) | 0 |
| Mental status | 7 (11) | 1 (2) | 0 |
| Hyperkalemia | 6 (9) | 1 (2) | 0 |
| Hyponatremia | 6 (9) | 1 (2) | 0 |
| Hypophosphatemia | 6 (9) | 3 (5) | 0 |
| Pulmonary/upper respiratory, other | 6 (9) | 2 (3) | 0 |
| Agitation | 4 (6) | 1 (2) | 0 |
| Hearing | 4 (6) | 1 (2) | 0 |
| Hemoglobin | 4 (6) | 1 (2) | 0 |
| QTc interval | 4 (6) | 2 (3) | 0 |
| Thrombosis/thrombus/embolism | 4 (6) | 2 (3) | 1 (2) |
| Creatinine | 2 (3) | 1 (2) | 0 |
| Leukocytes | 2 (3) | 2 (3) | 0 |
| Atrial fibrillation | 1 (2) | 1 (2) | 0 |
| Infection, other | 1 (2) | 1 (2) | 0 |
| Stomach hemorrhage | 1 (2) | 1 (2) | 0 |
Including 34 (52%) patients with grade 1 and 18 (27%) with grade 2 neuropathy, sensory.
One dose-limiting toxicity.
Response to treatment
Table 4 summarizes the response rates in both the overall population (N = 66) and in the phase 2 population (n = 35). All 66 patients achieved a PR or better to lenalidomide-bortezomib-dexamethasone treatment, including 26 (39%) with CR + nCR and an additional 18 (27%) with VGPR, for a rate of VGPR or better of 67%. In the phase 2 population, 20 (57%) patients achieved CR + nCR and an additional 6 (17%) achieved a VGPR, for a rate of VGPR or better of 74% (Table 4).
Table 4.
Best response to treatment for the treated population and the phase 2 population
| Response* | All patients (N = 66) |
Phase 2 population (n = 35) |
||||
|---|---|---|---|---|---|---|
| n | % | 90% CI | n | % | 90% CI | |
| CR | 19 | 29 | 20-39 | 13 | 37 | 24-52 |
| nCR | 7 | 11 | 5-19 | 7 | 20 | 10-34 |
| VGPR | 18 | 27 | 18-38 | 6 | 17 | 8-31 |
| PR | 22 | 33 | 24-44 | 9 | 26 | 14-41 |
| CR + nCR | 26 | 39 | 29-50 | 20 | 57 | 42-71 |
| CR + nCR + VGPR | 44 | 67 | 56-76 | 26 | 74 | 59-86 |
| At least PR | 66 | 100 | 96-100 | 35 | 100 | 92-100 |
CI indicates confidence interval; CR, complete response; nCR, near-complete response; PR, partial response; VGPR, very good partial response.
Per EBMT criteria,23 all response categories, including VGPR, required a confirmatory assessment at 6 weeks.
Responses to lenalidomide-bortezomib-dexamethasone after 4 cycles of treatment included 4 (6%) CR + nCR, 3 (5%) VGPR, 42 (64%) PR, 13 (20%) minimal response, and 4 (6%) stable disease. Improvement in response to lenalidomide-bortezomib-dexamethasone (by at least 1 response category) from cycle 4 through cycle 8 was observed in 42 (75%) of 56 patients who continued lenalidomide-bortezomib-dexamethasone treatment beyond cycle 4, with further improvement in 20 (54%) of 37 patients who continued lenalidomide-bortezomib-dexamethasone treatment beyond cycle 8 in the maintenance phase. Twenty-eight (42%) patients proceeded to ASCT. Among these patients, best responses to lenalidomide-bortezomib-dexamethasone treatment before undergoing ASCT were 6 (21%) CR + nCR, 10 (36%) VGPR, and 12 (43%) PR.
Rates of VGPR or better according to disease stage, baseline characteristics, and the presence of baseline cytogenetic abnormalities, including those associated with high risk of adverse outcome, are shown in Table 5. Median follow-up is 21 months. Median DOR has not been reached; 68% (95% CI 55%-79%) of patients have remained in response for more than 18 months.
Table 5.
Percentages of patients achieving a best response of VGPR or better to lenalidomide-bortezomib-dexamethasone and estimated 18-month PFS rates after lenalidomide-bortezomib-dexamethasone with/without ASCT, according to disease stage, baseline β2-microglobulin, baseline albumin, and presence of baseline cytogenetic abnormalities
| Characteristic/value | n | Response of VGPR or better, n (%) | Estimated 18-month PFS, % (95% CI) |
|---|---|---|---|
| Baseline characteristic | |||
| ISS disease stage | |||
| I | 29 | 21 (72) | 89 (70-96) |
| II/III | 37 | 23 (62) | 65 (47-78) |
| Durie-Salmon disease stage | |||
| I | 22 | 12 (55) | 82 (59-93) |
| II/III | 44 | 32 (73) | 72 (56-83) |
| β2-microglobulin, mg/L | |||
| < 3.5 | 44 | 32 (73) | 77 (61-87) |
| ≥ 3.5 | 22 | 12 (55) | 73 (49-87) |
| Albumin, g/dL | |||
| < 3.5 | 24 | 17 (71) | 63 (40-78) |
| ≥ 3.5 | 42 | 27 (64) | 83 (67-91) |
| Cytogenetics present | |||
| Abnormal metaphase cytogenetics | |||
| Yes | 6 | 5 (83) | 83 (27-97) |
| No | 60 | 39 (65) | 75 (62-84) |
| Del 13/13q by FISH | |||
| Yes | 24 | 18 (75) | 79 (57-91) |
| No | 27 | 16 (59) | 65 (43-80) |
| Del 17p by FISH | |||
| Yes | 5 | 3 (60) | 100 |
| No | 45 | 30 (67) | 68 (52-80) |
| t(4;14) by FISH | |||
| Yes | 2 | 2 (100) | 100 |
| No | 39 | 24 (62) | 63 (46-77) |
| t(11;14) by FISH | |||
| Yes | 11 | 7 (64) | 73 (37-90) |
| No | 40 | 28 (70) | 72 (55-83) |
| Del 17p and/or t(4;14) by FISH | |||
| Yes | 6 | 4 (67) | 100 |
| No | 44 | 29 (66) | 68 (51-79) |
FISH indicates fluorescence in situ hybridization; ISS, International Staging System; PFS, progression-free survival after lenalidomide-bortezomib-dexamethasone with/without transplantation; and VGPR, very good partial response.
Stem cell harvesting and engraftment
Among the 28 patients who proceeded to ASCT, median collection of CD34+ cells was 5.6 × 106 cells/kg (range, 2.3-20.1 × 106) in 2 (range, 1-5) collections. Median time-to-neutrophil engraftment (absolute neutrophil count > 500/μL for 2 consecutive days) and platelet engraftment (platelets > 20 000/μL for 2 consecutive days without platelet infusion within the previous week) was 12 days (range, 6-22 days) and 17 days (range, 2-25 days), respectively.
Outcomes
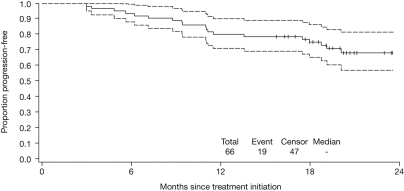
Forty-seven patients are alive and without disease progression; 1 patient with a history of significant coronary artery disease died as the result of cardiac ischemia (at 5.6 months, as described previously), which was considered unrelated to therapy by the treating physician, and 18 patients have since progressed, 3 of whom subsequently died from disease progression (at 17.6, 18.5, and 27.2 months). The estimated 18-month PFS rate after lenalidomide-bortezomib-dexamethasone with/without ASCT is 75% (95% CI 63%-84%; Figure 1). The estimated 18-month PFS rates according to disease stage, baseline characteristics, and the presence of baseline cytogenetic abnormalities are shown in Table 5. The rate appeared lower for patients with International Staging System (ISS) stage II or III (65%) versus stage I disease (89%).
Figure 1.
Kaplan-Meier estimate for PFS among all patients treated with lenalidomide-bortezomib-dexamethasone with/without ASCT (N = 66). Dashed lines indicate 95% CIs.
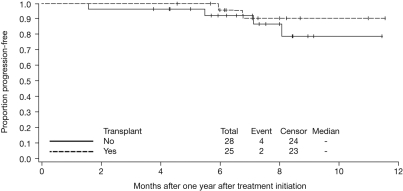
The risk of progression decreased after 12 months; in a post-hoc landmark analysis from 1 year after treatment initiation among 53 patients who had not progressed within 1 year, no difference (P = .38) was detected in PFS according to whether patients received ASCT (Figure 2), with 6 patients having progressed or died. Median OS has not been reached; the estimated 18-month OS rate after lenalidomide-bortezomib-dexamethasone with/without ASCT is 97% (95% CI 88%-99%).
Figure 2.
Kaplan-Meier estimate for PFS according to receipt of ASCT, from 1 year after treatment initiation.
Discussion
This phase 1/2 study, the first prospective investigation of the regimen of lenalidomide-bortezomib-dexamethasone in newly diagnosed MM, has shown the combination to have favorable tolerability during a lengthy period, with no treatment-related mortality. This regimen is the first of its kind to result in a 100% response rate.
The phase 1 portion of the study established the MPD for phase 2 investigation as lenalidomide 25 mg, bortezomib 1.3 mg/m2, and dexamethasone 20 mg. Dexamethasone at a dose of 40 mg proved less well tolerated, with 2 patients in dose level 4 experiencing DLT. Our findings reflect those from a phase 2 study of the same 3-drug regimen in patients with relapsed MM, in which the initial dexamethasone dose of 40 mg was reduced to 20 mg because of toxicity issues,20 and a phase 3 randomized trial of lenalidomide plus high-dose versus low-dose dexamethasone, which demonstrated better tolerability in the low-dose arm.17
The toxicities in our study were generally manageable; AEs reflected those reported with bortezomib plus dexamethasone14,15 and lenalidomide plus dexamethasone17,26 in newly diagnosed MM, and with bortezomib plus lenalidomide in relapsed MM.19 Notably, although 80% of patients reported sensory neuropathy, this was primarily (52%) grade 1, 18 (27%) patients had a grade 2 event, and only 1 (2%) patient had a grade 3 event. In addition, 2 (3%) and 7 (11%) patients had grade 2 motor neuropathy and neuropathic pain, respectively, and only 1 (2%) and 2 (3%) patients had grade 3 events, respectively. Similar rates of grade 3 neuropathic AEs were reported in a phase 2 study of single-agent bortezomib in front-line MM,27 but greater rates of grade 3 sensory neuropathy have been reported with a similar schedule of bortezomib in combination.7,28–30
These findings are in accordance with the low rates of grade 3 or greater neuropathy observed in the majority of other studies of bortezomib and lenalidomide,19,20,31 except for the greater rates seen in the phase 1/2 EVOLUTION (VELCADE in Combination With Other Drugs to Treat Previously Untreated Multiple Myeloma Patients) study.32 Importantly, in the EVOLUTION study, the steroid dosing schedule was different from in the present study, and dexamethasone was not partnered with bortezomib dosing. Interestingly, this difference may therefore have potentially decreased any effect of dexamethasone on reducing the inflammatory component of peripheral neuropathy, which in turn may otherwise at least reduce the severity, if not the overall rate reported, and to which an effect from lenalidomide could also contribute, through its modulation of proinflammatory cytokines.33 The rate of thrombosis in the present study also appeared limited (6%); it was lower than the 12% rate reported in the lenalidomide plus low-dose dexamethasone arm of an Eastern Cooperative Oncology Group phase 3 trial in the front-line setting.17 This result was attributable to the required use of aspirin prophylaxis,34,35 use of low-dose dexamethasone in approximately one half of patients,17,36 and the hypothesized protective effect of coadministration with bortezomib.37,38
All patients achieved at least a PR, with high rates of CR + nCR and VGPR or better. Our data on quality and rate of response according to the presence of adverse cytogenetic abnormalities39 appear consistent with reports from other studies of bortezomib in previously untreated MM7,15,27,28,30,40 and lenalidomide-bortezomib-dexamethasone in relapsed MM,20 in which response rates were unaffected by these cytogenetic abnormalities. Moreover, although no definite conclusions can be drawn from the present study because of the relatively small sample size of patients with adverse cytogenetics, the results are encouraging, especially as preliminary clinical outcome data are promising, with an 18-month PFS rate of 75% and OS rate of 97% after lenalidomide-bortezomib-dexamethasone with/without ASCT overall. As with response rates, our data on PFS rates according to the presence of adverse cytogenetic abnormalities also appear favorable and are consistent with other studies of bortezomib-based therapy in previously untreated MM.7,29
Of note, our post-hoc landmark analysis showed a low risk of progression after 1 year regardless of ASCT status, but with only 6 events having occurred, this must be considered preliminary. Longer follow-up is required for determination of OS and to determine the influence of lengthy lenalidomide-bortezomib-dexamethasone treatment in previously untreated MM patients on the activity of salvage therapies; the OS data will need to be interpreted in the context of the relatively favorable demographics and disease characteristics of the patients enrolled in this study.
It should be noted that the PFS rate appeared lower among patients with advanced (ISS stage II/III) disease, although the proportion of patients with ISS stage III disease at treatment initiation was relatively low, at 9%. Nonetheless, a key issue to be addressed is reducing the risk of progression within 1-year of treatment initiation in patients with advanced (ISS stage II/III) disease, with or without ASCT. Thus, an important area for future exploration is the addition of other novel therapies and conventional cytotoxic agents, including cyclophosphamide or liposomal doxorubicin,12,13,32,41,42 as well as newer agents such as histone deacetylase43–45 and heat shock protein 90 inhibitors,46 which have the potential of not only improving activity by overcoming resistance but also improving the therapeutic index of the combination by reducing toxicity, such as neuropathy.46,47
In conclusion, lenalidomide-bortezomib-dexamethasone is a highly effective regimen for previously untreated MM, and may represent the basis of future standards-of-care in this setting. Phase 3 studies are comparing bortezomib-dexamethasone with or without lenalidomide (NCT00522392) and lenalidomide-dexamethasone with or without bortezomib (NCT00644228) to assess the benefit of the 3-drug approach. An international prospective study is planned to assess this combination with or without ASCT, followed by maintenance.
Acknowledgments
We thank Katherine Redman for administrative assistance and Fangxin Hong and Raffaella Marcheselli for help with data analysis. Funding for this assistance was provided in part by the Rick Corman Multiple Myeloma Research Fund. We gratefully acknowledge the writing support provided by Steve Hill during the development of this publication, which was funded by Millennium Pharmaceuticals Inc, and Johnson & Johnson Pharmaceutical Research & Development LLC. We also gratefully acknowledge the contribution to the study of Thomas Myers, MD, the research nurses, pharmacists, research coordinators, and, most importantly, the participation of the patients and their families.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.G.R., E.W., T.H., C.S.M., R.K., D.-L.E., and K.C.A. designed the research; P.G.R., S.L., A.J.J., S.J., N.S.R., D.E.A., I.M.G., R.L.S., A.M., N.C.M., D.H.V., R.J., J.L.K., D.D., C.D., and K.C.A. performed the research; D.L.W., L.E.L., and S.K. collected and assembled the data; P.G.R., E.W., W.X., and C.S.M. analyzed and interpreted the data; E.W. and W.X. performed statistical analyses; P.G.R., E.W., and K.C.A. wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: P.G.R.: advisory boards for Millennium Pharmaceuticals, Celgene, and Johnson & Johnson group companies. S.L.: consultancy/advisory committee for and research funding from Millennium Pharmaceuticals, Celgene, Bristol-Myers Squibb, and Novartis. A.J.J.: consultancy/member of speakers' bureau/advisory committee for and received honoraria from Millennium Pharmaceuticals, Celgene, Exelixis, Bristol-Myers Squibb, and Centocor OrthoBiotech. S.J.: honoraria from Millennium Pharmaceuticals, Celgene, and Merck. N.S.R.: consultancy for Celgene, Novartis, and Amgen; member of speakers' bureau for, and honoraria from, Celgene, Millennium Pharmaceuticals, and Novartis; and research funding from AstraZeneca. D.E.A.: consultancy for and honoraria from Millennium Pharmaceuticals. I.M.G.: consultancy, member of speaker bureaus for, and honoraria from Millennium Pharmaceuticals, Celgene, and Novartis, and research funding from Millennium Pharmaceuticals. R.L.S.: consultancy for Celgene and honoraria from Celgene and Millennium Pharmaceuticals. A.M.: member of speakers' bureau/advisory committee for and honoraria from Millennium Pharmaceuticals and Celgene and research funding from Millennium Pharmaceuticals. N.C.M.: consultancy for and honoraria from Millennium Pharmaceuticals, Celgene, and Novartis. D.H.V.: member of speakers' bureau/advisory committee for Celgene and Millennium Pharmaceuticals. J.L.K.: consultancy for and honoraria and research funding from Millennium Pharmaceuticals and Celgene. D.D.: member of speakers' bureau/advisory committee for and honoraria from Millennium Pharmaceuticals. T.H.: consultancy for Biotest AG. C.S.M.: consultancy for and honoraria from Millennium Pharmaceuticals, Novartis, Pharmion, and Centocor. R.K.: employee of and equity ownership in Celgene. D.-L.E.: employee of Millennium Pharmaceuticals and equity ownership in Millennium/Takeda and Johnson & Johnson. K.C.A.: consultancy for and research funding from Millennium Pharmaceuticals, Celgene, and Novartis.
Correspondence: Dr Paul G. Richardson, Dana-Farber Cancer Institute, 44 Binney St, Dana 1B02, Boston, MA 02115; e-mail: paul_richardson@dfci.harvard.edu.
References
- 1.Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114(15):3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Leong T, Li S, et al. Complete response in multiple myeloma: clinical trial E9486, an Eastern Cooperative Oncology Group study not involving stem cell transplantation. Cancer. 2006;106(9):1958–1966. doi: 10.1002/cncr.21804. [DOI] [PubMed] [Google Scholar]
- 3.van de Velde H, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 4.Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia. 2009;23(6):1152–1157. doi: 10.1038/leu.2008.402. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23(3):449–456. doi: 10.1038/leu.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 10.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076. [PubMed] [Google Scholar]
- 12.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99(12):4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 14.Harousseau J-L, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91(11):1498–1505. [PubMed] [Google Scholar]
- 15.Harousseau JL, Mathiot C, Attal M, et al. Bortezomib/dexamethasone versus VAD as induction prior to autologous stem cell transplantation (ASCT) in previously untreated multiple myeloma (MM): updated data from IFM 2005/01 trial. J Clin Oncol. 2008;26(suppl):455s. Abstract 8505. [Google Scholar]
- 16.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82(10):1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zonder JA, Crowley JJ, Bolejack V, et al. A randomized Southwest Oncology Group study comparing dexamethasone (D) to lenalidomide + dexamethasone (LD) as treatment of newly-diagnosed multiple myeloma (NDMM): impact of cytogenetic abnormalities on efficacy of LD, and updated overall study results. J Clin Oncol. 2008;26:159s. Abstract 8521. [Google Scholar]
- 19.Richardson PG, Weller E, Jagannath S, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27(34):5713–5719. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson KC, Jagannath S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma (MM): encouraging outcomes and tolerability in a phase II study. J Clin Oncol. 2009;27(suppl):442s. Abstract 8536. [Google Scholar]
- 21.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 23.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 25.Collett D. Modelling Survival Data in Medical Research. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 26.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27(21):3518–3525. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonneveld P, van der Holt B, Schmidt-Wolf IGH, et al. First analysis of HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, adriamycin, dexamethasone (PAD) vs VAD as induction treatment prior to high dose melphalan (HDM) in patients with newly diagnosed multiple myeloma (MM). Haematologica. 2009;94(suppl):191. Abstract 0473. [Google Scholar]
- 29.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib, melphalan, prednisone and thalidomide (VMPT) followed by maintenance with bortezomib and thalidomide for initial treatment of elderly multiple myeloma patients. Blood (ASH Annual Meeting Abstracts) 2009;114(suppl):58a. Abstract 128. [Google Scholar]
- 30.Rosiñol L, Cibeira MT, Martinez J, et al. Thalidomide/dexamethasone (TD) vs. bortezomib (Velcade)/thalidomide/dexamethasone (VTD) vs. VBMCP/VBAD/Velcade as induction regimens prior autologous stem cell transplantation (ASCT) in multiple myeloma (MM): results of a phase III PETHEMA/GEM trial. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):59a. Abstract 130. [Google Scholar]
- 31.Wang M, Delasalle K, Giralt S, Alexanian R. Rapid control of previously untreated multiple myeloma with bortezomib-lenalidomide-dexamethasone (BLD). Blood (ASH Annual Meeting Abstracts) 2007;110(11 suppl):1057a. doi: 10.1179/102453310X12583347010133. Abstract 3611. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Flinn IW, Hari PN, et al. Novel three- and four-drug combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for newly diagnosed multiple myeloma: encouraging results from the multi-center, randomized, phase 2 EVOLUTION study. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):57a–58a. Abstract 127. [Google Scholar]
- 33.Richardson PG, Bruna J, Amato AA, et al. Bortezomib-associated peripheral neuropathy: relationship between clinical neurophysiologic evidence in previously untreated multiple myeloma patients and preclinical characterization in a mouse model. Blood (ASH Annual Meeting Abstracts) 2009;114(suppl 22):1485a. Abstract 3860. [Google Scholar]
- 34.Zonder JA, Barlogie B, Durie BG, et al. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: benefit of aspirin prophylaxis. Blood. 2006;108(1):403. doi: 10.1182/blood-2006-01-0154. [DOI] [PubMed] [Google Scholar]
- 35.Niesvizky R, Martinez-Banos D, Jalbrzikowski J, et al. Prophylactic low-dose aspirin is effective antithrombotic therapy for combination treatments of thalidomide or lenalidomide in myeloma. Leuk Lymphoma. 2007;48(12):2330–2337. doi: 10.1080/10428190701647887. [DOI] [PubMed] [Google Scholar]
- 36.Menon SP, Rajkumar SV, Lacy M, Falco P, Palumbo A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer. 2008;112(7):1522–1528. doi: 10.1002/cncr.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonial S, Richardson PG, San MJ, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol. 2008;143(2):222–229. doi: 10.1111/j.1365-2141.2008.07321.x. [DOI] [PubMed] [Google Scholar]
- 38.Zangari M, Fink LM, Zhan F, Tricot GJ. Bortezomib does not increase thromboembolic risk in multiple myeloma and may offer a protective effect with thalidomide/lenalidomide-based therapy: review of data from phase 3 trials and studies of novel combination regimens. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):743a–744a. Abstract 1873. [Google Scholar]
- 39.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 40.Harousseau J-L, Avet-Loiseau H, Attal M, et al. High complete and very good partial response rates with bortezomib—dexamethasone as induction prior to ASCT in newly diagnosed patients with high-risk myeloma: results of the IFM2005-01 phase 3 trial. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):149a–150a. Abstract 353. [Google Scholar]
- 41.Kumar S, Flinn I, Noga S, et al. Bortezomib, dexamethasone, cyclophosphamide, lenalidomide (VDCR) has high efficacy in frontline MM. Clin Lymphoma Myeloma. 2009;9(suppl):S43–S44. Abstract 247. [Google Scholar]
- 42.Jakubowiak AJ, Reece DE, Hofmeister CC, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: updated results of phase I/II MMRC trial. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):60a. doi: 10.1182/blood-2011-02-334755. Abstract 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.San-Miguel JF, Sezer O, Siegel D, et al. A phase IB, multi-center, open-label dose-escalation study of oral panobinostat (LBH589) and I.V. bortezomib in patients with relapsed multiple myeloma. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):1481a–1482a. Abstract 3852. [Google Scholar]
- 44.Siegel D, Jagannath S, Lonial S, et al. Update on the phase IIb, open-label study of vorinostat in combination with bortezomib in patients with relapsed and refractory multiple myeloma. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):1497a. Abstract 3890. [Google Scholar]
- 45.Siegel D, Weber DM, Mitsiades CS, et al. Combined vorinostat, lenalidomide and dexamethasone therapy in patients with relapsed or refractory multiple myeloma: a phase I study. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):129a. Abstract 305. [Google Scholar]
- 46.Badros AZ, Richardson PG, Albitar M, et al. Tanespimycin + bortezomib in relapsed/refractory myeloma patients: results from the Time-2 study. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):742a–743a. Abstract 1871. [Google Scholar]
- 47.Richardson PG, Chanan-Khan AA, Lonial S, et al. Tanespimycin + bortezomib demonstrates safety, activity, and effective target inhibition in relapsed/refractory myeloma patients: updated results of a phase 1/2 study. Blood (ASH Annual Meeting Abstracts) 2009;114(22 suppl):1128a–1129a. Abstract 2890. [Google Scholar]