Abstract
Increasingly, invitro culture of adherent cell types utilizes three-dimensional (3D) scaffolds or aggregate culture strategies to mimic tissue-like, microenvironmental conditions. In parallel, new flow cytometry-based technologies are emerging to accurately analyze the composition and function of these microtissues (i.e., large particles) in a non-invasive and high-throughput way. Lacking, however, is an accessible platform that can be used to effectively sort or purify large particles based on analysis parameters. Here we describe a microfluidic-based, electromechanical approach to sort large particles. Specifically, sheath-less asymmetric curving channels were employed to separate and hydrodynamically focus particles to be analyzed and subsequently sorted. This design was developed and characterized based on wall shear stress, tortuosity of the flow path, vorticity of the fluid in the channel, sorting efficiency and enrichment ratio. The large particle sorting device was capable of purifying fluorescently labelled embryoid bodies (EBs) from unlabelled EBs with an efficiency of 87.3% ± 13.5%, and enrichment ratio of 12.2 ± 8.4 (n = 8), while preserving cell viability, differentiation potential, and long-term function.
INTRODUCTION
Adherent cell types require spatial and temporal cues from the surrounding microenvironment to maintain proper function.1, 2, 3, 4 Thus, when adherent cell types are removed from an organism and propagated invitro they are often cultured as cell aggregates (e.g., cardiospheres, neurospheres, tumor spheroids, and embryoid bodies (EBs)). In addition, biomaterial approaches have been developed to generate surrogate, 3D matrices to house extracted cells with the goal of preserving cell function.5, 6, 7, 8, 9 Analysis of cell populations within aggregates or 3D matrices had been limited to low-throughput technologies, which typically require destruction of the sample (e.g., immunofluorescent staining and imaging, western blot, polymerase chain reaction (PCR)). However, newer, flow cytometry-based technologies are emerging that can rapidly and accurately analyze the composition and function of large particles (∼50–1000 μm) without compromising cell viability.10, 11 Still needed are accessible platforms for purification or sorting of large particles based on analysis parameters. Purification of cell aggregates or 3D engineered tissues would be beneficial for many basic biomedical research and clinical applications; examples include, enabling large-scale screens of 3D phenotypes and transplantation of more refined, therapeutically beneficial cell fractions.
For several decades, single cells have been efficiently purified using fluidic sorting techniques, however, purification of intact cellular aggregates or 3D microtissues (i.e., large particles, >100 μm) has been difficult. Purification of large particles requires a coordinated interplay between controlled particle delivery, detection of particles or attributes of particles, and subsequent movement of particles based on those detection parameters. Common cell separation techniques such as magnetic activated cell sorting (MACS) and affinity chromatography columns rely on antibodies or capture molecules to sort cells. Typically, size exclusion prohibits antibodies or other capture molecules from penetrating the center of large particles, which limits labeling to cells at or near the surface. Consequently, purification is based on characteristics of cells at or near the surface and does not necessarily accurately reflect the characteristics of the entire particle. For these reasons, sorting of large particles has been restricted to characteristics derived from optical-based, cellular resolution detection of basic extrinsic cues (i.e., cell permeant dyes12), genetically modified cell lines expressing fluorescent reporters or intrinsic properties (i.e., size, shape, autofluorescence).
The most common form of optical-based, particle sorting is fluorescence activated cell sorting (FACS). FACS is accomplished by applying a positive or negative charge to a droplet containing a single particle (e.g., cell) based on selected optical parameters. The particle-containing droplet is subsequently deflected by an electric field based on its inherited charge. However, the conventional, charged-based sorting used in commercial FACS machines has inherent limitations as particle size increases. Particle-induced surface disturbances can alter the predictability of droplet formations and this effect is amplified as particle size increases.13 In addition, the mass increase associated with increased size, decreases the angle of deflection induced by the electric field and thus greatly increases the distance required to separate particles.14 Any slight deviation in droplet trajectory (such as cell-induced surface disturbances) makes it difficult to predict particle position.14 Thus, charged-based droplet sorting is not a viable option for large particle sorting.
Currently, the only commercially available, large particle FACS system is the COPAS BioSorter (Union Biometric, Holliston, MA).14 This instrument is capable of measuring multiple parameters on each large particle including time of flight (size), extinction coefficient (optical density), and fluorescence parameters. The instrument is also capable of analyzing particles ranging from 10 to 1500 μm in diameter, by employing four different flow cells with varying inner bore diameters. The sorting mechanism is pneumatic and consists of a solenoid valve located outside the flow cell that is air pressurized and actuated (opened) in response to defined optical parameters. Unwanted particles are diverted into a waste container, while desired particles move freely through the system and are ultimately dispensed into microtiter plates or collection tubes. The system is a tremendous advance for sorting large particles, but is limited in that it employs conventional single photon optics and so cannot discern optical attributes of cells deep (> ∼ 50 μm) within an intact aggregate. In addition, the closed nature of the system limits the accessibility and flexibility desired for many basic research applications.
Microfluidic platforms have been utilized for several years to improve efficiency, accessibility, and flexibility of systems designed to purify small particles15, 16, 17, 18, 19 and have been utilized more recently to purify large particles based on particle size.20, 21 In particular, a microfluidic 1 mm glass capillary sorting mechanism was developed using diode laser bars to optically trap or deflect particles up to 200 μm in diameter.20 Theoretically, different size particles can be sorted by size into different compartments based on the supplied laser power.20 However, since the length of the laser bar must increase to accommodate increasingly large particles, practical application of this approach to particles larger than 400 μm is limited. In addition, the impact of required laser power on the viability and function of biologic particles in this system is unknown. Another approach utilizes a physical separation scheme to sort large particles based on size in a microfluidic device.21 In this case, mouse EBs could be separated into three size groups without compromised viability.
Future improvements to purify large particles would include sorting based on optical detection of fluorescent labels or autofluorescent molecules of cells of large particles. Microfluidic technology is a promising approach for optical-based, large particle sorting because (1) the microfluidic flow cell is inexpensive and fabrication is accessible to many research laboratories and (2) the flow cell can easily be added to a wide range of microscopy systems. We are particularly interested in developing a sorting system that can be coupled to our recently developed, enhanced-throughput multiphoton flow cytometry (MPFC) system which is capable of deep optical penetration of large aggregates in the context of flow.10 In this study, we focus on a microfluidic-based, electromechanical device for sorting large particles (i.e., large particle sorting device, LaPSD) that has the modularity and versatility needed in order to be coupled with our MPFC system and other custom and commercial microscopy platforms. This new device is capable of efficient large particle sorting while maintaining cell viability and long-term function.
MATERIALS AND METHODS
Device description
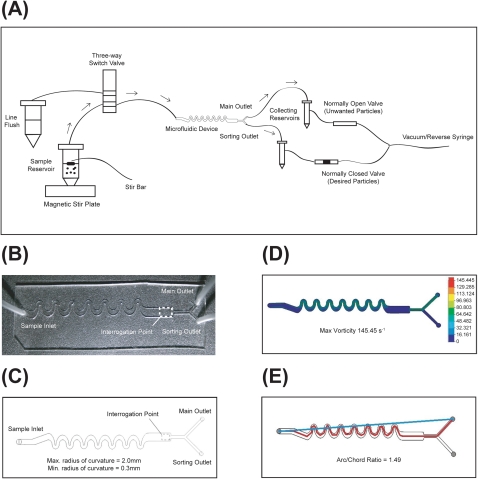
The LaPSD consists of a single sample input port and two output ports (main and sorting) (Figures 1a, 1b, 1c). Prior to entering the device, particles are gently and continuously mixed in a 50 ml conical tube to ensure homogeneous delivery of particles to the flow cell. Particles are drawn through the flow cell via a software controlled syringe pump in reverse mode coupled to the outlet ports (described below). Once delivered to the flow cell, particles are subjected to asymmetric curving channels that use hydrodynamic forces to self-focus and separate particles.22 This approach has been used successfully for analyzing small particles. The design reduces reagent consumption, reduces the complexity of the setup, and allows for higher throughput. We have modified the design to accommodate large particles, based on practical design rules for inertial focusing previously described.22 The height of the channel is 1 mm, the typical flow rates utilized range between 400–1000 μl/min (adjusted to obtain a particle speed between 1.5 and 2.0 mm/s), the length of the channel is 6.6 cm, and maximum and minimum radius of curvature of the curving channels are 2 mm and 0.3 mm, respectively. Theoretically, these flow rates and dimension parameters will accommodate particles in the range of 140 μm to 500 μm.23 The maximum particle size is limited only by the height of the channel and thus larger particles could be accommodated with altered channel dimensions. The procession of particles passes the optical interrogation point and upon optical detection of a desired event, sorting is triggered. Actuation of sorting is delayed to allow the particle to reach the outlet bifurcation. At the time of actuation, the main outlet closes and the sorting outlet opens (1–3 s, time delay; 0.1–1.0 s, actuation time). Main and sorting outlets are opened and closed via software-controlled microvalves that are linked to the syringe pump by way of an in-line y-connector. After sorting, the sorting outlet closes and main outlet opens. Sorted and unsorted particles are collected in reservoirs coupled to the fluidic lines prior to the microvalves to avoid clogging of the valves. The collection reservoirs are 15 ml conical tubes, which are filled with 1X phosphate buffered saline (PBS) to maintain driving pressure by avoiding air compression. A software controlled three-way solenoid pinch valve (Cole Parmer, Vernon Hills, IL) is used to rinse the flow cell and fluidic lines between large particle samples.
Figure 1.
Characterization of the LaPSD. (a) Schematic depicting the particle flow path. Black arrows denote particle path during main flow. Dotted arrows denote particle path during a sorting event. (b) Top view of the LaPSD design consisting of one inlet port, an interrogation region, a main outlet port, and a sorting outlet port. (c) Photograph depicting the fabricated LaPSD device, formed by bonding PDMS to glass cover slip. (d) 2D colorimetric vorticity plot of the LaPSD. The maximum vorticity during main flow (analogous to sorting flow) is 145 s−1. (e) Tortuosity of the LaPSD as defined by the arc to chord ratio is 1.49.
Sorting efficiency
Two separate polystyrene bead mixtures were employed to test the sorting efficiency of the LaPSD. For solution 1, the initial bead population consisted of ∼16% fluorescent beads and ∼84% nonfluorescent beads, for solution 2 the initial bead population consisted of ∼31% fluorescent beads and ∼67% nonfluorescent beads. Both solutions were prepared by mixing fluorescent polystyrene beads with non-fluorescent polystyrene beads measuring 160 μm in nominal diameter (Duke Scientific Corporation, Palo Alta, CA). Aliquots of solution 1 were counted with an Axiovert 40 CFL inverted fluorescence microscope (Zeiss, Thornwood, NY) and the concentration of fluorescent beads was determined to be 16% ± 11%. The total concentration of beads of solution 1 was determined to be 18 ± 5 beads/ml. Aliquots of solution 2 were also counted and the concentration of fluorescent beads determined to be 31% ± 3%. The total concentration of beads of solution 2 was 16 ± 5 beads/ml. Solutions were loaded into the sample reservoir of the LaPSD and the LaPSD was positioned on the stage to correspond to the interrogation point of a multiphoton microscopy system as previously described.10 Fluorescence levels of beads were determined using wiscscan acquisition software33 of the MPFC system with the following software and hardware modifications to accommodate electromechanical switching mechanisms required for sorting. In particular, particle size and intensity parameters were set to identify “positive” events. When positive events were detected, the software sent a 5 V signal to the data acquisition (DAQ) card (National Instruments, Austin, TX), which rapidly (0.3 ms) switched the microvalve state. A user defined positive event cued both outputs to switch states for a specified amount of time (actuation time). The time-delay functionality allowed a user to delay the switch of channel state up to 3 s (with millisecond discrimination) after optical interrogation. After sorting, beads were collected from sorting or main flow outlets and counted on a fluorescence microscope. Sorting efficiency or purity was defined as the number of desired particles divided by the total number of particles in the sorting outlet reservoir. To account for the initial concentrations of desired particles, we also report an enrichment ratio defined as the ratio of desired particles to undesired particles in the sorting outlet reservoir divided by the ratio of desired particles to undesired particles introduced into the sample inlet (determined by counting particles collected in both outlet ports, Table TABLE I.24). The average sorting efficiency and the average enrichment ratio were established by first determining the efficiency or enrichment per trial and then calculating the average.
TABLE I.
Sorting efficiency and enrichment ratio of large beads and EBs.
| Large beads | EBs | |||||
|---|---|---|---|---|---|---|
| Trial | Input concentration(% fluorescent particle of total) | Sorting efficiency(output concentration; % fluorescent particle of total) | Enrichment ratio | Input concentration(% fluorescent particle of total) | Sorting efficiency(output concentration; % fluorescent particle of total) | Enrichment ratio |
| 1 | 21 | 100 | 44.5 | 31 | 68 | 4.7 |
| 3 | 11 | 85 | 42.5 | 39 | 94 | 25.3 |
| 4 | 12 | 82 | 33.9 | 33 | 100 | 6.0 |
| 5 | 32 | 79 | 8.3 | 29 | 68 | 5.1 |
| 6 | 34 | 86 | 11.4 | 33 | 100 | 14.0 |
| 7 | 29 | 85 | 13.6 | 41 | 86 | 8.5 |
| 8 | 28 | 69 | 5.7 | 33 | 82 | 9.2 |
Preparation of cell aggregates
HM1 mouse embryonic stem cells (Open Biosystems, Huntsville, AL) were used to generate EBs as previously described.10 Briefly, EBs were left in hanging drops for 3 days and then introduced into the microfluidic device. Approximately one third of all harvested EBs were stained using CellTrackerTM green (Molecular Probes, Eugene, OR). Briefly, EBs were rinsed in 1X PBS, and next incubated for 30 min at 37 °C and 5% CO2 in cell culture medium without fetal bovine serum (FBS), containing 5 μM CellTrackerTM green (Molecular Probes). Stained EBs were mixed with unstained EBs to yield an EB suspension with ∼33% of EBs fluorescently labeled. This solution was loaded into the sample reservoir of the LaPSD. “Positive particles” were defined as those with fluorescent label and the software was set to actuate with detection of a positive event. Sorting efficiency and enrichment ratio were determined as defined above for sorting of fluorescent beads (Table TABLE I.).
Cell aggregate viability and functional analysis
EBs were generated as described above10 and were left in hanging drops for 3 days. EBs were then harvested and stained using CellTrackerTM green (Molecular Probes, Eugene, OR). Briefly, EBs were rinsed in 1X PBS, and next incubated for 30 min at 37 °C and 5% CO2 in cell culture medium without FBS, containing 5 μM CellTrackerTM green (Molecular Probes). Stained EBs were introduced into the LaPSD, optically scanned using multiphoton excitation and retrieved in the main outlet port. Collected EBs were counted, assessed for changes in morphology, and subsequently plated on culture surfaces to assess attachment and ability to form areas of spontaneous beating indicative of cardiomyocyte function.
Statistical analysis
For comparison of EB attachment, cardiomyocyte function in EBs with and without introduction into the LaPSD and for comparison of sorting efficiency and enrichment between devices and between types of large particles (i.e., beads and EBs), a normal distribution was assumed and one-way analyses of variance (anova) and Student t-test were used. Data were reported as average ± standard deviation and were analyzed with jmp 5.0.1 for Windows (SAS Institute, Inc., Carey, NC). A 99% confidence (P <0.01) interval was applied for statistical significance.
RESULTS
Fluid dynamic profiles of the LaPSD
To begin to characterize the fluid microenvironment experienced by large particles in the LaPSD, wall shear stress incurred by the fluid in the LaPSD was determined. Newtonian fluids moving along a solid wall of a microfluidic channel will sustain a shear stress near the wall surface that is proportional to the viscosity of the fluid and flow velocity and inversely proportional to the channel height. Wall shear stress has long been correlated to viability of cells that adhere to the wall surface25 and cells that are suspended in flow channels.26, 27 The level of wall shear stress found to cause death of adherent cell types (0.1–1 Pa)25 is much lower than that found to cause death of suspended cell types (100–500 Pa).26, 27 This is not surprising since cells in suspension rarely and transiently occupy the space near the wall (especially in this case in which suspended particles are hydrodynamically focused to the channel center-line) and so do not experience the maximum shear stress of a channel. The maximum wall shear stress of the microfluidic sorting device is 0.08 Pa, which is two orders of magnitude lower that the wall shear stress previously found to induce death of cells in suspension.26, 27 However, large particles are prone to rolling along the base of the channel if they are not hydrodynamically focused in the z plane. In this case, wall-induced lift forces, created by squeezing of fluid between the sphere and the wall during contact, tend to increase drag in the direction of motion.28 In addition, large particles tend to impact on each other at dense particle concentrations.28 Although relatively dilute particle concentrations were used in these studies, particle-particle interactions are still visible. A phenomenon known as drafting, kissing, and tumbling (DKT) is the dominant interaction of particles in the LaPSD.29 This interaction can directly impact on the viability and functional capacity of the cells of large particles and typically occurs in channel geometries that impose a high degree of vorticity and/or tortuosity. Thus these parameters were assessed in greater detail. (Additional microfluidic device designs were considered and these were evaluated as well, see Ref. 30.)
Vorticity is a local measure of fluid rotation with respect to adjacent fluid. Solidworks software (Solidworks Corp, Concord, MA) models vorticity by meshing a 3D volume of fluid into small voxels and computing vorticity for each voxel. Vorticity is a vector and has velocity component and rotational components. Vorticity calculations for the device were conducted using Solidworks Flow Simulation Software (Dassault Systemes, SolidWorks Corp, Concord, MA). The contour plot (Figure 1d) reports the vector as a positive number in rad/s (or s−1). The contour plot shown in Figure 1d is a 2D section of the 3D fluid volume in the LaPSD. These sections are located at the mid-height of the channel. The fluid in the LaPSD exhibits a relatively high level of vorticity during main flow and with sorting (145 s−1), but not substantially different from alternative device designs known to maintain cell viability10 (See Ref. 30).
Tortuosity is a measure of the divergence of a path from a straight line and is defined here as the ratio of the length of the particle path to the length of the straight-line path from the particle inlet to the particle outlet (i.e., arc/chord ratio). A depiction of the device showing the particle path and the straight line path is shown in Figure 1e. The arc/chord ratio of the LaPSD was 1.49 and so substantially higher than alternative device designs known to preserve viability. Given the increased tortuosity of the microfluidic sorting device and the propensity for fluid dynamic forces to compromise cell viability by interrupting cell-cell and cell-matrix interactions and/or by damaging cell membranes, we sought to test the viability of cells of large particles after transit through the LaPSD.
Viability and function of stem cells of large aggregates after flow through the LaPSD
To test whether the additional forces imposed by the LaPSD, due to increased wall shear stress, vorticity and tortuosity would compromise viability of cell aggregates, we introduced fluorescently labelled, stem cell aggregates (i.e., EBs; n = 21, 28, 32 in each of three separate experiments) into the sample inlet at a maximum volumetric flow rate of 800 μl/min. EBs were optically detected at the interrogation point and then collected and counted at the main outlet. It was determined that 92% ± 8% of EBs were recovered and of those EBs recovered, 97% ± 3% attached to gelatin-coated culture plates. This percentage was not statistically different from attachment of control EBs (98% ± 2%, P = 0.60, Figure 2). The stem cell line used to generate the EBs has a strong capacity for forming spontaneously beating areas (indicative of cardiomyocyte differentiation) when exposed to qualified serum. Thus, we counted the number of EBs that gave rise to beating areas after flow through the device compared to controls (no flow). The capacity to form beating areas by day 8 of differentiation (5 days post plating) in EBs after flow through the LaPSD (16% ± 3%) was not statistically different from control EBs (14% ± 2%, P = 0.33, Figure 2). Thus EB attachment and differentiation potential did not differ between these groups suggesting flow through the LaPSD did not alter the viability and ultimate function of stem cells of large aggregates.
Figure 2.
Viability and long-term function of stem cell aggregates following flow through the LaPSD. Fluorescently labelled EBs were introduced into the device through the sample inlet, optically interrogated and removed via the main outlet. After removal, EBs were placed in culture plates and the number of EBs that attached were counted and reported as a percentage of the total number of EBs plated compared to control EBs that were generated at the same time, but placed immediately in static culture dishes. In addition, EBs were tracked over time and the percentage of EBs with functional cardiomyocytes (i.e., beating areas) was determined. Attachment and beating functionality were not significantly altered with flow through the LaPSD compared to controls (P = 0.60 and 0.33, respectively). Error bars correspond to standard deviation from the mean (enhanced online) .
Sorting efficiency of the LaPSD
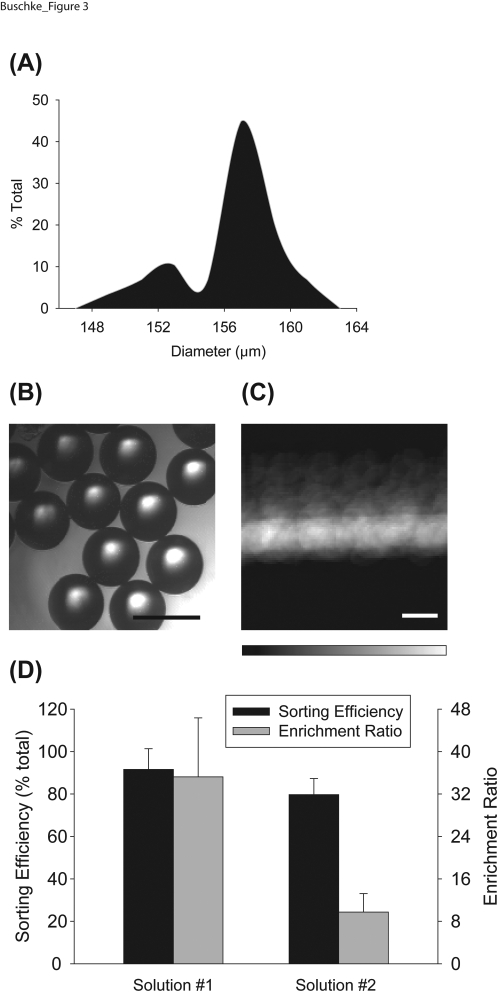
To test the ability of the LaPSD to purify populations of large particles, we introduced bead populations with a known fraction of fluorescent and non-fluorescent beads. The size distribution of the polystyrene beads, based on specifications of the manufacturer, was 158 ± 3 μm, with a coefficient of variation of 2.2%. We measured the cross sectional area of the beads using the oval tool in imagej,31 in a similar fashion to measurement of cell aggregate size, and determined the average diameter to be 157 ± 3 μm, with a coefficient of variation of 1.9%. The measured bead diameters were displayed in a histogram to demonstrate the relative uniformity of the samples (Figures 3a, 3b). The valve corresponding to the sorting inlet was actuated upon detection of a fluorescent bead and the final fraction of fluorescent particles in the sorting outlet was reported as the sorting efficiency (“positive” selection) and was the concentration used to determine the enrichment ratio. Sorting efficiencies of 92% ± 10% (solution 1, n = 4 trials) and 80% ± 8% (solution 2, n = 4 trials) were attained using the LaPSD. Moreover, enrichment ratios of 35.2 ± 11.1 (for solution 1) and 9.7 ± 3.5 (for solution 2) were achieved (Figure 3d).
Figure 3.
Sorting efficiency and enrichment ratio of polystyrene beads. (a) Histogram depicting the distribution of bead diameters in a representative fraction of fluorescent and non-fluorescent polystyrene beads. Measured bead diameter (157 ± 3 μm) did not differ statistically from manufacturer’s specifications (158 ± 3 μm). (b) Brightfield image of polystyrene beads. Scale bar = 200 μm. (c) Summation of temporal image series of beads flowing through the LaPSD. Fluorescence intensity images of beads were taken every 0.2 s and ∼3000 images were merged to create the image shown. The minimal deviation of fluorescence intensity from the center sample stream line conveys the ability of the LaPSD to effectively focus particles with uniform size. Scale bar = 200 μm. (d) Sorting efficiency and enrichment ratio of bead populations consisting of fluorescent and nonfluorescent beads. Solution 1 attained significantly higher enrichment ratios due to a low starting concentration of fluorescent beads; this result demonstrates the ability of the LaPSD to effectively purify particles from relatively dilute starting concentrations akin to rare cell populations. Error bars correspond to standard deviation from the mean.
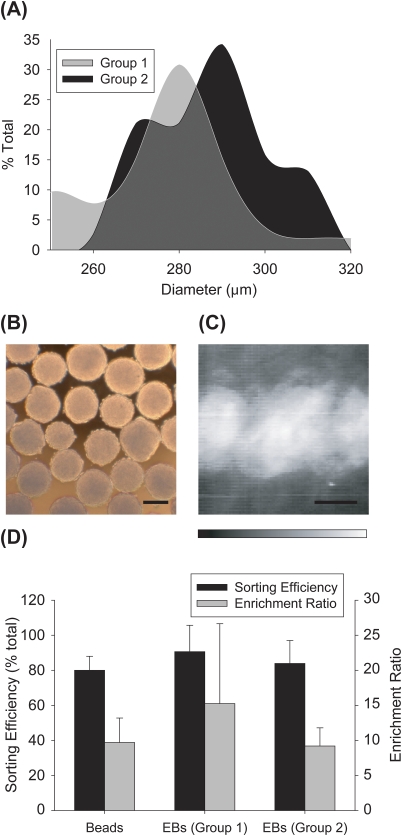
To test the ability of the LaPSD to purify populations of large cellular aggregates, we generated a mixed suspension of fluorescently labelled and unlabelled EBs (33% fluorescent:67% nonfluorescent, similar to bead solution 2 above). Two separate EB suspensions (or groups) were generated in this way and prior to each trial (n = 4 trials for each group, n = ∼ 75 EBs per trial) the relative size distribution of EBs was determined (group 1, mean diameter = 247 ± 20 μm; group 2, mean diameter = 295 ± 12 μm) (Figures 4a, 4b). Two separate EB groups were used to test the ability of the instrument to efficiently sort various size ranges of particles with inherent differences in particle velocities (Figure 4c). Sorting efficiency obtained for group 1 was 91% ± 15% and group 2 was 84% ± 13%. Group 1 EBs had an enrichment ratio of 15.2 ± 11.4 and group 2 EBs had an enrichment ratio of 9.2 ± 2.6 (Figure 4d). Sorting efficiencies and enrichment ratios of both groups of EBs were statistically similar to that of bead sorting results with similar starting concentrations of fluorescent particles (i.e., bead solution 2 compared to the 33% EB solution).
Figure 4.
Sorting efficiency and enrichment ratio of embryoid bodies. (a) Histograms depicting measured EB diameters for two separate groups of EBs. Measured mean EB diameters were 247 ± 20 μm for group 1, and 295 ± 12 μm for group 2. (b) Brightfield images of EBs. Scale bar = 200 μm. (c) Summation of temporal image series of EBs flowing through the LaPSD. Fluorescence intensity images of EBs were taken every 0.2 s and ∼2000 images were merged to create the image shown. The deviation of fluorescence intensity from the center sample stream line is more diffuse than that observed for beads reflecting the higher variability of size of EBs and therefore greater perturbation of flow. Scale bar = 200 μm. (d) Sorting efficiency and enrichment ratio of EB groups compared to that of bead solution 2. There was no significant difference between sorting efficiency or enrichment ratios when comparing beads to either group of EBs. Error bars correspond to standard deviation from the mean.
DISCUSSION
Motivated by the growing need to purify populations of large cellular aggregates or microtissues, a new microfluidic, electromechanical, enhanced-throughput sorting device, termed LaPSD, was designed and evaluated based on its ability to preserve cell health and yield high sorting efficiencies. Device optimization was driven by the primary objective of our laboratory, to assess all cells of a stem cell aggregate (even those at the very center of the aggregate) in an enhanced-throughput way and to purify aggregate populations based on this assessment. Several other applications can be envisioned which would benefit from the ability to rapidly purify large particles in the fields of basic cell biology and clinical therapy.
The LaPSD was designed with several important features to promote ease of use and elevated sorting efficiency. First, the asymmetric curving channel allowed for sheath-less particle focusing. Elimination of sheath fluid was important because it decreased the lateral distance required to divert a particle upon selection. When attempting to divert particles across the sheath stream (see alternative designs30), trailing particles were often diverted and so increased the percentage of contaminating or unwanted particles in the sorting outlet/reservoir. In addition, disrupted sheath flow could alter the flow path of the sample stream and thereby push subsequent particles outside of the interrogation region. In this case, wanted particles were often missed. Second, a time delay was incorporated between the time of optical detection and the time of actuation of the sorting valve to improve sorting efficiency. The time delay was necessary because channel dimensions at the point of optical interrogation (distal to the asymmetric curving channel) were increased three fold to effectively decelerate particle velocity to accommodate the relatively slow scan speeds (∼5 frames/s) of the MPFC system. Channel dimensions were subsequently decreased to accelerate particle velocity and decrease the volume of fluid that would change direction during a sorting pulse. In addition, a 5 mm separation distance from the interrogation region to the sorting bifurcation ensured that a sorting pulse would not alter the flow path of particles at the optical interrogation point. Third, the LaPSD functions without an additional pressure source to divert the particles, thereby reducing unnecessary stress that may be harmful to the structure of cellular aggregates. Instead, particles are diverted by simply switching the direction of the particle path while maintaining a uniform internal pressure within the microfluidic device and consistent particle positioning along natural streamlines. The exclusion of a pressure source to divert particles also reduces manufacturing complexity, reduces volumes of reagents needed and improves accessibility or the ability to be replicated in other laboratories.
One potential drawback of the LaPSD is the requirement of precise particle velocity to define the time delay from interrogation to sorting. If precise geometrical measurements can be made on the devices and inlet pressures can be tightly controlled, theoretical calculations may suffice, as was the case in these studies. Alternatively, a flow probe or video capture of particles in flow may be necessary to more accurately determine particle velocities and associated variability before sorting. In addition, as populations of cells (or large particles) become more diverse in size, their respective position along the horizontal and vertical velocity profile will vary. At our current flow rates, particles tend to flow along the bottom of the flow cell. Lateral displacement will be altered with changes to the input volumetric flow rate and the size of the particles. This might be especially problematic in the case of time-delayed sorting (as described here) if particle velocities vary outside of the predicted range. To further improve sorting efficiencies of this design, 3D focusing could be implemented to more precisely control particle position in the channel and thereby particle velocities.32
An additional important consideration for implementation of the devices reported here is the design of software that is capable of defining a positive event based on an optical input. One or more signals must be relayed to a DAQ card to execute a given sorting event. Future improvements will include gating based on brightfield images (size) to allow for spatial discrimination of fluorescence within the aggregate or microtissue. This type of detection and real-time image processing will allow for biologically relevant sorting studies including, for example, isolating aggregates with high ratios of living cells to dead cells based on live/dead fluorescence indicators or isolating microtissues containing differentiated cell types based fluorescent reporters coupled to lineage specific genes.
CONCLUSIONS
As efforts to recapitulate 3D environments invitro gain momentum, methods to accurately and rapidly analyze large particles and to purify populations based on parameters analyzed will be critical to advance these efforts. We expect that the device described here will provide solid footing on which to begin to purify large particles. The devices we describe can be easily manufactured and adapted by many research laboratories and will easily interface with most commercial and custom imaging systems. In addition, the sorting efficiencies rival those of commercially available small particle sorting systems.
ACKNOWLEDGMENTS
The authors would like to thank Dan Negrut, Department of Mechanical Engineering, for useful insights related to fluid mechanics within the devices, especially the impact of particles in flow and Erwin Berthier and Edmond Young for helpful discussions related to PMMA device manufacturing and bonding. The authors would also like to thank members of LOCI, especially Curtis Rueden and Mohit Chainani for software development, and members of the Brenda Ogle, Justin Williams and David Beebe laboratories for their input and support. Supported by National Institutes of Health grant RC1HL100014, Coulter Foundation Translational Research Partnership and Morgridge Institute for Research.
References
- Leong D. T., Nah W. K., Gupta A., Hutmacher D. W., and Woodruff M. A., Curr. Drug Discovery Technol. 5, 319 (2008). 10.2174/157016308786733537 [DOI] [PubMed]
- Phillips B. W., Horne R., Lay T. S., Rust W. L., Teck T. T., and Crook J. M., J. Biotechnol. 138, 24 (2008). 10.1016/j.jbiotec.2008.07.1997 [DOI] [PubMed]
- Carpenedo R. L., Bratt-Leal A. M., Marklein R. A., Seaman S. A., Bowen N. J., McDonald J. F., and McDevitt T. C., Biomaterials 30, 2507 (2009). 10.1016/j.biomaterials.2009.01.007 [DOI] [PMC free article] [PubMed]
- Stegemann J. P. and Nerem R. M., Exp. Cell Res. 283, 146 (2003). 10.1016/S0014-4827(02)00041-1 [DOI] [PubMed]
- Schmeichel K. L. and Bissell M. J., J. Cell Sci. 116, 2377 (2003). 10.1242/jcs.00503 [DOI] [PMC free article] [PubMed]
- Kong H. J. and Mooney D. J., Nat. Rev. Drug Discovery 6, 455 (2007). 10.1038/nrd2309 [DOI] [PubMed]
- Benoit D. S., Schwartz M. P., Durney A. R., and Anseth K. S., Nature Mater. 7, 816 (2008). 10.1038/nmat2269 [DOI] [PMC free article] [PubMed]
- Khetani S. R. and Bhatia S. N., Curr. Opin. Biotechnol. 17, 524 (2006). 10.1016/j.copbio.2006.08.009 [DOI] [PubMed]
- Ratner B. D. and Bryant S. J., Annu. Rev. Biomed. Eng. 6, 41 (2004). 10.1146/annurev.bioeng.6.040803.140027 [DOI] [PubMed]
- Buschke D. G., J. M. Squirrell, H. Ansari, M. A. Smith, C. T. Rueden, J. C. Williams, G. E. Lyons, T. J. Kamp, K. W. Eliceiri, and B. M. Ogle, Microsc. Microanal. 17, 540 (2011). 10.1017/S1431927610000280 [DOI] [PMC free article] [PubMed]
- Chen A. A., Underhill G. H., and Bhatia S. N., Integr. Biol. (Cambridge) 2, 517 (2010). 10.1039/c0ib00054j [DOI] [PMC free article] [PubMed]
- Fernandez L. A., Hatch E. W., Armann B., Odorico J. S., Hullett D. A., Sollinger H. W., and Hanson M. S., Transplantation 80, 729 (2005). 10.1097/01.tp.0000179105.95770.cd [DOI] [PubMed]
- Stovel R. T., J. Histochem. Cytochem. 25, 813 (1977). 10.1177/25.7.894007 [DOI] [PubMed]
- Hansen W. P., U. S. Patent, Union Biometrica, Inc., USA (1999).
- Johansson L., Nikolajeff F., Johansson S., and Thorslund S., Anal. Chem. 81, 5188 (2009). 10.1021/ac802681r [DOI] [PubMed]
- Huh D., Bahng J. H., Ling Y., Wei H. H., Kripfgans O. D., Fowlkes J. B., Grotberg J. B., and Takayama S., Anal. Chem. 79, 1369 (2007). 10.1021/ac061542n [DOI] [PMC free article] [PubMed]
- Holmes D., Sandison M. E., Green N. G., and Morgan H., IEE Proc.: Nanobiotechnol. 152, 129 (2005). 10.1049/ip-nbt:20050008 [DOI] [PubMed]
- Nieuwstadt H. A., Seda R., Li D. S., Fowlkes J. B., and Bull J. L., Biomed. Microdevices 13, 97 (2011). 10.1007/s10544-010-9474-6 [DOI] [PMC free article] [PubMed]
- Wei H., Chueh B. H., Wu H., Hall E. W., Li C. W., Schirhagl R., Lin J. M., and Zare R. N., Lab Chip 11, 238 (2011). 10.1039/c0lc00121j [DOI] [PubMed]
- R. W.Applegate, Jr., Marr D. W., Squier J., and Graves S. W., Opt. Express 17, 16731 (2009). 10.1364/OE.17.016731 [DOI] [PubMed]
- Lillehoj P. B., Tsutsui H., Valamehr B., Wu H., and Ho C. M., Lab Chip 10, 1678 (2010). 10.1039/c000163e [DOI] [PMC free article] [PubMed]
- Di Carlo D., Irimia D., Tompkins R. G., and Toner M., Proc. Natl. Acad. Sci. U.S.A. 104, 18892 (2007). 10.1073/pnas.0704958104 [DOI] [PMC free article] [PubMed]
- Di Carlo D., Lab Chip 9, 3038 (2009). 10.1039/b912547g [DOI] [PubMed]
- Gossett D. R., Weaver W. M., Mach A. J., Hur S. C., Tse H. T. K., Lee W., Amini H., and Di Carlo D., Anal. Bioanal. Chem. 397, 3249 (2010). 10.1007/s00216-010-3721-9 [DOI] [PMC free article] [PubMed]
- Stathopoulos N. A. and Hellums J. D., Biotechnol. Bioeng. 27, 1021 (1985). 10.1002/(ISSN)1097-0290 [DOI] [PubMed]
- Augenstein D. C., Sinskey A. J., and Wang D. I., Biotechnol. Bioeng. 13, 409 (1971). 10.1002/(ISSN)1097-0290 [DOI] [PubMed]
- McQueen A., Meilhoc E., and Bailey J. E., Biotechnol. Lett. 9, 831 (1987). 10.1007/BF01026191 [DOI] [PubMed]
- Lumholdt A., “Numerical investigations of macroscopic particle dynamics in microflows,” Dissertation (Riso National Laboratory, Roskilde, Denmark, 2001).
- Fortes A. F., Joseph D. D., and Lundgren T. S., J. Fluid Mech. 177, 467 (1987). 10.1017/S0022112087001046 [DOI]
- See supplementary material at http://dx.doi.org/10.1063/1.3692765 for a description of alternative designs.
- Collins T. J., BioTechniques 43, 25 (2007). 10.2144/000112517 [DOI] [PubMed]
- P. B.Howell, Jr., Golden J. P., Hilliard L. R., Erickson J. S., Mott D. R., and Ligler F. S., Lab Chip 8, 1097 (2008). 10.1039/b719381e [DOI] [PMC free article] [PubMed]
- See http://loci.wisc.edu/software/wiscscan. This website provides a comprehensive description of Wiscan software. Wiscan is a custom-made flexible package used to operate multiphoton microscopy systems and multiphoton flow systems.