Abstract
Case reports have linked varicella-zoster virus (VZV) to gastrointestinal disorders, including severe abdominal pain preceding fatal varicella and acute colonic pseudoobstruction (Ogilvie’s syndrome). Because we had previously detected DNA and transcripts encoding latency–associated VZV gene products in the human gut, we sought to determine whether latent VZV is present in the human enteric nervous system (ENS) and, if so, to identify the cells in which it is located and its route to the bowel. Neither DNA, nor transcripts encoding VZV gene products, could be detected in resected gut from any of 7 control children (< 1 year old) who had not received the varicella vaccine or experienced varicella; however, VZV DNA and transcripts were each found to be present in resected bowel from 6/6 of children with a past history of varicella and in that of 6/7 of children who received the varicella vaccine. Both wild-type (WT) and vaccine-type (vOka) VZV thus establish latent infection in human gut. To determine routes by which VZV might gain access to the bowel, we injected guinea pigs with human or guinea pig lymphocytes expressing green fluorescent protein (GFP) under the control of the VZV ORF66 gene (VZVOKA66.GFP). GFP-expressing enteric neurons were found throughout the bowel within 2 days and continued to be present for greater than 6 weeks. DNA encoding VZV gene products also appeared in enteric and dorsal root ganglion (DRG) neurons following intradermal administration of WT-VZV and in enteric neurons after intradermal injection of VZVOKA66.GFP; moreover, a small number of guinea pig DRG neurons were found to project both to the skin and the intraperitoneal viscera. Viremia, in which lymphocytes carry VZV, or axonal transport from DRG neurons infected through their epidermal projections are thus each potential routes that enable VZV to gain access to the ENS.
Introduction
Varicella-zoster virus (VZV) causes a primary infection, varicella (chickenpox), during which the virus becomes latent in neurons of dorsal root (DRG) and cranial nerve (CNG) ganglia (Hope-Simpson, 1965); however, evidence also suggests that VZV establishes latency in ganglia of the enteric nervous system (ENS) (Gershon et al, 2008b). VZV reactivates from latency in approximately 30% of individuals to give rise to a secondary cutaneous infection, herpes zoster (HZ). Individuals who are over the age of 50 and/or immunocompromised are most likely to manifest HZ; nevertheless, HZ also occurs in healthy young children or adults who have experienced varicella or been immunized against it (Gershon et al, 2008a).
Evidence suggesting that VZV establishes latency in the ENS includes clinical and experimental observations. In 1998, we reported an infant with the congenital varicella syndrome, Barrett’s esophagus, feeding difficulty, and frequent vomiting (Ussery et al, 1998). Because markers of VZV infection were detected in esophageal biopsies, we proposed that the gastrointestinal system might be a novel target for persistent or latent VZV infection. Acute VZV infection of the gastrointestinal tract has frequently been observed (Mallet et al, 2006) and VZV has been linked to acute colonic pseudoobstruction (Oglivie’s syndrome) (Alpay and Yandt, 1994; Nomdedeu et al, 1995; Pui et al, 2001). Experimental observations linking VZV to the ENS grew out of studies of VZV latency in an in vitro model system that utilized neurons in ganglia isolated from guinea pig ENS (Chen et al, 2003; Gershon et al, 2008b; Stallings et al, 2006; Walters et al, 2008). These neurons were to found to be readily infected with VZV in vitro and the type of infection depended on how the virus was presented to the neurons. Infection with cell-associated VZV led to lytic infection, while infection with cell-free VZV caused latency. Reactivation, moreover, was induced when latently infected neurons were made to express VZV ORF61 protein or its herpes simplex virus (HSV) orthologue, ICP0. Because guinea pig enteric neurons manifest each of the stages, latent, lytic and reactivated, of VZV infection, and clinical reports had linked VZV to the bowel, we postulated that VZV routinely infects the human ENS and establishes latency within enteric neurons. The current studies were carried out to test this hypothesis and also to determine potential routes that might enable VZV to gain access to the ENS during varicella. Our experiments suggest that VZV commonly establishes latency in the human ENS and can do so following either natural infection or as a result of varicella vaccination. Two routes to the ENS were found to be possible, viremia in which VZV-infected lymphocytes carry the virus to the ENS, or transport in axons of sensory neurons that become infected because they innervate VZV-infected regions of the skin.
MATERIALS AND METHODS
Cells and viruses
Human embryonic lung fibroblasts (HELF) were used for propagation of VZV stocks and preparation of cell free virus as previously described (Gabel et al, 1989). Guinea pig peripheral blood mononuclear cells (PBMCs) from Hartley guinea pigs were prepared by centrifugation through two successive Ficoll-Paque (Sigma, USA) gradients (Gan et al, 2011). Oka type VZV (vOka) labeled in ORF66 with green fluorescent protein (GFP) (VZVORF66.GFP) was a gift of Dr. Paul Kinchington (Uiversity of Pittsburgh).
Nested PCR and RT-PCR
DNA was extracted from tissue or PBMC (DNeasy Blood and Tissue Kit; Qiagen, Valencia, CA). DNA (100 ng) was subjected to PCR amplification (Eppendorf SmartCycler) in 20 μl of reaction mixture for 35 cycles. The PCR product (1.0 μl) was subjected to further amplification with sets of nested primers for each gene for an additional 32 cycles. Extraordinary precautions were taken to avoid contamination, which is a serious risk when nested amplification is employed. PCR tubes were opened only in a fume hood and negative control samples of tissue DNA (gut tissue removed from human neonates or uninfected guinea pigs) were always analyzed simultaneously with experimental samples. Cellular DNA was also examined; primers for human β-globin and guinea pig glyceraldehyde 3-phosphate dehydrogenase primers were used to verify the presence, respectively, of human and guinea pig DNA. In no experiment did the respective cellular gene fail to be amplified. Primers used for PCR and nested PCR were designed to amplify VZV ORFs 4, 29, 31, 61, 62, 63, 66, 67 and 68 (Gan et al, 2011; Gershon et al, 2007). PCR products were separated on 2% agarose gels, and digital images were obtained for documentation with a Kodak Image Station 440.
RNA was extracted with Trizol (Invitrogen, Carlsbad, CA; manufacturer’s instructions were followed) from the same tissue from which DNA was extracted (see above). RNA was treated with DNase I (4U/100 μl) to remove possible DNA contamination. Total RNA (3.0 μg) was subjected to reverse transcription with Maloney Murine Leukemia Virus reverse transcriptase (Promega, Madison, WI) in 25 μl of reaction mixture to obtain cDNA. The cDNA (1.0 μl) was subjected to PCR amplification in 20 μl of reaction mixture for each VZV gene. β-actin universal primers were used as controls both for RNA extraction and cDNA preparation.
In situ hybridization and immunocytochemistry
For in situ hybridization, frozen sections were permeabilized with proteinase K (100 μg/ml) for 20 min, washed with PBS, and post-fixed with 4% formaldehyde (from paraformaldehyde) for 10 min. The sections were then treated with 0.3M NaOH for 5 min and neutralized with 0.4M Tris-HCl (pH7.4) for 15 min. The sections were subsequently washed with PBS and dehydrated with 50% and 100% ethanol respectively. Prehybridization was carried out in buffer (50% formamide, 1 XSSC, 1X Denhardt’s, 500 μg/ml salmon sperm DNA) at room temperature for 2–3 hrs. Specimens were then covered with a hybridization buffer (prehybridization buffer supplemented with a digoxigenin-conjugated oligonucleotide probe targeted to VZV ORF54 and 10% dextran sulfate) and incubated for 10 min at 85°C and overnight at 37°C. Following hybridization, slides were washed with 2x and 0.1x SSC. Sites of bound probe were detected with alkaline phosphatase conjugated antibodies to digoxigenin (Roche Diagnostics, Mannheim, Germany), which were applied for 2 hrs and visualized after washing and histochemical detection of alkaline phosphatase activity (Lungu et al, 1995). Levamizol (0.5 mM) was added to the buffer solution in order to inhibit the endogenous alkaline phosphatase activity of neurons.
The intestinal wall of guinea pigs or mice (CD-1; Charles River) was dissected into layers to permit the ENS to be examined in whole mounts. This allows large sections of the enteric plexuses to be examined as flat laminar preparations. The layers examined consisted of the submucosa containing the submucosal plexus and the longitudinal muscle with adherent myenteric plexus. For immunocytochemistry, tissue sections or whole mounts of bowel were blocked with 10% normal horse serum for 30 min at room temperature and then incubated for 48 or 72 hrs at 4°C with primary antibodies. Bound primary antibodies were visualized with streptavidin-Alexa 594 for mouse monoclonal antibodies, and donkey antibodies to goat, rabbit, or sheep IgG coupled to Alexafluor 350, 488, 594, or 680 [infrared] (diluted 1:200; Invitrogen). The use of species-specific secondary antibodies coupled to contrasting fluorophores enabled as many as four primary antibodies to be located simultaneously. Preparations were washed with PBS, mounted in buffered glycerol, and examined with a Leica CTR 6000 microscope. Images were obtained with a cooled CCD camera and analyzed with computer assistance (Velocity 5.4 imaging software; Improvision, Waltham, MA). DNA was visualized with bisbenzimide (1 μm/ml; Sigma Aldrich, St. Louis, MO). Polyclonal rabbit antibodies to human protein kinase G1 alpha (PKG1; US Biological, Swampscott, MA) were diluted 1:100 and antibodies to calcitonin gene related peptide (CGRP; Sigma Aldrich, St. Louis, MO) were diluted 1:1200. Biotinylated mouse monoclonal antibody to the neuronal marker, Hu C/D, were obtained from Invitrogen, and diluted 1:50.
Transmission electron microscopy (TEM)
For TEM, 3 × 106 cells were fixed by immersion for 2 hr in 2.5% glutaraldehyde in 0.1M phosphate buffer and centrifuged to form a pellet. The pellets were post-fixed with 2% osmium tetroxide for 1 hr at room temperature, rinsed 5 times in PBS and maleate buffer, and stained en bloc with 5% aqueous uranyl acetate on ice for 1 hr. The fixed pellets were dehydrated with a graded series of ethanol solutions, cleared with propylene oxide and embedded in polymerized Epon 812 (Ted Pella). Ultra thin sections (1 micron) were cut with a microtome (Reichert-Jung) and collected on copper grids. Sections were examined with a JEOL 1200EX electron microscope.
Infection of guinea pigs in vivo
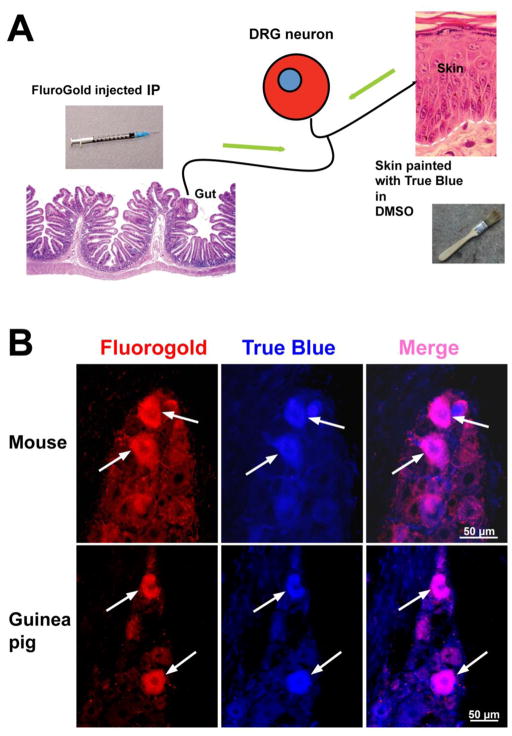
These experiments were designed to identify cutaneous and visceral projections of sensory neurons using retrograde tracers to determine whether any of these projections might constitute a conduit to the bowel that VZV might utilize to travel to the gut from the skin. Neurons projecting to the suprabasal epidermis were selectively labeled by painting the skin of mice (CD-1) and guinea pigs with the retrograde label, True Blue dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO)(Costas et al, 1998). At the same time, neurons projecting to the viscera were specifically labeled by injecting the retrograde tracer, hydroxystilbamidine bis(methanesulfonate) “Fluorogold” (Sigma-Aldrich, St. Louis, MO), into the peritoneal cavity (Phillips et al, 2004). DRG were analyzed 4 wks later. All experiments in guinea pigs were approved by Columbia’s Institutional Animal Care and Use Committee. In separate experiments, 100 intradermal injections, each containing ~ 50 plaque forming units (pfu) (10μl) of cell free VZV, were administered to guinea pigs in a belt between the front and rear extremities. Nested PCR and in situ hybridization were used to examine DRG and gut 3–6 weeks following injection.
Infection of guinea pig peripheral blood mononuclear cells (PBMC) and in vivo infection of guinea pigs
VZV-infected PBMCs were prepared by co-culture with VZVORF66.GFP -infected HELF (Gan et al, 2011; Soong et al, 2000). For infection of guinea pigs, 1 ×106 VZVORF66.GFP -infected PBMCs in 50μl were injected into the posterior ocular sinus of a 4 week-old Hartley guinea pigs (Charles River, MA).
Specimens of human gut
Surgical specimens of gut were obtained at New York-Presbyterian Hospital from 20 children; surgery was carried out for medically indicated reasons. Following removal of segments of bowel, specimens that normally would have been discarded were coded and sent for analysis, accompanied only with information about each child’s age, varicella vaccination status, and history of varicella. Ages of children in the control group (N = 7) were: newborn (1), 2 months (2), 4 months (3), and one year (1). Ages of children who had received varicella vaccine (N = 7) were: 1.3 years (1), 1.6 years (1), 3.5 years (2), 4.1 years (1), 5.7 years (1), and 12 years (1). Ages of children with a history of varicella (N = 6) were: 6 years (1), 15 years (1), 16 years (2), and 17 years (1). The Columbia University Institutional Review Board (IRB) approved this study. Specimens were transmitted immediately to the laboratory for processing. Each specimen was divided in two; one half was placed in RNAlater™ (Ambion, Applied Biosystems, Austin TX) for analysis of nucleic acids, while the other half was fixed for immunocytochemistry with 4% formaldehyde (from paraformaldehyde) at pH 7.4, for histological examination.
Results
VZV is present in the gut of children exposed to VZV
Three groups of children were studied. Controls consisted of infants, each of whom were less than one year of age, were not vaccinated, and had no history of varicella. The second group consisted of children (6–17 years of age) who had not received the varicella vaccine, but in whom a history of natural varicella was documented. The third group consisted of children (1.3–12 years of age) who were vaccinated against varicella but lacked a history of natural varicella. RNA isolated from each specimen was converted to cDNA and nested PCR was employed to determine whether transcripts encoding members of a panel of latency-associated (ORFs 4, 29, 62, 63, 66) and/or lytic infection-associated (ORFs 31, 61, 68) VZV gene products could be detected. Latency-associated gene products were considered to be those reported to be expressed during latency in human ganglia and in isolated guinea pig enteric neurons (Gershon et al, 2008b; Kennedy and Cohrs, 2010). Lytic infection-associated gene products were considered to be those reported to be expressed during lytic infection and but not during latency. At least one latency-associated gene product was detected in each the 6 specimens from children in whom there was a history of natural varicella (Table 1). The most commonly detected transcript was ORF63 followed by ORFs4 and 66. No lytic infection-associated transcripts were found in any of the 6 specimens. At least one latency-associated gene product was also found in most of the specimens from vaccinated children although one of seven lacked any transcripts encoding a VZV gene product (Table 1). The most commonly detected transcript again was ORF63, followed by ORFs 62 and 66. As after natural infection, no lytic infection-associated transcripts were found in any of the 7 specimens from vaccinated children. These observations are consistent with the ideas that VZV is commonly latent in the human bowel and that its presence depends on prior exposure to VZV, either in the form of natural varicella or receipt of the varicella vaccine.
Table 1.
Detection of transcripts encoding VZV gene products in human gut
| ORF | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| VZV exposure | 4 | 29 | 31* | 61* | 62 | 63 | 66 | 68 | Total |
| varicella | 4/6 | 0/6 | 0/6 | 0/6 | 0/6 | 5/6 | 1/6 | 0/6 | 6/6 |
| % | 67 | 0 | 0 | 0 | 0 | 83 | 17 | 0 | 100 |
|
| |||||||||
| vaccine | 4/7 | 0 | 0 | 0 | 2/7 | 6/7 | 1/7 | 0 | 6/7 |
| % | 57 | 0 | 0 | 0 | 29 | 86 | 14 | 0 | 86 |
|
| |||||||||
| No VZV | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 |
| % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Transcripts encoding VZV gene products were detected in specimens of human gut removed surgically for medically indicated reasons unrelated to VZV. The column at the left indicates the type of each patient’s exposure to VZV. The proportions and the percent of specimens in which each of the indicated transcripts listed in the table was detected and the totals for each type of exposure are presented in the column at the right. Each sample was analyzed at least 3 times. Positive samples were detected as migrating with the appropriate size in agarose gels as illustrated below for ORF62 (Lanes: M = markers, + = positive control [plasmid DNA], − =
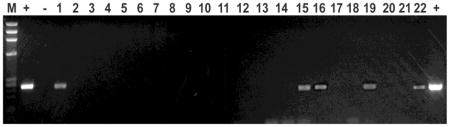
negative control [distilled water], 1–22 = individual samples from human patients). DNA encoding ORF62 was detected in lanes 1, 15, 16, 19 and 22.
Transcript encoding a VZV gene product that is an indicator of lytic infection.
A subset of DRG neurons projects both to the skin and to viscera
The epidermis is infected with VZV during episodes of varicella. Sensory nerves travel to the skin and lose their myelin sheaths as they cross the dermal-epidermal junction to meander within the epidermis. Naked nerve endings within VZV-infected regions of the skin thus become exposed to infectious cell free virions released in the suprabasal epidermis (Chen et al, 2004). It is conceivable that these nerve endings become infected with VZV, which is then transported in the retrograde direction within axons to cell bodies in DRG and/or CNG where VZV establishes latency. If some of the sensory neurons that become infected via retrograde transport from the skin were also to extend collaterals to the viscera, these neurons could provide a conduit for VZV to reach, infect, and establish latency in the ENS. This hypothesis was tested. First, double labeling with retrograde tracers applied to the skin and viscera of mice and guinea pigs were used to determine whether the putative sensory neuronal skin-to-viscera pathway exists (Fig. 1A). Two species were examined to verify that the putative route is not species-specific. Second, VZV was experimentally administered to guinea pig skin to determine whether VZV is able to utilize this route to the bowel in a mammal that it is known to be able to infect (Lowry et al, 1992; Myers and Stanberry, 1991; Myers et al, 1985; Myers et al, 1991; Sabella et al, 1993). The retrograde tracer, True Blue was dissolved in DMSO and applied with a paintbrush to the shaved guinea pig or mouse skin. When applied in this way, True Blue selectively enters intra-epidermal sensory nerve endings and is transported to their cell bodies (Costas et al, 1998). At the same time, the retrograde tracer, Fluorogold was injected intraperitoneally. When applied in this way, Flurogold enters and labels all neurons of the ENS and also enters sensory nerves that project to intraperitoneal targets, including the bowel (Phillips et al, 2004). Although most neurons in DRG in dermatomes corresponding to the painted epidermis were labeled only with True Blue or only with Fluorogold, coincident labeling of small numbers of DRG neurons with both True Blue and Fluorogold were found (Fig. 1). None of the non-neuronal cells of the DRG acquired either label. The existence of doubly labeled neurons demonstrates that a small subset of DRG neurons projects both to the suprabasal epidermis and the viscera. Interestingly, all of the neurons that projected to the viscera also expressed the immunoreactivities of the markers, CGRP, and PKG1 (not illustrated), which have been associated with the perception of pain (Kondo et al, 2010; Qiao and Grider, 2009).
Fig. 1.
Retrograde tracers reveal that a subset of DRG neurons projects both to the skin and the viscera. A. A cartoon outlines the method of approach. Fluorogold, injected intraperitoneally (IP) labels all neurons within the gut and sensory axons projecting to it. Simultaneously, True Blue in DMSO painted onto the skin labels neurons with intraepidermal projections. Both dyes move to DRG via retrograde axonal transport (green arrows). The presence of both dyes in an individual DRG neuron, confirms that the doubly labeled neuron projects both to intraperitoneal viscera and to the epidermis. B. In both mouse and guinea pig, individual DRG neurons (identified as large cells within the DRG that are neither satellite cells nor fibroblasts) can be found following the administration of Flurogold and True Blue as indicated in A (above) that contain both dyes. To enhance the sensitivity of detection of Flurogold, the dye was detected immunocytochemically and its red immunofluorescence is shown after its demonstration with secondary antibodies coupled to Alexafluor 594. The native fluorescence of True Blue is illustrated and the doubly labeled cells appear pink in the merged image. Neurons can be seen that contain true blue but not Flurogold; these neurons project to skin and not viscera. Neurons can also be seen that contain Flurogold but not True Blue; these neurons are visceral afferents that lack projections to the skin. Note that only neurons are stained.
Latent VZV appears in DRG and enteric neurons following intradermal injection of VZV
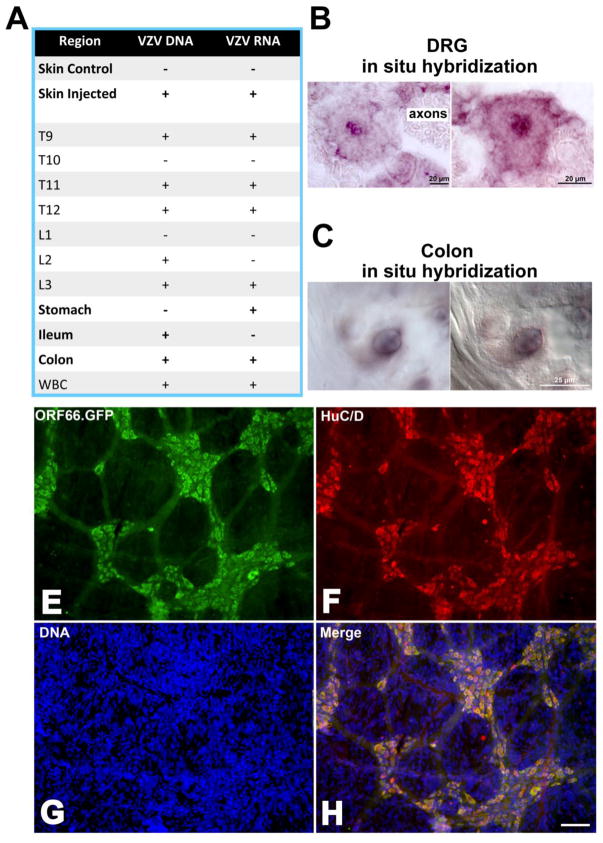
Cell-associated VZV was injected intradermally into guinea pigs. One hundred sites were injected (10μl/site) in a belt between the forelegs and the hindlegs. The skin, DRG (bilaterally from T9 to L3), stomach, ileum, colon, and white blood cells (buffy coat of centrifuged blood) were collected 7–28 days after injection. Control skin was obtained from distant non-injected regions of the same guinea pigs (Fig 2A). PCR was used to examine DNA (ORFs 40 and 67) and transcripts encoding latency-associated gene products (ORFs 4, 62, and 63) from all specimens. In situ hybridization was also used to investigate DNA (ORF54) encoding VZV gene products in DRG and the ENS. No VZV DNA or transcripts were detected in the non-injected control regions of skin; however, both were found in skin from the injected region, suggesting persistent VZV infection (Fig. 2A). VZV DNA and transcripts were detected in DRG at most of the axial levels examined; DRG at T10 and L1 were exceptions (Fig. 2A). In situ hybridization also detected viral DNA within the affected DRG (Fig. 2B). The hybridizing DNA was only observed in neurons; none was detected in axons, satellite cells, or connective tissue and, within neurons, the viral DNA was strikingly nuclear in its location. The neurons that contained VZV DNA appeared to be healthy and could not be distinguished from neighboring neurons that did not contain hybridizing DNA. Both DNA and transcripts were found in the colon (Fig. 2A), where as in the DRG, in situ hybridization revealed the presence of viral DNA in the nuclei of neurons (Fig. 2C). Transcripts encoding latency-associated VZV gene products were detected in the stomach and viral DNA was detected in the ileum (Fig. 2A). To verify that VZV reaches the bowel following its introduction into the skin and to improve determination of its location in the ENS, a second series of guinea pigs were injected intradermally with VZVORF66.GFP (Fig. 2D–H). This study confirmed again that VZV reaches and infects the ENS after its intradermal injection; moreover, the experiments revealed that VZVORF66.GFP was present in virtually all neurons of the colonic ENS (Fig. 2H) and in most of those in the ileal ENS. The degree of infection diminished proximally (not illustrated), suggesting a location in the bowel corresponding to the axial levels of the dermatomes injected with VZV. The location of VZVORF66.GFP was strikingly confined to neuronal cell bodies; GFP fluorescence was coincident with the immunofluorescence of the neuronal marker HuC/D (Fig. 2H). The ganglionic neuropil and intraganglionic connectives did not show any GFP fluorescence.
Fig. 2.
VZV infects DRG and enteric neurons following its intradermal injection at multiple sites in a band between the forelegs and hindlegs of guinea pigs. A. A table indicates the sites where either DNA or transcripts encoding VZV gene products can be found 7–28 days following injection. B. The presence of DNA encoding VZV ORF54 is shown by in situ hybridization to be present in the nucleus of neurons in two different DRGs. Because DRGs were not serially sectioned, hybridizing neurons were not quantified. Neurons were considered to contain hybridizing DNA only if their nucleus contained reaction product. The markers = 20 μm. C. The presence of DNA encoding VZV ORF54 is shown by in situ hybridization to be present in the nucleus of a myenteric neuron in the colon of an injected guinea pig. The image at the left shows the ganglion in bright field, while the same ganglion is viewed at the right with interference contrast optics in order to visualized non-labeled structures. D. Cutaneous injection of VZVORF66.GFP leads to the widespread presence of GFP (green) in neurons of ganglia of the guinea pig myenteric plexus. E. The same field (as in D) is illuminated to show the immunofluorescence (red) of the neuronal marker HuC/D. F. The same field (as in D) illuminated to show the fluorescence of bisbenzimide-stained DNA (blue). G. When viewed as a merged image, the coincident location of GFP and HuC/D in the cell bodies of virtually all neurons is apparent. Note the restriction of GFP to the ENS; there is no labeling at all in the vast number of non-neuronal cells, the presence of which is revealed by their blue nuclei. The marker = 50 μm.
Taken as a whole, experiments with intradermal injection of VZV show in the guinea pig that VZV infects neurons in both DRG and gut following its introduction into the skin. The experiments are consistent with the possibility that the virus is transported to DRG and bowel in the axonal projections of sensory neurons, possibly those that project both to skin and viscera. The failure of VZV to infect non-injected skin suggests that the virus may not be disseminated widely following its intradermal injection; nevertheless, the observation that VZV transcripts and DNA were found in white blood cells (Fig. 2A), indicates that a viremia did occur despite the local introduction of the virus into the skin. These experiments thus do not rule out viremic conduction of VZV to DRG and gut within infected lymphocytes.
VZV-infected lymphocytes establish latency in enteric neurons
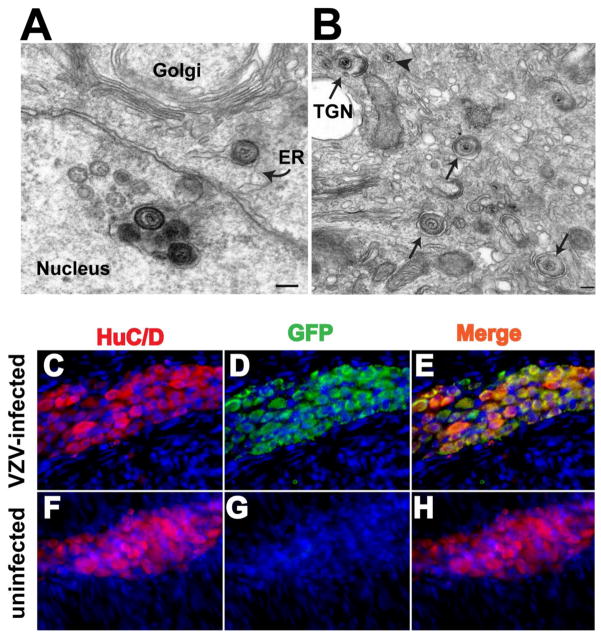
The experiments described above revealed that guinea pig white blood cells become infected with VZV. Guinea pig and human PBMC were isolated and co-cultured on a layer of HELF infected with VZVORF66.GFP. Both types of PBMC acquire VZV infection when subjected to this procedure. The GFP fluorescence of the virus was coincident with the immunofluorescence of CD3, suggesting that the infected subset of PBMC were T lymphocytes (Gan et al. 2011). The VZVORF66.GFP-infected cells were further examined electron microscopically to characterize the infection. Infected cells all had the appearance of activated lymphocytes. Extracellular encapsulated virions adhered to the exterior surface of plasma membranes and nucleocapsids were abundant in euchromatic nuclei (Fig. 3A). Enveloped virions were observed within the perinuclear cisternae and endoplasmic reticulum (Fig. 3A); however, these virions appeared to be immature and deficient in tegument, a morphology that has been noted to characterize virions prior to release to the cytosol and final envelopment in the trans-Golgi network (TGN) (Gershon et al, 1994; Zhu et al, 1995). Mature virions, that appeared to be post-TGN, were found in single cytoplasmic vesicles (Fig. 3B). Notably lacking was the accumulation of virions in endosomes that characterizes viral maturation and intracellular viral degradation in other types of infected cell (Chen et al, 2004). These observations suggest that VZV-infected HELF transfer infection to co-cultured T lymphocytes and that the infection is lytic in nature; however, the morphology of the infected cells, which does not indicate that virions are degraded intracellularly (Fig. 3B), is consistent with the possibility that infected T lymphocytes secrete infectious VZV (Moffat et al, 1995). We therefore determined whether T lymphocytes infected with VZVORF66.GFP were capable of infecting and establishing latency in neurons of the guinea pig ENS.
Fig. 3.
VZV-infected lymphocytes establish latency in enteric neurons. A. A VZV infected lymphocyte contains nucleocpasids assembling the a euchromatic nucleus. A cluster of immature enveloped virions can be seen within an invagination of the pernuclear cisterna. A similar immature virion has exited from the perinuclear cisterna and lies within the endoplasmic reticulum (ER) of the infected cell. B. A number of re-enveloped virions can be seen within individual vesicles (arrows). A virion is caught in a wrapping structure of the trans-Golgi network (TGN). An arrowhead points to a naked nucleocapsid in the cytosol that has emerged from the ER and is awaiting envelopment with tegument in the TGN. The marker = 20 nm. C. A myenteric ganglion of the ileum of a guinea pig that was injected with VZVORF66.GFP-infected lymphocytes immunostained with HuC/D to identify neurons (red). D. The same field has been illuminated to visualize GFP (green). E. In the merged image (yellow) virtually every neuron can be seen to contain GFP. F. Neurons, identified by the immunofluorescence of HuC/D, are abundant in a ganglion of the myenteric plexus of the ileum of a guinea pig. G. The same field has been illuminated to reveal GFP fluorescence, which is absent (control). H. In the merged image, no yellow fluorescence is seen because none of the neurons contain GFP. The blue nuclei that can be seen in all fields are due to staining of DNA with bisbenzimide to show the cellularity of the fields.
Guinea pigs were injected with VZVORF66.GFP-infected lymphocytes, prepared by co-culture with infected HELF. The VZVORF66.GFP-infected lymphocytes were injected into the retro-orbital sinus. Infection of enteric neurons was detected within 2 days and persisted for over 6 weeks. The fluorescence of GFP, which was coincident with the immunofluorescence of the neuronal marker, HuC/D, marked the presence of infection of enteric neurons (Fig. 3C–E). Again, no GFP fluorescence was observed in the neuropil or in intraganglionic connectives. In preparations in which neurons were marked with antibodies to 3-tubulin and glial cells with antibodies to GFAP, concidence of GFP and 3-tubulin fluorescence was limited to nerve cell bodies and no coincident GFP fluorescence with GFAP immunofluorescence was detected (not illustrated). No GFP fluorescence was ever observed in the ENS of control uninfected guinea pigs (Fig. 3F–H).
Discussion
Prior to undertaking the current investigation, we detected DNA encoding VZV genes in 77% of archived paraffin sections of human intestine from 30 patients (6 of each type) with Crohn’s disease, ulcerative colitis, diverticulitis, chronic constipation, and adenocarcinoma of the colon (Chen et al, 2005). We also detected transcripts encoding VZV proteins in 88% (21/24) of cDNA extracts from surgically removed adult human colon and small intestine (Gershon et al, 2008b). Although these observations are consistent with the possibilities that the ENS is a target for VZV and a site of viral latency, no history of VZV exposure was available for any of the individuals examined. In the current study, we prospectively examined specimens of colon and small intestine from children who either had experienced an episode of natural varicella or had been immunized against varicella. We again found evidence that the ENS is a target for VZV; moreover, the data suggest that not only wild-type VZV, but also vOka can infect the ENS. These data thus confirm and extend our earlier observations and they suggest that almost everyone who is exposed to VZV, either through natural infection or through varicella vaccination, acquires latent VZV in the ENS.
Infection with VZV can be either lytic or latent. The simple detection of VZV DNA in the ENS, therefore, would not serve, by itself to establish whether or not an infection is latent. Neither would the persistence of infection for long periods of time be sufficient to indicate that viral infection is latent, although persistence would be consistent with latency. In the case of the ENS, the fact that none of the children in the study were disabled by gastrointestinal disease and none exhibited the acute pseudoobstruction that has been associated with VZV suggests that the persistence of VZV that was detected in their bowel did not impair the function of the ENS. That failure to give rise to symptoms is certainly consistent with viral latency, which allows VZV to persist in neurons without blocking their function. The observation that transcripts as well as DNA were present in the bowel implies that the ENS was indeed infected with VZV and not a passive bystander that had just taken up and stored viral DNA from a prior encounter with the virus. The pattern of the viral genes expressed, moreover, also indicates that the type of infection seen in the ENS after either natural infection or varicella vaccination is latent infection.
Latency of VZV is characterized by the expression of a number of immediate early (IE) and early (E) genes of the viral cascade without expression of late (L) genes that encode structural elements of the virus (Cohrs and Gilden, 2003; Gershon et al, 2008b; Kennedy and Cohrs, 2010). During latency, at least 6 viral transcripts encoding ORFs 4, 21, 29, 62, 63, and 66 have all been reported. Expression of viral genes may be even more extensive (Nagel et al, 2011) and there is evidence for expression of corresponding viral proteins as well (Ambagala et al, 2009; Cohrs et al, 2003; Lungu et al, 1998). Latently infected guinea pig enteric neurons in vitro express the same latency-associated viral transcripts as do latently infected human DRG neurons (Gershon et al, 2008b). During latent infection, ORFs 31 (gB), 61 (the VZV orthologue of HSV ICP0), 67 (gI), and 68 (gE) are not expressed (but see also below for ORF68); therefore, their presence, should it be detected, would constitute evidence of lytic infection. In fact, the expression of ORF61 (via an adenoviral vector) in latently infected enteric neurons in vitro reactivates VZV (Gershon et al, 2008b). There is also evidence that the location of viral proteins expressed in an infected cell differs in lytic and latent infection. The latency-associated proteins are all restricted to the cytoplasm during latency but translocate to the nucleus when VZV reactivates and are intranuclear during lytic infection (Chen et al, 2003; Gershon et al, 2008b; Lungu et al, 1998; Stallings et al, 2006; Walters et al, 2008). There are some possible exceptions in that expression of gE has recently been reported as a component of the latency transcriptome (Nagel et al, 2011) and the multiple reports that viral proteins are expressed during latency have been challenged as possibly an artifact due to the confusion of pigment in DRG neurons with the dark immunocytochemical reaction product used to visualize antibodies labeled with horseradish peroxidase or alkaline phosphatase (Zerboni et al, 2010). Of course, this objection does not apply to the neurons of children, which have not accumulated pigment, or to immunofluorescence. The expression of the six common latency-associated transcripts, the absence of transcripts encoding L gene products, and the restriction of the corresponding proteins, when detected, to the cytoplasm thus constitutes a latency signature that allows latent infection to be distinguished. Because we have found that latency-associated transcripts are expressed in the human bowel after natural VZV and that viral transcripts associated with lytic infection cannot be detected, we conclude that VZV does, in fact, establish latency in the human ENS after natural varicella or varicella vaccination.
Because VZV may be an important pathogen of the human ENS, we investigated possible pathways by which VZV might reach enteric neurons to establish latent infection. It is well established that retrograde transport in sensory axons that project to the skin can enable VZV to reach and establish latency in DRG and CNG during varicella (Arvin and Cohen, 2007). Direct neuronal pathways from the skin to the bowel, however, are not known to exist. Somatic afferent neurons from DRG project to the skin and visceral afferent neurons exist in many of the same ganglia. VZV-infected somatic afferent neurons might potentially infect neighboring visceral afferent neurons and then travel to the ENS via anterograde transport in the axons of those neurons; however, that would involve movement of the virus from one cell to another, which would be difficult to conceive of happening in the absence of lytic infection. We therefore tested an alternative hypothesis that a putative viral conduit exists in a subset of hybrid neurons that project both to the epidermis and the viscera. Such neurons indeed were found and became doubly labeled with Flurogold injected into the peritoneal cavity and True Blue in DMSO painted onto the surface of the guinea pig or mouse skin. Tests confirmed that the sensory neuronal conduit is a possible route to the bowel because the ENS readily became infected with VZV when the virus was introduced intradermally. Latency was established both in DRG neurons and in the ENS; moreover, injections of VZVORF66.GFP established that infection of the bowel was limited to neurons of the ENS and because the ORF66-driven GFP expression in these neurons was restricted to their cytoplasm, the infection was probably latent. This conclusion explains why the guinea pigs displayed no symptoms or signs of ENS dysfunction. Collaterals from infected DRG neurons projecting to the gut, therefore, could provide VZV with a pathway enabling it to reach the ENS during varicella or after vaccination; nevertheless, intradermal injection of VZV also led to persistent infection of the local skin and the infection of white blood cells. That means that the alternative possibility, that VZV establishes latency in the ENS following a viremia in which the virus is carried in infected T lymphocytes is not ruled out.
Because intradermal injections of VZV infected white blood cells, we tested the ability of VZV-infected lymphocytes to establish latency in the ENS when given intravenously to guinea pigs. Both guinea pig and human PBMC were readily infected with VZV when they were co-cultured with VZV-infected HELF. The infected PBMC were predominantly T lymphocytes and co-expressed CD3. When VZVORF66.GFP-infected lymphocytes were injected into guinea pigs, virtually the entire ENS became infected and almost all enteric neurons expressed GFP fluorescence. Again, as after the intradermal injection of VZVORF66.GFP, the GFP fluorescence was limited to the cytoplasm of neurons and was confined to their cell bodies. This distribution, as well as the absence of signs or symptoms of ENS dysfunction in the infected guinea pigs, suggests that the infection was latent. Viremia occurs during both varicella and zoster (Satyaprakash et al, 2009). Children who have been vaccinated in the arm have been reported to manifest zoster due to the Oka strain on their face (Galea et al, 2008). The facial zoster suggests that the trigeminal ganglion was the source of the zoster, but because the trigeminal ganglion does not innervate the arm, it is highly likely that a vaccination-induced viremia infected the trigeminal ganglion, which subsequently reactivated to cause the facial zoster. Because the infection of the gut was relatively restricted to the caudal bowel following intradermal administration of VZV between the forelegs and hindlegs of the guinea pigs, but generalized from stomach to colon after experimental viremia, it is likely that both a sensory neuronal conduit of virus from skin to gut and viremia are able to establish latency in the bowel and the two routes of infection are not mutually exclusive. Both might function either after varicella or after administration of varicella vaccine.
The presence of VZV as a common pathogen in the human ENS suggests that enteric zoster, which would not be suspected clinically because of the absence of an accompanying rash, probably occurs in humans. Whatever leads to the reactivation of VZV in DRG is likely to lead to reactivation in the ENS as well. The manifestations of enteric zoster have yet to be identified but, because reactivation is lethal to the neurons in which it occurs, these manifestations may include multiple intestinal disorders of unknown pathogenesis, such as irritable bowel syndrome, inflammatory bowel disease, idiopathic gastroparesis, and chronic intestinal idiopathic pseudoobstruction. Acute colonic pseudoobstruction, which is associated with VZV, is uncommon and related to immunodeficiency. Reactivation of VZV in the ENS of an individual who is not immunodeficient may be severe enough to cause pseudoobstruction, but it may also be symptomatic and should be investigated. It is also possible that low asymptomatic reactivations of VZV in the gut, the largest lymphoid organ in the body, stimulates immunity to VZV intermittently, leading to long-term protection and maintenance of resistance to infection. The absence of exogenous exposure to VZV has recently been demonstrated not to weaken protection against zoster, strongly suggesting that an internal stimulus maintains long-term immunity to VZV (Gaillat and al., 2011). Enteric zoster may thus be beneficial if it remains benign and below a clinical threshold. Whether that is so, or whether enteric zoster is an unsuspected cause of distress remains to be determined.
Acknowledgments
Supported by NIH grants DK093094 and NS12969; National Science Foundation of China, 30872253
References
- Alpay K, Yandt M. Herpes zoster and Ogilvie’s syndrome. Dermatology. 1994;189:312. doi: 10.1159/000246870. [DOI] [PubMed] [Google Scholar]
- Ambagala AP, Bosma T, Ali MA, Poustovoitov M, Chen JJ, Gershon MD, Adams PD, Cohen JI. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J Virol. 2009;83:200–9. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM, Cohen J. Varicella-Zoster Virus. In: Fields, editor. Virology. Raven Press; 2007. pp. 2773–2818. [Google Scholar]
- Chen J, Gershon A, Bischoff S, Blaszyk H, Ciolino A, Mawe G, Gershon MD. Latency of VZV in ganglia of the human small and large intestines. 30th IHW; Turku, Finland. 2005. p. abstract 7.19. [Google Scholar]
- Chen J, Gershon A, Silverstein SJ, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric and dorsal root ganglia by varicella zoster virus. J Med Virol. 2003;70:S71–78. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH. Varicella zoster virus transcription in latently-infected human ganglia. Anticancer Res. 2003;23:2063–9. [PubMed] [Google Scholar]
- Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PG. Varicella-Zoster Virus Gene 66 Transcription and Translation in Latently Infected Human Ganglia. J Virol. 2003;77:6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas PD, Sabin TD, Wang KK, Jones DS, Seckel BR. Transcutaneous access to retrograde axonal flow. Muscle & nerve. 1998;21:531–2. doi: 10.1002/(sici)1097-4598(199804)21:4<531::aid-mus13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Gabel C, Dubey L, Steinberg S, Gershon M, Gershon A. Varicella-zoster virus glycoproteins are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillat J, et al. Does Monastic Life Predispose to the Risk of Saint Anthony’s Fire (Herpes Zoster)? Clin Infect Dis. 2011;53:405. doi: 10.1093/cid/cir436. [DOI] [PubMed] [Google Scholar]
- Galea SA, Sweet A, Beninger P, Steinberg SP, Larussa PS, Gershon AA, Sharrar RG. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(Suppl 2):S165–9. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- Gan L, Wang M, Chen J, Gershon M, Gershon AA. New persectives on varicella-zoster infection in a guinea pig model. 2011 In preparation. [Google Scholar]
- Gershon A, Chen J, LaRussa P, Steinberg S. Varicella-zoster virus. In: Murray PR, Baron E, Jorgensen J, Landry M, Pfaller M, editors. Manual of Clinical Microbiology. ASM Press; Washington, D.C: 2007. pp. 1537–1548. [Google Scholar]
- Gershon A, Takahashi M, Seward J. Live attenuated varicella vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. WB Saunders; Philadelphia: 2008a. pp. 915–958. [Google Scholar]
- Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis. 2008b;197(Suppl 2):S61–5. doi: 10.1086/522149. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Sherman DL, Zhu Z, Gabel CA, Ambron RT, Gershon MD. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–90. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson RE. The nature of herpes zoster: a long term study and a new hypothesis. Proc Roy Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. Journal of neurovirology. 2010;16:411–8. doi: 10.1007/BF03210846. [DOI] [PubMed] [Google Scholar]
- Kondo T, Oshima T, Obata K, Sakurai J, Knowles CH, Matsumoto T, Noguchi K, Miwa H. Role of transient receptor potential A1 in gastric nociception. Digestion. 2010;82:150–5. doi: 10.1159/000310836. [DOI] [PubMed] [Google Scholar]
- Lowry PW, Solem S, Watson BN, Koropchak C, Thackeray H, Kinchington P, Ruyechan W, Ling P, Hay J, Arvin A. Immunity in strain 2 guinea pigs inoculated with vaccinia virus recombinants expressing varicella-zoster virus glycoproteins I, IV, V, or the protein product of the immediate early gene 62. J Gen Virol. 1992;73:811–819. doi: 10.1099/0022-1317-73-4-811. [DOI] [PubMed] [Google Scholar]
- Lungu O, Panagiotidis C, Annunziato P, Gershon A, Silverstein S. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Nat Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Sun XW, Wright TC, Ferenczy A, Richart RM, Silverstein S. A polymerase chain reaction-enzyme-linked immunosorbent assay method for detecting human papillomavirus in cervical carcinomas and high-grade cervical cancer precursors. Obstet Gynecol. 1995;85:337–342. doi: 10.1016/0029-7844(94)00399-x. [DOI] [PubMed] [Google Scholar]
- Mallet E, Maitre M, Mouterde O. Complications of the digestive tract in varicella infection including two cases of erosive gastritis. Eur J Pediatr. 2006;165:64–5. doi: 10.1007/s00431-005-1748-2. [DOI] [PubMed] [Google Scholar]
- Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Stanberry L. Drug testing for activity against varicella-zoster virus in hairless guinea pigs. Antiviral Res. 1991;15:341–344. doi: 10.1016/0166-3542(91)90015-j. [DOI] [PubMed] [Google Scholar]
- Myers M, Stanberry L, Edmond B. Varicella-zoster virus infection of strain 2 guinea pigs. J Infect Dis. 1985;151:106–113. doi: 10.1093/infdis/151.1.106. [DOI] [PubMed] [Google Scholar]
- Myers MG, Connelly B, Stanberry LR. Varicella in hairless guinea pigs. J Infect Dis. 1991;163:746–751. doi: 10.1093/infdis/163.4.746. [DOI] [PubMed] [Google Scholar]
- Nagel MA, Choe A, Traktinskiy I, Cordery-Cotter R, Gilden D, Cohrs RJ. Varicella-zoster virus transcriptome in latently infected human ganglia. Journal of virology. 2011;85:2276–87. doi: 10.1128/JVI.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomdedeu JF, Nomdedeu J, Martino R, Bordes R, Portorreal R, Sureda A, Domingo-Albos A, Rutllant M, Soler J. Ogilvie’s syndrome from disseminated varicella-zoster infection and infarcted celiac ganglia. J Clin Gastroenterol. 1995;20:157–9. doi: 10.1097/00004836-199503000-00020. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Pui JC, Furth EE, Minda J, Montone KT. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie’s syndrome) Am J Gastroenterol. 2001;96:1627–30. doi: 10.1111/j.1572-0241.2001.03808.x. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Grider JR. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Experimental neurology. 2009;219:93–103. doi: 10.1016/j.expneurol.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabella C, Lowry P, Abbruzzi G, Koropchek C, Kinchington P, Sagedh-Zadeh M, Hay J, Ruyechan W, Arvin A. Immunization with immediate-early tegument protein (open reading frame 62) of varicella-zoster virus protects guinea pigs against virus challenge. J Virol. 1993;67:7673–7676. doi: 10.1128/jvi.67.12.7673-7676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyaprakash AK, Tremaine AM, Stelter AA, Creed R, Ravanfar P, Mendoza N, Mehta SK, Rady PL, Pierson DL, Tyring SK. Viremia in Acute Herpes Zoster. J Infect Dis. 2009 doi: 10.1086/599381. [DOI] [PubMed] [Google Scholar]
- Soong W, Schultz JC, Patera AC, Sommer MH, Cohen JI. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J Virol. 2000;74:1864–70. doi: 10.1128/jvi.74.4.1864-1870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings CL, Duigou GJ, Gershon AA, Gershon MD, Silverstein SJ. The cellular localization pattern of Varicella-Zoster virus ORF29p is influenced by proteasome-mediated degradation. J Virol. 2006;80:1497–512. doi: 10.1128/JVI.80.3.1497-1512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussery XT, Annunziato P, Gershon A, Reid B, Lungu O, Langston C, Silverstein S, Lee K, Baker CJ. Congenital varicella-zoster infection and Barrett’s esophagus. J Infect Dis. 1998;178:539–543. doi: 10.1086/517469. [DOI] [PubMed] [Google Scholar]
- Walters MS, Kyratsous CA, Wan S, Silverstein S. Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J Virol. 2008;82:8673–86. doi: 10.1128/JVI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerboni L, Sobel RA, Ramachandran V, Rajamani J, Ruyechan W, Abendroth A, Arvin A. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J Virol. 2010;84:3421–30. doi: 10.1128/JVI.02416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Gershon MD, Gabel C, Sherman D, Ambron R, Gershon AA. Entry and egress of VZV: role of mannose 6-phosphate, heparan sulfate proteoglycan, and signal sequences in targeting virions and viral glycoproteins. Neurology. 1995;45:S15–17. doi: 10.1212/wnl.45.12_suppl_8.s15. [DOI] [PubMed] [Google Scholar]