Abstract
In this issue of Kidney International, Linkermann, et al. provide the first evidence for a possible biochemical mechanism of necrotic kidney cell death associated with renal ischemia/reperfusion-induced acute kidney injury. The mechanisms of several pathways resulting in programmed necrosis were recently elucidated and rely on receptor-interacting protein kinases 1 and 3. Using an inhibitor of one of these kinases, Linkermann was able to ameliorate functional and morphologic kidney damage after ischemia/reperfusion.
Acute kidney injury (AKI) is defined by a rapid loss of kidney function, and it is also associated with kidney cell death. In animal models of AKI the morphology is predominantly characterized as necrosis, and acute tubular necrosis is associated with human AKI arising from several causes. However, most extensively studied in vitro models of AKI, such as cisplatin exposure, result in apoptosis rather than necrosis (1). The morphologic differences between these two types of cell death were first described in 1972 by Kerr, et al. (2). Subsequently, apoptosis was characterized as “programmed” and biochemical pathways resulting in apoptotic cell death were delineated (3); necrosis was assumed to be “accidental” rather than the result of defined pathways. Evidence was emerging that depending on the stimulus, apoptosis and necrosis represented a continuum of cell death morphologies (4), and recently, unique biochemical pathways of necrosis were elucidated (5, 6). A type of programmed necrotic cell death, initially found to be the result of stimulation of cell death receptors such as Fas, was given the name “necroptosis” and required the activity of receptor-interacting protein 1 (RIP1), a novel protein kinase (7). Similarly, a programmed necrosis pathway independent of cell death receptor activation can be initiated by the assembly of a heteromultimeric protein scaffold of 2 MDa in the cytosol, termed the “ripoptosome” (8). The protein complexes found in these pathways contain RIP1 and require its kinase activity, but can result in either apoptosis or necrosis. The decision to engage the necrosis pathway was found to be dependent on the kinase activity of another enzyme, RIP3.
RIP kinase activity is associated with the pathogenesis of several diseases, including brain ischemia, head trauma, myocardial infarction, neuronal cell death, cerulein-induced pancreatitis, viral infections, and it plays a role in lipopolysaccharide-induced cell death (9). RIP1 knock-out mice die at 1-3 days after birth and display extensive apoptosis in their lymphoid and adipose tissue indicating that this kinase is also important during development (10). In this issue, Linkermann, et al. (11) present evidence that RIP1 kinase activity also contributes to the damage in a mouse model of renal ischemia/reperfusion (I/R). Administration of the drug necrostatin-1, a specific RIP1 kinase inhibitor (7), reduced kidney damage and attenuated AKI after I/R. A necrostatin derivative that was not able to inactivate the kinase activity had no effect on AKI. Significantly, they also found that treating mice with the pan-caspase inhibitor zVAD-fmk did not prevent kidney damage and did not reduce I/R-induced elevations of serum creatinine or urea, and did not reduce renal cell death. Electron microscopic examination of kidney tissue after I/R showed typical hallmarks of necrotic but not apoptotic cell death. However, other parameters that could have provided evidence of apoptosis, such as TUNEL staining to detect apoptosis-specific DNA damage, were not reported. These findings suggest that caspase-dependent apoptosis does not play a significant role in this setting. Similarly, caspase-3 knockout mice were not protected from I/R AKI (Kaushal GP, personal communication) although these mice were reported to be partially protected from polycystic kidney disease (12). Caspase-8 activation promotes cell death in receptor-mediated apoptosis, and caspase-8 is also found in multimeric protein complexes in programmed necrosis pathways. At the same time, caspase-8 has a role in survival, likely by preventing RIP-dependent necrosis during development, and caspase-8 knockout mice are not viable (6). Whether caspase-8 is a protective protein or promotes kidney cell death in I/R AKI would therefore be interesting to study.
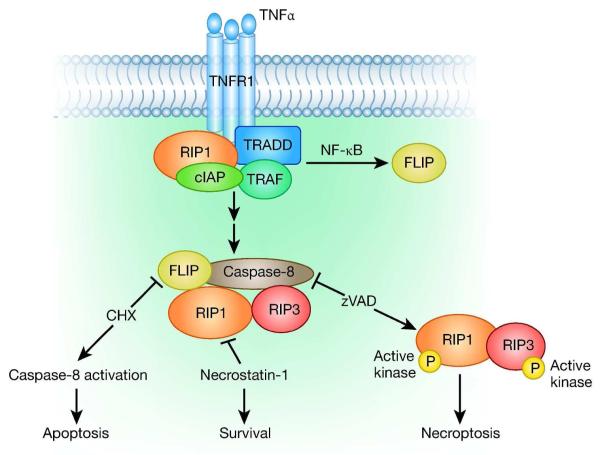
The best described pathways of programmed necrosis are associated with the activation of death receptors (Figure 1). After ligand binding, the activated receptor promotes the assembly of multimeric protein scaffolds ultimately containing caspase-8, RIP1 and RIP3. The activation of NF-κB and transcription of downstream proteins usually promotes cell survival, which paradoxically could be dependent on caspase-8 (6). When the NF-κB signal is blocked, as with cycloheximide addition, caspase-8 activation predominates and apoptosis results. When caspase-8 is inhibited, as with zVAD addition, RIP3 kinase is activated, resulting in programmed necrosis, or necroptosis. Linkermann, et al. have demonstrated that necrosis resulting from these protein assemblies can occur in cultured kidney cells, indicating that these pathways could also occur in kidney in vivo. Using several cell lines derived from kidney tissues, and inducing cell death by stimulating the tumor necrosis factor (TNFα) death receptor pathway in the presence of cycloheximide and zVAD-fmk, the authors showed that glomerular endothelial cells were sensitive to necroptosis, whereas mesangial cells and podocytes were not. Cell death in a mouse kidney tubular cell line (TKPTS) was protected both by necrostatin-1 and by RIP1 knockdown, but only the glomerular endothelial cells were protected by RIP3 knockdown.
Figure 1.
Binding of the TNFα ligand to its receptor, TNFR1, leads to recruitment of TNF receptor-associated death domain (TRADD). These proteins associate with other cytoplasmic proteins, including RIP1, TNF receptor-associated factors (TRAFs), and cellular inhibitor of apoptosis proteins (cIAPs) to form Complex 1. Complex 1 can also activate NF-κB and thereby transcription of FLICE-like inhibitory protein long (FLIP). Several steps later, the RIP1-associated complex can functionally interact with RIP3 and caspase-8, forming Complex 2. With high levels of FLIP, the complex does not result in RIP3 signaling and cell death is averted. Without FLIP, as with cycloheximide (CHX) addition, caspase-8 activation predominates and apoptosis results. When caspase-8 is inhibited, as with zVAD addition, RIP3 kinase is activated, resulting in programmed necrosis, or necroptosis. Necrostatin-1 blocks the kinase activity of RIP1, which is necessary for both apoptotic and necrotic pathways directed by Complex 2. Not shown here, in a similar death receptor mediated pathway, ligand binding to CD95/Fas induces a conformational change in the receptor, which promotes the assembly of a complex with Fas-associated death domain (FADD) and caspase-8. Similarly to the TNF pathway, FLIP can prevent caspase-8 apoptosis and cycloheximide addition can promote cell death.
Since exposure of cultured cells to TNFα, cycloheximide, and zVAD has been used to demonstrate the existence of the necroptosis pathway (8, 9), it is significant that several kidney cell types were also sensitive to these stimuli, showing that necroptosis could be a potential cell death pathway in the kidney. However, it is unclear whether common molecular pathways are shared between I/R-induced renal cell death in vivo and death receptor-initiated cell death occurring in vitro in the presence of protein synthesis and caspase inhibitors. It is also possible that the recently described “ripoptosome” (8) participates in the in vivo mechanism of I/R-induced kidney cell death. This multimeric protein complex assembles in the cytosol independent of cell death receptor activation, and its formation could be stimulated by exposure to etoposide, a DNA damaging agent, or by depletion of cellular Inhibitor of Apoptosis Proteins (cIAPs). Its assembly was dependent on RIP1 and the complex could induce either caspase-8-dependent apoptosis or RIP3 kinase-dependent necrosis. The cellular stresses promoting ripoptosome formation, its cell-type specificity, as well as the ultimate consequences of its formation are still being elucidated.
The results of Linkermann, et al. are provocative and could encourage future examination of programmed necrosis pathways in AKI. Although they provide sufficient evidence that a RIP1 kinase-dependent pathway plays a role in renal I/R, the implication that necroptosis is the causative mechanism is not yet convincing. RIP1 kinase activity is most likely involved in the pathobiology, but necrostatin-1, a RIP1 kinase inhibitory drug, can also inhibit apoptosis. Cultured cells were made to undergo necroptosis showing that this pathway could exist in kidney in vivo, but attempts to demonstrate by coimmunoprecipitation the associations of RIP1 and RIP3 kinases thought to be necessary for necroptosis, were not successful.
It is hoped that these studies will provide the stimulus for further investigations on pathways of programmed necrosis in kidney disease, so that for AKI, to paraphrase Winston Churchill, it is, perhaps, the end of the beginning.
Contributor Information
Peter M. Price, University of Arkansas for Medical Sciences-Internal Medicine 4300 W 7th Street Little Rock, Arkansas 72205 501-257-4800
Rawad Hodeify, University of Arkansas for Medical Science Internal Medicine Little Rock, Arkansas.
References
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green DR. Means to an end: Apoptosis and other cell death mechanisms. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2010. [Google Scholar]
- 4.Zong W-X, Thompson CB. Necrotic death as a cell fate. Genes & Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 5.Golstein P, Kroemer G. Cell death by necrosis: toward a molecular definition. Trends Biochem Sci. 2006;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS. RIPK-Dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand MJM, Vandenabeele P. The ripoptosome: death decision in the cytosol. Mol Cell. 2011;43:323–325. doi: 10.1016/j.molcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered explosion. Nature Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 10.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 11.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. RIP1-mediated ripoptosis essentially contributes to renal ischemia/reperfusion injury. 2012. [DOI] [PubMed]
- 12.Tao Y, Zafar I, Kim J, Schrier RW, Edelstein CL. Caspase-3 gene deletion prolongs survival in polycystic kidney disease. J Am Soc Nephrol. 2008;19:749–755. doi: 10.1681/ASN.2006121378. [DOI] [PMC free article] [PubMed] [Google Scholar]