Abstract
Many tumors, including lung cancers, promote immune tolerance to escape host immune surveillance and facilitate tumor growth. Tumors utilize numerous pathways to inhibit immune responses, including the elaboration of immune-suppressive mediators such as PGE2, TGF-β, IL-10, VEGF, GM-CSF, IL-6, S100A8/A9 and SCF, which recruit and/or activate myeloid-derived suppressor cells (MDSCs). MDSCs, a subset of heterogeneous bone marrow-derived hematopoietic cells, are found in the peripheral blood of cancer patients and positively correlate to malignancy. Solid tumors contain MDSCs that maintain an immune-suppressive network in the tumor microenvironment. This review will focus on the interaction of tumors with MDSCs that lead to dysregulation of antigen presentation and T-cell activities in murine tumor models. Specific genetic signatures in lung cancer modulate the activities of MDSCs and impact tumor progression. Targeting MDSCs may have a long-term antitumor benefit and is at the forefront of anticancer therapeutic strategies.
Keywords: APC, arginase 1, immune suppression, immune therapy, lung cancer, myeloid-derived suppressor cells, nitric oxide synthase, T lymphocytes, tumor genetic signature
The tumor microenvironment consists of tumor cells, stroma, blood vessels, immune infiltrates and the extracellular matrix. Genetic alterations in oncogenes and tumor suppressor genes, or epigenetic changes in the tumor that modulate tumor growth and invasion into the surrounding tissue orchestrate the persistence of inflammatory infiltrates. These cellular infiltrates modulate tumor development and progression. The tumor infiltrates vary by size and composition in diverse tumor types and at different stages of tumor development. The tumor programs the cellular infiltrates to sustain a dysregulated inflammation that is hyporesponsive to the tumor. Characterization of the complex interactions among the infiltrates and tumor will aid in defining their role in tumor progression. This understanding will be important for the development of novel anticancer therapies. Although this is not a trivial undertaking, the information garnered will take us a step closer to personalized medicine. If we know an individual’s tumor inflammatory infiltrates, we will be able to predict the risk of tumor progression and then give specific treatment to reprogram the tumor microenvironment to control the disease.
Contributing to the inflammatory infiltrates of the innate system are NK cells and the myelomonocytic cell lineage consisting of immature macrophages, granulocytes and dendritic cells (DCs), as well as myeloid cells at earlier stages of differentiation [1–4]. The downregulation of MHC expression by tumors enables them to evade T-cell immune responses. The presence of NK cells in the infiltrates can contribute to antitumor activity because NK effectors recognize tumor targets independent of MHC expression [5]. However, there is usually a paucity of NK cells in the tumor microenvironment, suggesting evasion mechanisms are preventing their recruitment. Macrophages in the tumor microenvironment play an important modulatory role in the generation of antitumor responses. The production of chemotactic factors such as CCL2, VEGF and M-CSF in the tumor microenvironment recruits macrophages [6,7]. The type of macrophages infiltrating the tumor correlates with favorable or unfavorable prognoses [8]. The M1 macrophages have a potent antigen presentation function and stimulate type 1 immune responses that lead to tumor rejection, tissue destruction and host defense. M1 macrophage density in the tumor islets is positively associated with extended survival of non-small-cell lung cancer (NSCLC) patients [9]. The M1 macrophages produce high levels of IL-12, CXCL10 and inducible nitric oxide synthase (iNOS) [10]. By contrast, M2 macrophages are thought to promote tumor formation by enhancing wound healing and tissue remodeling via inhibition of type 1 immune responses by IL-10 and TGF-β secretion [10–13]. The M2 macrophages express high levels of IL-10 and arginase, which suppress antitumor immune responses. These macrophages increase metastatic potential by increasing tumor cell migration, invasion, angiogenesis and metastases. The tumor microenvironment also consists of T and B lymphocytes of the adaptive immune system. The phenotypes of the T and B subsets evoked in a chronic inflammatory state of the tumor microenvironment are regulatory in nature and dampen immune responses against the tumor. B cells and antibodies have a key role in orchestrating macrophage-driven, tumor-promoting inflammation [14], suggesting that modulating the pathways involved might be of therapeutic benefit in cancers driven by chronic inflammation.
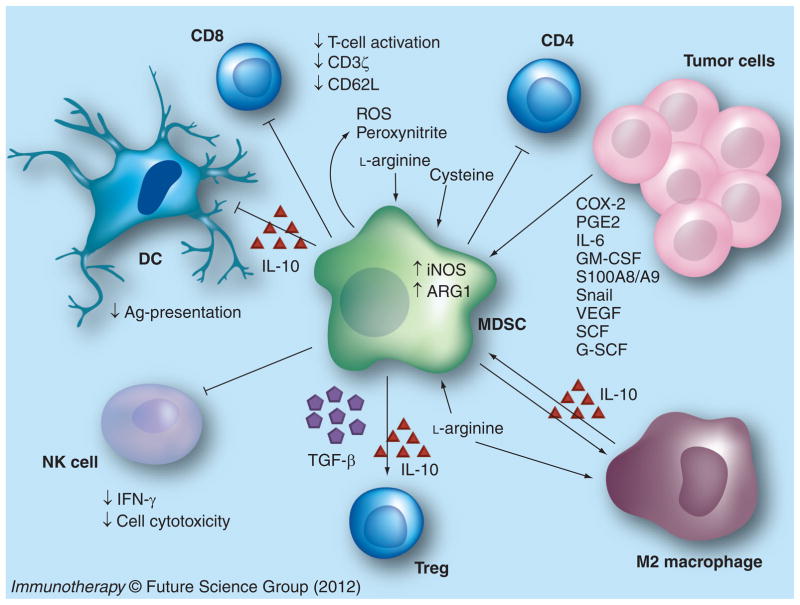
Solid tumors contain a significant population of tumor-infiltrating myeloid cells, which promote tumor growth by suppressing the immune system (Figure 1). This review will focus on the interaction between the tumors and myeloid-derived suppressor cells (MDSCs), which suppress host APC and T-cell activities and contribute to tumor progression.
Figure 1. Myeloid-derived suppressor cell accumulation in tumors suppresses antitumor activity.
MDSCs are recruited to and expanded in the tumor through the induction/production of COX-2, PGE2, IL-6, GM-CSF, S100 proteins and snail in the tumor. T-cell activation is suppressed by MDSC-mediated deprivation of l-arginine and cysteine from the environment, the production of ROS and peroxynitrite, downregulation of CD62L and the T-cell receptor-associated ζ chain, and the induction of Tregs via MDSC-mediated IL-10 and TGF-β production. MDSCs suppress NK cell cytotoxicity and NK cell IFN-γ production, and induce tumor-associated macrophages with a type 2 phenotype. MDSC expansion and IL-10 production inhibits DC antigen presentation.
Ag: Antigen; DC: Dendritic cell; MDSC: Myeloid-derived suppressor cell; ROS: Reactive oxygen species; SCF: Stem cell factor.
MDSC-mediated immune modulation in the tumor microenvironment
MDSCs are a heterogeneous population of immature myeloid cells consisting of myeloid progenitors and precursors of macrophages, granulocytes and DCs. In mice, MDSCs are identified by antibodies that detect cell surface expression of Gr1 and CD11b. Gr1 includes the macrophage and neutrophil markers Ly6C and Ly6G, respectively, while CD11b is characteristic of macrophages. More recently, MDSCs have been subdivided into different subtypes based on their expression of Ly6C and Ly6G. CD11b+Ly6G+Ly6Clo cells with granulocytic-like morphology and multilobed nuclei are called granulocytic MDSCs, whereas CD11b+Ly6G−Ly6Chi cells with monocytic-like morphology are referred to as monocytic MDSCs [15]. In cancer patients, MDSCs are identified by surface expression of the myeloid marker CD33 and the lack of expression of markers of mature myeloid and lymphoid cells. They are typically CD11b+CD33+CD34+CD14− cells that vary in CD15, CD124, CD66 and MHC class II expression, along with other markers [16]. A monocytic MDSC with a phenotype of CD14+CD11b+HLA−DRlo/neg in melanoma patients [17] and CD11b+CD14−CD15+CD33+ in NSCLC patients [18,19] has been identified. Because of the variation in MDSC gene expression between different tumor microenvironments, it has been challenging to identify a unique set of markers for human MDSCs. Thus, along with phenotypic characterization, the functional ability of MDSCs to suppress T cells is the defining hallmark of a MDSC.
MDSC accrual in the tumor microenvironment is dependent on tumor-derived soluble factors including growth factors, cytokines and chemokines. GM-CSF supports the survival and expansion of MDSCs in the tumor microenvironment [20]. The sources of GM-CSF are tumors or activated immune effectors such as T cells, NK cells and DCs. IL-1β has been demonstrated to accumulate MDSCs in the tumors of mice with mammary carcinoma or fibrosarcomas. Mice inoculated with tumor cells secreting IL-1β accumulate elevated levels of MDSCs in comparison with tumor controls lacking IL-1β secretion. In turn, the IL-1β-induced MDSCs have elevated reactive oxygen species (ROS) with enhanced suppressive activity against T cells [21,22]. The elaboration of IL-6 from tumors increases the accumulation of MDSCs in the tumor microenvironment with a similar impact on immune suppression [21].
Proinflammatory S100 proteins promote MDSC accumulation and suppressive activity [23,24]. The S100A8 and S100A9 proteins are members of a large family of proteins that includes inflammatory and noninflammatory molecules. Heterodimeric S100A8/A9 complexes are calcium-binding proteins that are released by neutrophils, activated monocytes, tumor cells and MDSCs. MDSCs have receptors for S100A8/A9 complexes and enhance the levels of S100A8/A9 in the tumor microenvironment through an autocrine loop. Antibody blockade of the receptors reduces the number of MDSCs in the tumors and secondary lymphoid organs of tumor-bearing mice. Mice genetically deficient for S100A9 are resistant to challenge with colon carcinoma but become susceptible if MDSCs are adoptively transferred from wild-type mice to S100A9-deficient mice. S100A8/A9 heterodimers mediate these effects through at least two mechanisms: they block the differentiation of myeloid precursors to differentiated DCs and macrophages through a STAT3-dependent mechanism, and they chemoattract MDSCs into the tumor through a NF-κB-dependent pathway [23,24]. These studies suggest that S100 proteins mediate the accumulation of MDSCs in cancer and may serve as a useful therapeutic target to reduce tumor-induced immune suppression. Inflammation-mediated S100A8/A9 induction, MDSC accumulation and immune suppression provide the link through which inflammation can contribute to cancer.
Molecular & cellular mechanisms of MDSC-mediated immune suppression
MDSCs suppress immune responses to newly displayed tumor antigens, and promote tumor progression and the metastatic potential of the tumor. MDSCs suppress T-cell activation in tumor tissues and draining lymph nodes through several mechanisms. Murine MDSCs use two enzymes involved in L-arginine metabolism to control T-cell responses: arginase 1 (ARG1), which depletes the milieu of L-arginine and iNOS2, which generates nitric oxide (NO) [25,26]. L-arginine is essential for T-cell function, including the optimal use of IL-2 and the development of a T-cell memory phenotype. MDSC ARG1 is induced by cytokines such as TGF-β and IL-10 within the tumor microenvironment. The MDSC-mediated depletion of arginine suppresses CD4+ and CD8+ T-cell activation. IFN-γ and TNF-α in the tumor microenvironment induce iNOS in MDSCs, releasing NO, which blocks the phosphorylation and activation of several targets in the IL-2 receptor signaling pathway and induces T-cell apoptosis [27].
Cysteine, another essential amino acid for T-cell activation, is depleted by MDSCs [28]. T cells lack both the enzyme to convert methionine to cysteine and the membrane transporter to import cystine for intracellular reduction to cysteine. T cells obtain their cysteine from extracellular sources. During normal antigen processing and presentation activity, DCs and macrophages synthesize cysteine from methionine and import extracellular cystine for cysteine conversion. Cysteine is then exported by APCs during antigen presentation, and imported by T cells. Like T cells, MDSCs are unable to convert methionine to cysteine and are dependent on importing cystine for conversion to cysteine. In the tumor microenvironment MDSCs are present in high concentration and import most of the available cystine, depriving DCs and macrophages. Since MDSCs do not export cysteine, they deprive T cells of cysteine, which is necessary for synthesizing the proteins required for T-cell activation [28].
MDSC-mediated downregulation of T-cell L-selectin (CD62L) further impairs T-cell activity [29]. CD62L is a plasma membrane molecule necessary for the homing of naive T cells to lymph nodes for activation by tumor antigens. MDSCs downregulate CD62L on naive T cells, which reduce the T cells’ capacity to migrate to lymph nodes [29].
MDSC-produced ROS and peroxynitrite in the tumor microenvironment inhibit CD8+ T cells by catalyzing the nitration of the T-cell receptor and thereby preventing T cell–peptide–MHC interactions [30]. MDSCs also downregulate the T-cell receptor-associated ζ chain, a phenomenon common in cancer patients [31]. In the absence of the ζ chain, T cells are unable to transmit the required signals for activation.
MDSCs impair T-cell activation by directly inducing Tregs through the production of IL-10 and TGF-β, or arginase, which is independent of TGF-β [1]. The Tregs actively downregulate the activation and expansion of antitumor-reactive T cells [32–34] and NK cells [35]. MDSCs affect tumor immunity by polarizing T cells towards a tumor-promoting type 2 phenotype, by producing IL-10 and downregulating macrophage production of IL-12 [13]. The suppressive activity of MDSCs on T cells can be antigen-specific or nonspecific and can vary depending on the MDSC subpopulation. MDSCs impair NK cells by inhibiting their cytotoxicity ability and IFN-γ production [36,37].
Tumor-specific genetic signatures as drivers of immune suppression in lung cancer
The authors’ laboratory has been evaluating tumor signatures that maintain tumor growth kinetics through the modulation of immune activity [38,39]. Many tumors, including those of lung cancer, have the capacity to promote immune tolerance and escape host immune surveillance [40,41]. Tumors utilize numerous pathways to inhibit immune responses including the elaboration of immune-inhibitory cytokines. In addition to directly secreting immunosuppressive cytokines, lung cancer cells may induce host cells to release immune inhibitors [38,39,42–44]. In previous studies, the authors found an immune-suppressive network in NSCLC that is due to overexpression of tumor COX-2 [39,45], which is constitutively expressed in a variety of malignancies. The authors, and others, have reported that COX-2 is frequently constitutively elevated in human NSCLC [39,46–48]. Although multiple genetic alterations are necessary for lung cancer invasion and metastasis, COX-2 may be a central element in orchestrating this process [39,47–51]. Overexpression of COX-2 is associated with apoptosis resistance [52,53], promotion of angiogenesis [54,55], enhanced tumor invasion and metastasis [55–57] and decreased host immunity [39,45,58]. In murine lung cancer models, the authors reported that specific genetic or pharmacological inhibition of COX-2 reduced tumor growth [45]. In other related studies, the authors documented that COX-2 inhibition prevented tumor-induced suppression of DC activities [58]. In recent studies, they have also demonstrated that treatment of mice with a COX-2 inhibitor promoted a type 1 cytokine response, inducing IFN-γ, IL-12 and CXCL10, and augmented the vaccination response to tumor challenge [59].
Tumor COX-2 can also modulate MDSC activity through ARG1 in lung carcinoma [60]. MDSCs producing high levels of ARG1 block T-cell function by depleting arginine. Until recently, the mechanism by which ARG 1 in MDSCs is induced in cancer was unknown. Rodriguez et al., utilizing the mouse Lewis lung carcinoma model (3LL, which spontaneously arose in the C57BL/6 mice), showed that ARG1 expression is independent of T-cell-produced cytokines and that tumor-derived PGE2 maintains ARG1 expression in MDSCs [60]. 3LL tumor cells constitutively express COX-1 and COX-2, and produce high levels of PGE2. Genetic or pharmacological inhibition of COX-2, but not COX-1, blocked ARG1 induction in vitro and in vivo. Signaling through the PGE2 receptor E-prostanoid (EP) 4 expressed in MDSCs induced ARG1. Furthermore, blocking ARG1 expression using COX-2 inhibitors elicited a lymphocyte-mediated antitumor response [60]. These results demonstrate a new pathway of prostaglandin-induced immune dysfunction and provide a novel mechanism to help explain the antitumor benefits of COX-2 inhibitors, which-target the major immune-suppressive pathways mediated by MDSCs.
The complex nature of the interactions between MDSCs and Tregs has yet to be fully defined; however, it is evident that MDSCs promote Treg development in vivo. Tumor-reactive T cells have been shown to accumulate in lung cancer tissues but fail to respond to the tumors because of suppressive tumor cell-derived factors [61,62] and because high proportions of NSCLC tumor-infiltrating lymphocytes are CD4+CD25+ Tregs [63]. CD4+CD25+ Tregs play an important role in the maintenance of immunological self-tolerance [64]. Treg activities increase in lung cancer, and appear to play a role in suppressing antitumor immune responses. Tregs actively downregulate the activation and expansion of self-reactive lymphocytes [32]. Given that many of the tumor-associated antigens recognized by autologous T cells are antigenically normal self-constituents, Tregs engaged in the maintenance of self-tolerance may impede the generation and activity of antitumor-reactive T cells [32,33]. Thus, reducing the number of Tregs or abrogating their activity within the tumor environment may induce effective tumor immunity in otherwise nonresponding hosts by activating tumor-specific as well as -nonspecific effector cells [65–67]. In recent studies, the authors have demonstrated that tumor COX-2 expression contributes to decreased host antitumor immune responses by impacting the frequency and activity of CD4+CD25+FOXP3+ Tregs [68,69]. The definition of the pathways controlling Treg activities will enhance our understanding of limitations of the host antitumor immune responses. The authors demonstrated that lung tumor-derived COX-2/PGE2 induced expression of the Treg-specific transcription factor, Foxp3, and increased Treg activity. Assessment of EP receptor requirements revealed that PGE2-mediated induction of Treg Foxp3 gene expression was significantly reduced in the absence of the EP4 receptor and ablated in the absence of EP2 receptor expression. Invivo, COX-2 inhibition reduced Treg frequency and activity, attenuated Foxp3 expression in tumor-infiltrating lymphocytes and decreased tumor burden. The transfer of Tregs or administration of PGE2 to mice receiving COX-2 inhibitors reversed these effects. The authors’ studies were the first to report that COX-2 inhibition downregulates tumor-induced Treg activity, leading to the restoration of antitumor responses.
Tumor snail knockdown reduces MDSCs & increases CD107a-activated effector T cells in the tumor microenvironment
The authors are defining genetic programs in lung cancer that modulate tumor growth and metastases. Cancer cells acquire the ability to progress, invade and metastasize by undergoing the process of epithelial–mesenchymal transition (EMT), by activating transcription factors (e.g., snail, twist, zeb and slug) that repress E-cadherin, a transmembrane glycoprotein essential for epithelial cell–cell adhesion [70,71]. These transcriptional repressors are normally active during embryogenesis, where they program the EMT to enable various morphogenetic steps. The EMT is involved in tumor progression [72,73]. Snail expression in primary NSCLC has been associated with a shorter overall survival [74]. Tumor snail expression has recently been demonstrated to be important in EMT-induced metastases in melanoma [75]. The authors are evaluating the mechanistic role of tumor snail expression in the modulation of tumor growth and metastases in immune-competent mice. Their data demonstrate that tumor snail expression alters tumor growth and metastasis by impacting on MDSCs in the tumor microenvironment [76]. 3LL, 3LL snail knockdown and 3LL control vector cells were implanted in C57BL/6 mice. Compared with controls, 3LL snail knockdown mice had:
Decreased MDSCs;
Reduced MDSCs, as well as the non-MDSC populations intracellular expression of ARG1 in the tumors;
Increased expression of the CD107a cytolytic marker in tumor-infiltrating CD8+ T cells;
Increased tumor infiltrates of CD4+ and CD8+ T lymphocytes that elaborated enhanced IFN-γ but reduced levels of IL-10;
Augmented frequencies of innate NK effectors and DCs.
Accompanying the inflammatory signature, snail knockdown cells demonstrated reduced subcutaneous tumor growth and lung metastases. Current experiments are mechanistically delineating the genetic program(s) induced by tumor snail knockdown that alter the balance and activity of immune effectors and suppressors in the tumor, as well as the impact of adoptive transfer of MDSCs on the tumor growth kinetics of snail knockdown cells. An adequate understanding of the genetic signatures in tumor and tumor–host interactions that induce immune evasion and promote tumor growth, invasion and metastases will be crucial for the development of effective therapies for lung cancer.
Impact of depleting Gr1 or Ly6G myelomonocytic cells on 3LL tumor growth kinetics
Increases in the number of MDSCs evoke strong natural suppressive activity in cancer patients [77,78] or tumor-bearing mice [77,79,80]. It has been demonstrated that Gr+CD11b+ immune-suppressive cells are capable of inhibiting the T-cell proliferative response induced by alloantigens [81], CD3 ligation [82] or various mitogens [83,84], and can also inhibit IL-2 utilization [85], as well as NK cell activity [78]. These studies indicate that progressive tumor growth is associated with the downregulation of T-cell responses and that the Gr+CD11b+ myeloid cells are involved in negative immunoregulatory mechanisms in the tumor-bearing host. In murine tumor models there is an increase in the MDSC populations of the tumors, spleen, bone marrow and blood as the tumor progresses. In the 3LL lung cancer model, as the tumors progress, the frequency and activity of immune-suppressive cells are enhanced in the tumor microenvironment. The authors have found that as many as 45% of tumors have infiltrates that are predominantly of the GR1+ CD11b+ immature myeloid phenotype. As has been recently reported for murine glioblastoma [86] and colon [87] cancer models, the authors evaluated the contribution of the Gr1- and Ly6G-expressing myelomonocytic cells on 3LL tumor growth in C57BL/6 mice, by depleting cells expressing these markers with anti-Gr1 (RB6-8C5) or anti-Ly6G (1A8) administered every other day via an intraperitoneal route starting on day 5 post tumor inoculation. Compared with isotype-matched control antibody, the anti-Gr1 antibody or anti-Ly6G led to a 85–90% decrease in the Gr1hiCD11b-expressing myeloid subset and a subsequent increase in the CD107a-expressing CD3+ T lymphocytes (two- to threefold) and NK cells (threefold) in the tumors. Accompanying the decrease in the Gr1hiCD11b-expressing myeloid subset was an eightfold decrease in tumor weight. The Ly6G-expressing cells are a subset of the Gr1-positive population, and depletion of either of the cell populations expressing these markers led to similar antitumor activity in the 3LL cancer model. The rationale for using these two different antibodies to target MDSCs was to confirm that depletion of these cells leads to reduced tumor growth rate and progression. Although these depletion antibodies impact other Gr1- or Ly6G-expressing monocytes, the authors’ data suggest that the broad targeting of MDSCs along with other myeloid cell types is beneficial in eliciting anticancer effects. These data are consistent with studies by several groups [86,87]. It would be interesting to evaluate the impact of MDSC depletion on DC and tumor-associated macrophage functional activity. This may further resolve compensatory pathways of immune suppression. Currently, the authors are evaluating strategies that target the myeloid-suppressor subsets in combination with various immune-potentiating strategies to increase the antitumor benefit.
Critical role of antigen presentation in lung cancer: T-cell tolerance versus T-cell priming
The inadequate function of the host immune system is one of the major mechanisms of tumor escape from the immune system. Effective anti-tumor responses require APC, lymphocyte and NK cell effectors, as well as the elaboration of effector molecules that promote antitumor activity. Although lung cancer cells express tumor antigens, the limited expression of MHC antigens, defective transporter associated with antigen processing and the lack of costimulatory molecules, make them ineffective APCs [88]. Many tumors, including lung cancer, have the capacity to promote immune tolerance and escape host immune surveillance [40,41]. Tumors utilize numerous pathways to inhibit immune responses, including reduction in APC activity. T-cell nonresponsiveness to specific antigens has been shown to be an early event in tumor progression in animal models of cancer and in cancer patients [89]. Although T-cell tolerance in cancer has been shown to be mediated by host APCs [90,91], the nature of these APCs has not been clear. Recent studies provide evidence that MDSCs may represent a population of APCs responsible for induction of antigen-specific CD8 T-cell tolerance in cancer [30,92].
The central importance of functional APCs in the immune response against cancer has been well defined [90]. This study revealed that even highly immunogenic tumors require host APCs for antigen presentation. Thus, host APCs, rather than tumor cells, present tumor antigens. This is consistent with a study indicating that CD8+ T-cell responses can be induced in vivo by professional APCs that present exogenous antigens in a MHC I-restricted manner [93]. This has been referred to as cross-priming or representation and may be critical for effective antitumor responses [94]. DCs have been demonstrated to be the host APC responsible for cross-priming by presenting epitopes obtained from apoptotic cells [95].
However, in tumor-bearing hosts, there is a state of T-cell unresponsiveness [96–98]. Both clonal deletion and active suppression by antigen-specific suppressor T cells appear to contribute to the maintenance of the tolerant state [99–102]. The cellular mechanisms that lead to the induction of the tolerogenic state are not well understood. The dominant mechanism underlying the development of antigen-specific T-cell unresponsiveness is thought to involve tumor-antigen processing and presentation by APCs [91]. The intrinsic APC capacity of tumor cells has little influence over T-cell priming versus tolerance, an important decision that is regulated by bone marrow-derived APCs. DCs, macrophages and B cells are all bone marrow-derived cells that express both the MHC and the costimulatory molecules CD80 and CD86, and present tumor antigens to antigen-specific Tcells.
Several studies have shown that DCs play a critical role in leading to either T-cell tolerance or T-cell priming [103–106], which is dictated by the environmental context in which the DCs encounter the antigen. Antigen capture by DCs in an inflammatory context triggers their maturation to a phenotype capable of generating strong immune responses, whereas antigen capture in a noninflammatory environment leads instead to the development of T-cell tolerance. The tumor microenvironment not only fails to provide the inflammatory signals needed for efficient DC activation, but also inhibits DC differentiation and maturation through IL-10 [107] and VEGF [108]. Myeloid precursor differentiation into granulocytes, macrophages and DCs is frequently dysregulated in the tumor, leading to a decreased generation of fully competent APCs, and the accumulation of MDSCs. Activation of MDSC by various soluble factors produced by the tumor renders them immunosuppressive [109]. Thus, myeloid cells that accumulate in the tumor are blocked in their immature state and do not differentiate into mature DCs. DCs, which are pivotal for T-cell priming, remain immature and become dysfunctional in hosts bearing growing tumors, acquiring tolerogenic properties that induce T-cell tolerance to tumor antigens. Immature DCs have little or no expression of costimulatory molecules such as CD80, CD86 and CD40 on their surface, and produce little or no IL-12, which is required to support T-cell proliferation. Immature DCs are unable to induce an antitumor immune response but can induce T-cell tolerance. If APCs fail to provide an appropriate costimulatory signal for T cells, tolerance or anergy can develop. The importance of restoring APCs with immune-stimulating activity in the tumor microenvironment will be crucial for immunotherapeutic strategies. In a recent study, ectopic lymph node or tertiary lymphoid structures were retrospectively identified within human NSCLC specimens and it was demonstrated that there is a correlation of cellular content with clinical outcome [110]. The density of DC-Lamp, indicating mature DCs within these structures, was a predictor of long-term survival within the selected lung cancer patient population. The authors observed that a low density of tumor-infiltrating CD4+ and T-bet+ T lymphocytes present in tumors poorly infiltrated by DC-Lamp+ mature DCs appears to provide additional supporting evidence for the prognostic importance of an adaptive immune reaction to a solid tumor. The authors have previously demonstrated that elements from the tumor microenvironment can suppress DC function [58]. They found that bone marrow-derived DCs, stimulated with GM-CSF and IL-4 in the presence of tumor supernatants, failed to generate antitumor responses and caused immunosuppressive effects that correlated with enhanced tumor growth. Functional analyses indicated that tumor supernatants cause a decrease in the capacity of DCs to process and present antigens, induce alloreactivity and secrete IL-12. The tumor supernatants caused a reduction in cell surface expression of CD11c, DEC205, MHC I antigen, MHC II antigen, CD80 and CD86, as well as a reduction in TAP-1 and -2 proteins [58].
IL-7–IL-7Rα–Fc promotes the M1 macrophage phenotype in lung cancer
Although tumor growth and invasion leads to inflammatory responses, the immune system generally develops tolerance to cancer. One way to induce potent immune responses against tumors is to activate key innate and immune effector mechanisms. Toward this end, the authors have evaluated the utility of a chimeric γc homeostatic cytokine, IL-7–IL-7Rα–Fc, to restore host APC and T-cell activities dysregulated in cancer patients [111,112]. It is evident from previous studies that intratumoral infiltration by relatively high numbers of activated T lymphocytes [113,114] and APCs [110] leads to a better prognosis in lung cancer patients.
The authors evaluated the utility of the chimeric γc homeostatic cytokine, IL-7–IL-7Rα–Fc, to restore host APC and T-cell activities in lung cancer [115]. Utilizing murine lung cancer models they determined the antitumor efficacy of IL-7–IL-7Rα–Fc. Its administration inhibited tumor growth and increased survival in lung cancer. Accompanying the tumor growth inhibition were increases in APC and T-cell activities. In comparison with controls, IL-7–IL-7Rα–Fc treatment of tumor-bearing mice led to:
Increased tumor macrophage infiltrates characteristic of the M1 macrophage phenotype, with increased IL-12 and iNOS but reduced IL-10 and arginase;
Increased frequencies of T and NK cells;
Increased T-cell activation markers CXCR3, CD69 and CD127low;
Increased effector memory T cells.
IL-7–IL-7Rα–Fc treatment abrogated the tumor-induced reduction in splenic functional APC activity to T responder cells. Our findings demonstrate that IL-7–IL-7Rα–Fc promotes the afferent M1 macrophage phenotype and the efferent (CXCR3–CXCR3 ligand biological axis) limbs of the immune response for sustained antitumor activity in lung cancer. IL-7–IL-7Rα–Fc provides the cues to address the deficits in the lung tumor microenvironment andachieve the requirements for the inhibition of tumor growth kinetics by:
Generating sufficient numbers of T cells systemically;
Increasing the activated T-cell infiltrates in the tumor;
Activating the innate and immune cells in the tumor to manifest antitumor benefit.
Although IL-7–IL-7Rα–Fc is potent at reducing tumor growth kinetics, it does not lead to complete tumor eradication. This may in part be due to the presence of MDSCs in the tumor microenvironment, which dampens the antitumor activity of IL-7–IL7Rα–Fc. This remains to be resolved.
Drug targets impacting MDSCs
As MDSCs have a major immune-suppressive role in cancer, several pharmacological approaches that target MDSCs are currently being explored in tumor-bearing hosts. The drugs can be divided based on their ability to control the following [116]:
MDSC differentiation into mature DCs and macrophages capable of APC activity (all-trans retinoic acid [ATRA] and vitamin D3);
MDSC maturation from precursors (STAT 3 inhibitors, bevacizumab, anti-BV8 monoclonal antibodies, amino-biphosphonates and MMP9 inhibitors);
MDSC proliferation (tyrosine kinase inhibitors [TKI], e.g., sunitinib and sorefnib);
MDSC accumulation (CXCR2 and CXCR4 antagonists);
MDSC cytotoxicity (gemcitabine, 5-fluoro-uracil);
MDSC function/activation (ROS scavengers and ARG and NOX inhibitors, e.g., nitro-aspirin, PDE-5, COX-2 inhibitors and cytokines) [116].
One approach to therapeutic targeting of MDSCs is the use of agents that promote the differentiation of myeloid cells. Gabrilovich et al. demonstrated that differentiating MDSCs to DCs and macrophages by using ATRA reduced MDSC numbers and augmented the responses to cancer vaccines [92]. ATRA induced differentiation of MDSCs primarily via neutralization of high ROS production in these cells. The mechanism involves specific upregulation of glutathione synthase and accumulation of glutathione in the MDSC and could be used in developing and monitoring therapeutic application of ATRA [92].
Recent advances in targeted therapy for cancer have provided small-molecule kinase inhibitors that recognize specific targets on the surface of or inside cancer cells. These inhibitors have shown efficacy against several hematopoietic malignancies and solid tumors. Most TKI drugs generally have inhibitory effects on several kinases, including tyrosine kinases, which are critical for the survival, proliferation, migration and invasion of tumor cells. With regard to the effects of TKI on tumor immunity, some studies have demonstrated immune-stimulatory effects (e.g., imatinib) [117], whereas others report the immune-suppressive effects of the same inhibitor [118].
Several studies have evaluated the effect of the TKI sunitinib on MDSCs [119,120]. The administration of sunitinib, a receptor TKI, has been shown to reduce the frequency of MDSCs and reverse T-cell immune suppression in the peripheral blood of patients with metastatic renal cell carcinoma [119] and in several murine tumor models [120]. However, sunitinib has a variable impact in reducing MDSCs and restoring T-cell activity in the tumor microenvironment, which seems to be tumor dependent. The authors suggest that the persistence of MDSCs in the tumor following sunitinib treatment in renal cell carcinoma may be due in part to increased GM-CSF expression by the tumors, which prolongs the survival of MDSCs and protects from sunitinib through the pSTAT5 pathway. The authors contend that GM-CSF-mediated MDSC survival in patient tumors is supported by the observation that GM-CSF produced by renal cell carcinoma cultures protects MDSCs from sunitinib-induced cell death. However, transducing tumors with GM-CSF in several tumor models has been shown to lead to strong immune-dependent rejection. It would be interesting to see the activity of MDSCs in the microenvironment of the GM-CSF-secreting tumors in these models. Additionally, an alternate explanation for the persistence of MDSCs may be associated with increased expression of proangiogenic proteins, such as MMP9, MMP8 and IL-8, produced by tumor stromal cells or infiltrating MDSCs [120,121]. More studies are required to evaluate the role of TKIs (sunitinib, sorafenib, imatinib and dasatinib) on MDSC activity in the tumor microenvironment and tumor immunity in several tumor models and in clinical samples.
Blocking the recruitment of MDSCs in the tumor may reduce the immune-suppressive effects of these cells. GW2580, a selective molecule TKI of colony-stimulating factor 1 receptor (CSF1R), blocks the recruitment of CSF1R-expressing tumor-associated macrophages as well as monocytic MDSCs in different tumor models without having an impact on tumor burden [122]. PLX3397, another TKI of CSF1R, has also been used to efficiently deplete CD11b+Ly6G−LY6ClowF4/80+ tumor-associated macrophages without altering the presence of granulocytic MDSCs. The treatment of mammary tumor-bearing mice with PLX3397 led to a decrease in tumor burden [123].
MDSC expansion is regulated by tumor-derived factors, and neutralization of the effects of those factors has recently been evaluated. Studies by Pan et al. have demonstrated that the expression of c-kit ligand (stem cell factor [SCF]) by tumor cells may be important for MDSC accumulation in tumor-bearing mice, and that blocking the c-kit ligand–c-kit receptor interaction can reverse MDSC-mediated immune suppression [124]. Mice bearing tumor cells with SCF siRNA knockdown exhibited significantly reduced MDSC expansion and restored proliferative responses of tumor-infiltrating T cells. The blockade of SCF receptor (c-kit)–SCF interaction by antic-kit antibodies prevented tumor-specific T-cell anergy, Treg development and tumor angiogenesis. The authors found that the prevention of MDSC accumulation in conjunction with immune activation therapy showed synergistic therapeutic effect when treating mice bearing large tumors. Their data suggest that modulation of MDSC development may be essential to enhance immune therapy against advanced tumors [124].
N-acetyl cysteine (NAC) has been proposed as an antitumorigenic agent because of its ability to reduce the oxidative stress that promotes genetic instability. NAC treatment of mice with progressively growing tumors has demonstrated therapeutic efficacy [125]. NAC may have the additional benefit of facilitating T-cell activation by increasing extracellular pools of cysteine in the presence of high levels of MDSCs in cancer patients. Although NAC targets the cysteine pathway of MDSC-mediated T-cell suppression, MDSC production of arginase and NO can still maintain the suppressive effects of MDSCs. However, administration of NAC, an already US FDA-approved drug, in combination with other agents that block additional MDSC suppressive pathways (ARG1 and NO), may be more effective at inhibiting MDSCs and facilitate the treatment of cancers.
Several tumors (e.g., lung, breast, colon, pancreatic and prostate) have upregulated COX-2 [126]. COX-2 is required for PGE2 synthesis; drugs that specifically block COX-2 and reduce PGE2 delay tumor growth by reducing MDSC activation. Therefore, inhibition of PGE2 biosynthesis in tumor-bearing mice blocks MDSC activation through EP receptors 1, 2 and 4, and subsequently retards tumor progression [60,127].
Another possibility for targeting MDSCs in cancer is through chemotherapeutic drugs that eliminate this population. Studies have demonstrated that the chemotherapeutic agent gemcitabine enhances T-cell responsiveness by reducing the number of MDSC in the spleens of tumor-bearing mice [128]. In this study, gemcitabine, administered at a dose similar to the equivalent dose used in patients, was able to specifically reduce the number of MDSCs found in the spleens of animals bearing large tumors without significant reductions in CD4+ T cells, CD8+ T cells, NK cells, macrophages or B cells. The decrease in MDSCs was accompanied by an increase in the antitumor activity of CD8+ T cells and activated NK cells. Since all measurements of MDSC frequency and activity in this study were performed on the spleens of tumor-bearing animals, it is not clear from this work as to the extent of depletion of MDSCs from the tumor microenvironment following gemcitabine treatment and the restoration of immune responses in the tumor microenvironment. The authors did observe, however, that combining gemcitabine with cytokine immunogene therapy using IFN-β markedly enhanced antitumor efficacy, leading to a greater reduction in tumor burden than when either therapy was administered alone [128].
Future perspective
Tumor growth and invasion into surrounding tissue promotes an inflammatory response that is important for tumor development and progression. Dysregulated inflammation in cancer leads to hyporesponsiveness of the tumor. MDSCs play a major role in suppressing T-cell activation in the tumor microenvironment and sustain overall tumor growth, proliferation and metastases. Regulating MDSC recruitment, differentiation and expansion, and inhibiting MDSC suppressive function, will serve as a multifaceted approach to control cancer. However, the therapeutic benefits of agents that regulate MDSCs are only evident when they are combined with immune therapy and not when they are administered alone. Thus, cancer immune therapy offers an attractive therapeutic addition, delivering treatment of high specificity, low toxicity and prolonged activity. Despite the identification of a repertoire of tumor antigens, hurdles remain for immune-based therapies. Tumor-induced immune suppression may be contributing to the limited efficacy of the current approaches. Effective immunotherapeutic strategies for cancer will result from a basic understanding of the mechanisms that sustain tumor growth kinetics. Strategies that reprogram the tumor niche could alter the inflammatory infiltrate in the tumor microenvironment making it permissive for immune destruction of tumors. It is likely that combination therapies focusing on methods to address immune deficits in the cancer microenvironment will be required to develop effective therapies for this disease. Targeting MDSC-induced immune suppression is at the forefront of these therapeutic approaches. The future of immune therapy for cancer holds promise, with combined approaches that simultaneously downregulate MDSC suppressor pathways, restore APC immune-stimulating activity and expand tumor-reactive T cells with γc homeostatic cytokines such as IL-7, IL-15 and IL-21 to generate effective therapy. The optimal way to integrate novel immune-targeted combinations will be the major focus of future studies and will require a coordinated and cooperative multidisciplinary effort by the international scientific community. Objective cancer regressions and extensions in survival should be correlated with multiple predictive and prognostic molecular and cellular biomarkers of response. This information will prove useful in improving therapy.
Executive summary.
Myeloid-derived suppressor cells (MDSCs) mediate immune modulation in the tumor microenvironment by negatively impacting APC and T-cell activities.
Molecular and cellular mechanisms are utilized by MDSCs to mediate immune suppression in T cells.
Tumor-specific COX-2 or genetic signatures are drivers of immune suppression in lung cancer.
Tumor snail genetic knockdown reduces MDSCs and increases CD107a-activated effector T cells in the lung tumor microenvironment.
Depleting Gr1 or Ly6G myelomonocytic cells reduces mouse Lewis lung carcinoma tumor growth kinetics.
T-cell tolerance versus T-cell priming is impacted by MDSCs that skew T cells toward tolerance, playing a critical role in antigen presentation in lung cancer.
IL-7–IL-7Rα–Fc promotes the M1 macrophage phenotype that facilitates antitumor responses by improving T-cell activity in lung cancer.
Drug targets that control the differentiation, expansion, accumulation and activation of MDSCs impact antitumor responses in tumor-bearing hosts.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1▪.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. Comprehensive review of myeloid-derived suppressor cells in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 4.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37(1):14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 14.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer. 2010;17(2):121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. Detailed explanation of molecular mechanisms that impact myeloid-derived suppressor cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 17.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57(10):1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 20.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 21.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176(1):284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Krelin Y, Dvorkin T, et al. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175(12):8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181(7):4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng P, Corzo CA, Luetteke N, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183(2):937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaraj S, Gupta K, Pisarev V, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13(7):828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagaraj S, Collazo M, Corzo CA, et al. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69(19):7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 33.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Yu JP, Cao S, et al. CD4 +CD25 + regulatory T cells decreased the antitumor activity of cytokine-induced killer (CIK) cells of lung cancer patients. J Clin Immunol. 2007;27(3):317–326. doi: 10.1007/s10875-007-9076-0. [DOI] [PubMed] [Google Scholar]
- 35.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176(3):1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182(1):240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109(10):4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M, Sharma S, Mao JT, Dubinett SM. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol. 1996;157:5512–5520. [PubMed] [Google Scholar]
- 39.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58(6):1208–1216. [PubMed] [Google Scholar]
- 40.Chouaib S, Assellin-Paturel C, Mami-Chouaib F, Caignard A, Blay J. The host–tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today. 1997;18:493–497. doi: 10.1016/s0167-5699(97)01115-8. [DOI] [PubMed] [Google Scholar]
- 41.Smyth MJ, Trapani JA. Lymphocyte-mediated immunosurveillance of epithelial cancers? Trends Immunol. 2001;22(8):409–411. doi: 10.1016/s1471-4906(01)01977-9. [DOI] [PubMed] [Google Scholar]
- 42.Alleva DG, Burger CJ, Elgert KD. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production: role of tumor-derived IL-10, TGF-beta and prostaglandin E2. J Immunol. 1994;153:1674. [PubMed] [Google Scholar]
- 43.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59(4):911–917. [PubMed] [Google Scholar]
- 44.Maeda A, Hiyama K, Yamakido H, Ishioka S, Yamakido M. Increased expression of platelet-derived growth factor A and insulin-like growth factor-1 in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest. 1996;109:780–786. doi: 10.1378/chest.109.3.780. [DOI] [PubMed] [Google Scholar]
- 45.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164(1):361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 46.Hida T, Kozaki K, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6(5):2006–2011. [PubMed] [Google Scholar]
- 47.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58(17):3761–3764. [PubMed] [Google Scholar]
- 48.Hosomi Y, Yokose T, Hirose Y, et al. Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000;30(2):73–81. doi: 10.1016/s0169-5002(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 49.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58(22):4997–5001. [PubMed] [Google Scholar]
- 50.Achiwa H, Yatabe Y, Hida T, et al. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res. 1999;5(5):1001–1005. [PubMed] [Google Scholar]
- 51.Riedl K, Krysan K, Pold M, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat. 2004;7(3):169–184. doi: 10.1016/j.drup.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Tsujii M, Dubois R. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase-2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 53.Lin MT, Lee RC, Yang PC, Ho FM, Kuo ML. Cyclooxygenase-2 inducing Mcl-1-dependent survival mechanism in human lung adenocarcinoma CL1.0 cells. Involvement of phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2001;276(52):48997–49002. doi: 10.1074/jbc.M107829200. [DOI] [PubMed] [Google Scholar]
- 54.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60(5):1306–1311. [PubMed] [Google Scholar]
- 55.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, Dubois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 56.Dohadwala M, Batra RK, Luo J, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277(52):50828–50833. doi: 10.1074/jbc.M210707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dohadwala M, Luo J, Zhu L, et al. Non small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276(24):20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma S, Stolina M, Yang SC, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9(3):961–968. [PubMed] [Google Scholar]
- 59.Sharma S, Zhu L, Yang SC, et al. COX-2 inhibition promotes IFN gamma-dependent enhancement of anti-tumor responses. J Immunol. 2005;175(2):813–819. doi: 10.4049/jimmunol.175.2.813. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez PC, Hernandez CP, Quiceno D, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202(7):931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batra RK, Lin Y, Sharma S, et al. Non-small cell lung cancer-derived soluble mediators enhance apoptosis in activated T lymphocytes through an I kappa B kinase-dependent mechanism. Cancer Res. 2003;63(3):642–646. [PubMed] [Google Scholar]
- 62.Yoshino I, Yano T, Murata M, et al. Tumor-reactive T-cells accumulate in lung cancer tissues but fail to respond due to tumor cell-derived factor. Cancer Res. 1992;52(4):775–781. [PubMed] [Google Scholar]
- 63.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61(12):4766–4772. [PubMed] [Google Scholar]
- 64.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+CD4 + regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu J, Suda T, Yoshioka T, Kosugi A, Fujiwara H, Hamaoka T. Induction of tumor-specific in vivo protective immunity by immunization with tumor antigen-pulsed cells. J Immunol. 1989;142:1053–1059. [PubMed] [Google Scholar]
- 66.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 67.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪▪.Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. First report of lung tumor COX-2 signature for the functional induction of Tregs. [DOI] [PubMed] [Google Scholar]
- 69.Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175(3):1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 70.Bussemakers MJ, Van Bokhoven A, Mees SG, Kemler R, Schalken JA. Molecular cloning and characterization of the human E-cadherin cDNA. Mol Biol Rep. 1993;17(2):123–128. doi: 10.1007/BF00996219. [DOI] [PubMed] [Google Scholar]
- 71.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 72.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 73.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27(55):6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yanagawa J, Walser TC, Zhu LX, et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15(22):6820–6829. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 76▪▪.Srivastava MK, Zhu L, Huang M, et al. Tumor Snail knockdown reduces tumor burden and metastases by inducing antitumor immune responses in lung cancer. Presented at: AACR Frontiers in Basic Cancer Research; San Francisco, CA, USA. 14–18 September 2011; p. Abstract A17. Importance of the lung cancer genetic signature for the functional induction of myeloid-derived suppressor cells. [Google Scholar]
- 77.Young MR, Wright MA, Pandit R. Myeloid differentiation treatment to diminish the presence of immune-suppressive CD34+ cells within human head and neck squamous cell carcinomas. J Immunol. 1997;159(2):990–996. [PubMed] [Google Scholar]
- 78.Kusmartsev SA, Kusmartseva IN, Afanasyev SG, Cherdyntseva NV. Immunosuppressive cells in bone marrow of patients with stomach cancer. Adv Exp Med Biol. 1998;451:189–194. doi: 10.1007/978-1-4615-5357-1_30. [DOI] [PubMed] [Google Scholar]
- 79.Subiza JL, Vinuela JE, Rodriguez R, Gil J, Figueredo MA, De La Concha EG. Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int J Cancer. 1989;44(2):307–314. doi: 10.1002/ijc.2910440220. [DOI] [PubMed] [Google Scholar]
- 80.Kusmartsev SA, Ogreba VI. Suppressor activity of bone marrow and spleen cells in C57Bl/6 mice during carcinogenesis induced by 7,12-dimethylbenz(a)anthracene. Eksp Onkol. 1989;11(5):23–26. [PubMed] [Google Scholar]
- 81.Schmidt-Wolf IG, Dejbakhsh-Jones S, Ginzton N, Greenberg P, Strober S. T-cell subsets and suppressor cells in human bone marrow. Blood. 1992;80(12):3242–3250. [PubMed] [Google Scholar]
- 82.Young MR, Wright MA, Matthews JP, Malik I, Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J Immunol. 1996;156(5):1916–1922. [PubMed] [Google Scholar]
- 83.Sugiura K, Inaba M, Ogata H, et al. Wheat germ agglutinin-positive cells in a stem cell-enriched fraction of mouse bone marrow have potent natural suppressor activity. Proc Natl Acad Sci USA. 1988;85(13):4824–4826. doi: 10.1073/pnas.85.13.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angulo I, Rodriguez R, Garcia B, Medina M, Navarro J, Subiza JL. Involvement of nitric oxide in bone marrow-derived natural suppressor activity. Its dependence on IFN-gamma. J Immunol. 1995;155(1):15–26. [PubMed] [Google Scholar]
- 85.Brooks JC, Hoskin DW. The inhibitory effect of cyclophosphamide-induced MAC-1+ natural suppressor cells on IL-2 and IL-4 utilization in MLR. Transplantation. 1994;58(10):1096–1103. [PubMed] [Google Scholar]
- 86.Fujita M, Kohanbash G, Fellows-Mayle W, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71(15):5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabrilovich D, Pisarev V. Tumor escape from immune response: mechanisms and targets of activity. Curr Drug Targets. 2003;4(7):525–536. doi: 10.2174/1389450033490849. [DOI] [PubMed] [Google Scholar]
- 90▪▪.Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. Importance of bone marrow-derived cells as APCs for tumor antigens. [DOI] [PubMed] [Google Scholar]
- 91▪▪.Sotomayor EM, Borrello I, Rattis FM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98(4):1070–1077. doi: 10.1182/blood.v98.4.1070. Importance of APCs in the mechanism of T-cell tolerance. [DOI] [PubMed] [Google Scholar]
- 92.Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67(22):11021–11028. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- 93.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 94.Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 96.Staveley-O’Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuenca A, Cheng F, Wang H, et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63(24):9007–9015. [PubMed] [Google Scholar]
- 98.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437(7055):141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 99.Nossal GJ. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 100.Roser B, Dorsch S. The cellular basis of transplantation tolerance in the rat. Immunol Rev. 1979;46:55–86. doi: 10.1111/j.1600-065x.1979.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 101▪.Steinmuller D. Suppressor cells and transplantation tolerance. Transplantation. 1978;26(1):2–3. Original concept of the existence of suppressor cells and immune tolerance. [PubMed] [Google Scholar]
- 102.Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- 103.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 104.Belz GT, Behrens GM, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196(8):1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 106.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8(5):271–280. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 107.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol. 2004;165(6):1853–1863. doi: 10.1016/S0002-9440(10)63238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 109.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 110.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 111.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 112.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 113.Johnson SK, Kerr KM, Chapman AD, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27(1):27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 114.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94(2):275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andersson A, Srivastava MK, Harris-White M, et al. Role of CXCR3 ligands in IL-7/IL-7R{alpha}-Fc-mediated antitumor activity in lung cancer. Clin Cancer Res. 2011;17(11):3660–3672. doi: 10.1158/1078-0432.CCR-10-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ugel S, Delpozzo F, Desantis G, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9(4):470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 117.Wang H, Cheng F, Cuenca A, et al. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood. 2005;105(3):1135–1143. doi: 10.1182/blood-2004-01-0027. [DOI] [PubMed] [Google Scholar]
- 118.Seggewiss R, Lore K, Greiner E, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(6):2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 119.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 120.Ko JS, Rayman P, Ireland J, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70(9):3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P. MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol. 2011;11(7):856–861. doi: 10.1016/j.intimp.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Priceman SJ, Sung JL, Shaposhnik Z, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenictherapy. Blood. 2010;115(7):1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Denardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan PY, Wang GX, Yin B, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111(1):219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao P, Zhang H, Dinavahi R, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12(3):230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 127.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]