Abstract
Extracellular matrix remodeling is an important mechanism in the initiation and progression of cardiovascular diseases. Cysteine protease cathepsins are among the important proteases that affect major events in the pathogenesis of atherosclerosis and abdominal aortic aneurysm, including smooth muscle cell transmigration through elastic lamina, macrophage foam cell formation, vascular cell and macrophage apoptosis, and plaque rupture. These events have been studied in cathepsin deficiencies and cathepsin inhibitor deficiencies in mice and have provided invaluable insights regarding the roles of cathepsins in cardiovascular diseases. Pharmacological inhibitions for cathepsins are under evaluation for other human diseases and may be used as clinical treatments for cardiovascular diseases in the near future. This article reviews different mechanisms for cathepsins in atherosclerosis and abdominal aortic aneurysm that could be targeted by selective cathepsin inhibitors.
Keywords: Cathepsin, Atherosclerosis, Abdominal aortic aneurysm, Protease inhibitor, Cathepsin inhibitor
Introduction
Proteolysis of the extracellular matrix in the vessel wall is important in many biological processes. Imbalance in proteolytic activities and uncontrolled remodeling of the extracellular matrix, however, can lead to pathological conditions such as atherosclerosis and abdominal aortic aneurysm (AAA) [1]. Destruction of the major components of the arterial wall extracellular matrix—elastin and collagen—weakens blood vessels, which eventually can lead to rupture of the vasculature [2]. Cysteine protease cathepsins have been identified as major elastolytic and collagenolytic enzymes in the arteries [1] that cause arterial wall pathological changes.
Cathepsins are known classically as enzymes that degrade proteins within the acidic environment in late endosomes and lysosomes. Expressed and mobilized by macrophages, smooth muscle cells (SMCs), and endothelial cells (ECs) in the arterial wall, these enzymes also may participate in physiological and pathophysiological processes outside the acidic compartments [3, 4]. The exact mechanisms behind which cathepsins are released from lysosomes to the extracellular environment are incompletely understood, but pH changes between the extracellular environment and lysosomes directly affect cathepsin activities. For example, cathepsin K (CatK) has a narrow pH profile [5], and its activity decreases at neutral pH in extracellular environment; it therefore requires acidic pericellular space for extracellular activity. In contrast, cathepsin S (CatS) remains partially active at neutral pH [6]. Some cells, including macrophages, can create an acidic pericellular space by vacuolar-type H+ ATPase, leading to an optimal environment for many cathepsins [3], thus explaining observations that extracellular cathepsins effectively degrade elastin, collagen, laminin, and fibronectin [6–9]. Cathepsin activities are regulated by their endogenous inhibitors, many of which belong to the cystatin family [10]. The most thoroughly studied member of this family in cardiovascular diseases is cystatin C, which has the highest inhibitory effects on cathepsins L and S [11].
This review focuses on the role of cathepsins in atherosclerosis and AAA based on our current understanding from human pathological studies and animal studies. It also discusses the possible mechanisms of cathepsins, followed by the potential benefits of developing selective cathepsin inhibitors for the treatment of human cardiovascular diseases.
Cathepsins in Atherosclerosis
Extracellular Remodeling in Atherosclerosis
Atherosclerosis is a chronic inflammatory disease affecting the vasculature of medium and large arteries. It is characterized by a thickening of the intima, leading to the formation of bloodstream-obstructing plaques [12]. The accumulation of low-density lipoprotein (LDL) particles in the vessel wall by retention to the extracellular matrix is a major feature in the development of atherosclerosis. Such accumulation is mediated by the binding of apolipoprotein (apo) B-100—present on the surface of LDL particles—to extracellular matrix components [13]. ECs respond to retained LDL by expression of adhesion molecules, which increase the infiltration of inflammatory cells from the bloodstream into the vessel wall. Monocyte-derived macrophages and SMCs infiltrate to the intima and contribute to the formation of atherosclerotic plaques [12]. Within atherosclerotic lesions, these cells produce a large number of extracellular degrading enzymes, including cysteine protease cathepsins [3, 4, 14, 15] and matrix metalloproteinases [16, 17].
As atherosclerosis progresses, macrophages engulf LDL particles and become lipid-rich foam cells [12]. In the center of the atheroma, these cells undergo apoptosis and form necrotic cores, consisting of cell debris, lipids, and cholesterol crystals. An atherosclerotic lesion is covered by a fibrous cap containing extracellular matrix components, which protects the content of the lesion from coming into contact with the bloodstream. Proteolysis of fibrous cap components may contribute to plaque rupture and, consequently, to exposure of the thrombotic-prone lesion content [12]. Atherosclerosis also involves loss of arterial wall elasticity due to degradation of arterial elastin matrix [18].
Expression of Cathepsins in Human Atherosclerosis
In nonatherosclerotic arteries, the expression of many cathepsins—including cathepsins F, S, and K—is negligible [4, 19], although some SMCs express low levels of cathepsin F (CatF) [19]. Cathepsin L (CatL), which is expressed at low levels in most tissues and cells [20, 21], also has a low expression level in the vessel wall of healthy arteries [22]. But as an atherosclerotic lesion advances and inflammatory cells infiltrate, the expression of many cathepsins is enhanced (for overview, see ref [23]). In early and less fibrous lesions, CatS and CatK are localized to the expanding intima and in medial SMCs [4]. In more advanced lesions, many cathepsins are expressed in macrophage-rich regions and are often located around the fibrous cap. CatS and CatK are expressed in macrophages and SMCs near the fibrous cap and in regions of foam cell accumulation [4]. They also are expressed by ECs [18, 24]. CatL is expressed by macrophages, SMCs, and ECs and is located in the fibrous cap, tunica media, and macrophage-rich shoulder regions [22]. CatF is localized to areas with inflammatory infiltrates but not to necrotic lipid cores; it also appears in the fibrous cap and in SMCs from the tunica media [19]. Interestingly, the strongest expression of CatF occurs in macrophages devoid of intracellular lipid deposition. Some ECs and SMCs, but no T cells, express CatF [19]. Furthermore, cathepsin B (CatB) mRNA level increases in unstable regions of human plaque [25], cathepsin V (CatV) protein is expressed in macrophages in human atheroma, and CatV accounts for most (if not all) macrophage intracellular activities and some extracellular elastolytic activities [26].
Cathepsin Studies in Atherosclerosis in Mice
Similar to humans, cathepsins are expressed in atherosclerotic lesions in mice. In apolipoprotein E (ApoE)-deficient mice, CatB, CatL, and CatS are all expressed in the intima of atherosclerotic lesions [15, 27]. Of these, CatS was the one most strongly expressed in medial SMCs. CatB and CatL, but not CatS, were present in lipid-rich areas [15]. Mice that are double deficient for an atherosclerosis-prone gene (e.g., ApoE or LDL receptor [LDLr]) and a cathepsin gene have been useful in studying the role of cathepsins in atherosclerosis. Cathepsin-deficient mice on an atherogenic background have been generated for CatK, CatL, and CatS [7, 14, 24, 28].
In CatK and ApoE double-deficient mice, decreased numbers of large advanced lesions reduced the total atherosclerotic plaque areas, though the total numbers of lesions were not affected by CatK deficiency [24]. Advanced plaques from these mice showed increased collagen content and decreased medial elastin breaks. Although the relative macrophage contents were not altered, macrophage sizes in atherosclerotic lesions were increased due to enhanced cellular storage of cholesterol esters [24]. When mice were fed a cholate-containing diet, CatK deficiency led to an increased fibrous cap thickness and collagen content but a decreased number of buried fibrous caps [29]. Buried fibrous caps in mice have been considered as a model for human plaque vulnerability [30], though not without controversy. Mice lacking CatK only in leukocytes, stemmed from a bone marrow transplantation technique, exhibited no changes in lesion area but showed a reduction in medial elastic breaks [31]. Contrary to that in the CatK-deficient mice, the collagen content in these mice was decreased. The reduction was attributed to the hindrance of SMC migration over the internal elastic media, combined with increased macrophage content, which may have enhanced the release of other proteolytic enzymes [31]. Together, these studies provide no clear picture of CatK’s effects on atherosclerotic plaque stability.
CatL deficiency on a LDLr-deficient background decreased the size of atherosclerotic lesions and lipid cores [7]. Lesions from these mice exhibited reduced levels of collagen and less elastin degradation than in those from the control mice (wild type for CatL). Macrophage, CD4+ T cell, and SMC contents were significantly lower in lesions from CatL-deficient mice than in those from control mice after 12 weeks of an atherogenic diet. After 24 weeks on an atherogenic diet, however, these cellular differences could no longer be detected [7].
The effects of CatS deficiency in atherogenesis were studied in both apoE-deficient and LDLr-deficient mice. CatS deficiency in LDLr-deficient mice slowed the progression of atherosclerosis and decreased lesion sizes [14]. In addition, these lesions exhibited reduced collagen content, elastin degradation, and fibrous cap thickness. CatS deficiency also led to reduced numbers of intimal macrophages, CD4+ T cells, and SMCs [14]. Absence of CatS in ApoE-deficient mice reduced lesion size and buried fibrous layer [28]. Lesion sizes did not differ between mice with leukocyte-specific CatS deficiency and control mice [32], but leukocyte-specific CatS deficiency altered plaque morphology. Lesions in these mice had reduced necrotic core area and apoptotic cells, decreased amounts of large foam cells, and increased macrophage content but fewer SMCs, compared with those in wild-type leukocyte control mice. The leukocyte-specific CatS-deficient mice also exhibited less degradation of collagen and elastin, which suggests that leukocyte-derived CatS is important for elastic lamina disruption [32]. In line with these studies from CatS-deficient cells and whole-body deficient animals, pharmacological treatment with a specific CatS inhibitor reduced lesion size and preserved the integrity of the elastic lamina [33]. Taken together, these studies propose a direct and pro-atherosclerotic effect for CatS.
The increased expression of cathepsins in atherosclerotic lesions and the altered atherogenesis in cathepsin-deficient animals provide strong evidence for the direct participation of cathepsins in atherogenesis. But further studies with specific inhibitors are needed to understand the mechanisms behind individual cathepsins and to develop therapeutic strategies for targeting each cathepsin.
Cathepsins in Abdominal Aortic Aneurysm
Extracellular Remodeling in Abdominal Aortic Aneurysm
Imbalanced proteolytic activities, resulting in excessive matrix destruction and progressive weakening of the arterial wall, are one of the hallmarks of abdominal aortic aneurysm (AAA) pathology. AAA formation is characterized by extensive medial and adventitial inflammatory cell invasion, medial SMC depletion, and degradation of elastin and collagen in the media [34–37]. Inflammatory cells—including macrophages, lymphocytes, mast cells, dendritic cells, and neutrophils—accumulate in the aortic wall and contribute to AAA development [35, 38].
Loss of elastin is important in the formation of aneurysm dilatation [39], and stabilization of elastin delays AAA formation and attenuates aneurysmal expansion in rats [40]. Collagen synthesis increases during the early stages of aneurysm formation, but in more mature lesions, collagen degradation exceeds its synthesis and eventually may lead to AAA lesion rupture [35]. Many cathepsins have potent elastolytic and/or collagenolytic activities [18] and thus may participate in the development of AAA.
Cathepsin Expression Pattern in AAA and Experimental Animals
In human AAA, protein levels of CatK, CatL, and CatS are increased, whereas the level of their endogenous inhibitor, cystatin C, is decreased [4, 22, 41, 42]. Macrophages and SMCs express CatK and CatS [4], whereas CatL is mainly localized to macrophage-rich areas and only weakly in SMCs [22]. In addition, CatB, CatK, and CatS are highly expressed in cerebral aneurysms, whereas cystatin C is sparse [43]. CatC (also called dipeptidyl peptidase I [DPPI]), a lysosomal protease required for post-translational processing of serine proteases [44], is expressed in elastase-induced AAA [45]. CatC-deficient mice are resistant to elastase-induced AAA, have a reduced inflammatory response, and have preservation of medial elastic lamina. Interestingly, the recruitment of neutrophils to aneurysmal lesions in these mice is diminished [46].
Even though CatK is expressed in human AAA, CatK deficiency in angiotensin II-induced mice did not protect against aneurysm formation or elastic lamina breaks [47]. But CatS-expressing and CatC-expressing cells were increased in aneurysms from these mice, suggesting a compensatory mechanism. Similar to atherosclerotic lesions, CatK-deficient mice exhibited increased collagen content compared with wild-type controls [24]. Mice deficient for cystatin C developed increased lumen diameter and AAA lesion size, compared with wild-type controls, in mice with angiotensin II-induced AAA [48]. These mice also exhibited enhanced inflammatory cell accumulation, more severe elastin fragmentation, and fewer SMCs in the tunica media than the wild-type control mice. Interestingly, cystatin C-deficient mice had increased numbers of microvessels in their AAA lesions [48], suggesting a potential role for cathepsins in microvessel formation. Although cathepsins are present in human and animal AAA lesions, the exact role each specific cathepsin plays in human AAA development and the mechanisms and significance behind their functions are yet to be elucidated.
Potential Mechanisms of Cathepsin Activity
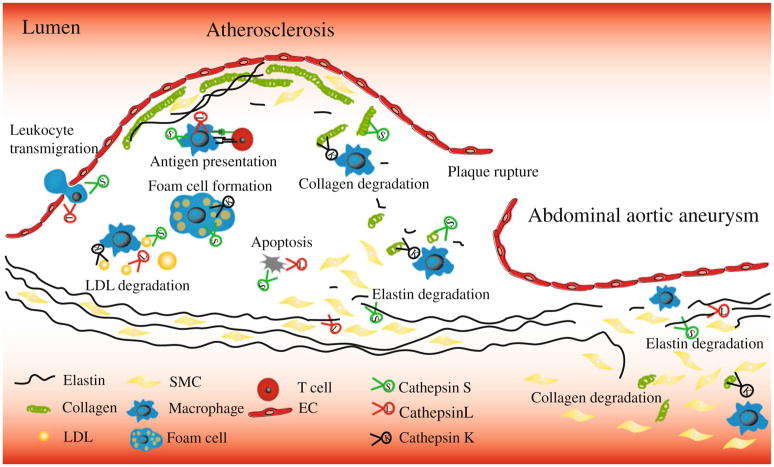
Cathepsins belong to lysosomal proteins, with their optimal activities under acidic pH. Due to the acidic condition in atherosclerotic lesions [49], both intracellular and extracellular activities can cause the action mechanisms of cathepsins in atherosclerosis [18]. Potential mechanisms for cathepsins in atherosclerosis and AAA are summarized in Fig. 1.
Fig. 1.
Overview of potential mechanisms for cathepsins in the pathogenesis of atherosclerosis and abdominal aortic aneurysms. Cathepsins expressed in inflammatory cells, vascular smooth muscle cells (SMC), and endothelial cells (EC) degrade elastin and collagen, which may affect lesion stability, SMC migration, and leukocyte transmigration. Cathepsins are involved in antigen presentation and may increase low-density lipoprotein (LDL) retention to extracellular matrix, foam cell formation, and vascular cell apoptosis
Extracellular Remodeling
Many events in the development of atherosclerotic and AAA lesions depend on the degradation of extracellular matrix proteins, including transmigration of leukocytes through the endothelium [14], intimal SMC accumulation by migration through the elastic lamina [7, 32], and neo-vascularization [50]. Thrombosis formation due to exposure of plaque content to the blood—leading to clinical manifestation—also may result from breaks in fibrous caps caused by extracellular remodeling [51, 52].
Leukocyte Infiltration
Leukocyte penetration of the subendothelial basement layer (composed primarily of collagen and laminin) requires protease activities [53]. Decreased numbers of macrophages and CD4+ T cells were found in atherosclerotic lesions from CatL-deficient and CatS-deficient mice [7, 14]. In transwell cell migration assays in vitro, transmigrations of CatL-deficient monocytes and T lymphocytes through a collagen matrix or EC monolayer-coated collagen matrix were impaired [7]. Similarly, CatS-deficient macrophage migration through an EC monolayer-coated mixture of collagen types I and IV was also reduced [14]. Mice with CatS leukocyte-specific deficiency, however, exhibit no suppression of monocyte infiltration; thus, endothelial CatS expression may be required for leukocyte transmigration [32]. In contrast, CatK might be less important than CatS in leukocyte transmigration. In an in vitro assay, CatK-deficient macrophage migration through matrigel matrix was not altered, compared with those of wild-type control mice; the CatK-deficient mice also did not have altered macrophage or T-cell lesion content [24]. One explanation for these observations is that deficiency of one cathepsin may lead to compensatory expression of other cathepsins. We therefore need further studies of cathepsin inhibitors, to understand cathepsin function in leukocyte transmigration through the endothelium without the interference of compensatory effects, as in the cells from cathepsin-deficient mice.
Smooth Muscle Cell Accumulation
Multiple mechanisms, such as proliferation, apoptosis, extracellular matrix remodeling, and migration, lead to SMC accumulation in atherosclerotic lesions. SMC transmigration through the elastic lamina depends largely on elastin degradation—a process mediated by a spectrum of proteases, including cathepsins [54]. Reduced elastin degradation in CatS-deficient and CatL-deficient atherosclerotic mice associated with decreased intimal SMC accumulation [7, 14, 32]. Collagen content and fibrous-cap thickness depend on the balance between collagen synthesis and degradation. As SMCs produce most collagen, a decreased accumulation of SMCs may explain the reduced collagen content and thinner fibrous caps seen in CatS-deficient and CatL-deficient mice [7, 14, 32]. In line with these results, cystatin C-deficient mice on an ApoE background had increased elastic lamina degradation and SMC content, compared with control mice [55]. In addition, pharmacological inhibition of CatS reduces SMC migration through the elastic lamina into the intima [33], further indicating a role for cathepsins in SMC accumulation.
Lesion Stability
Although the cause and molecular explanation of atherosclerotic lesion instability and plaque rupture are yet to be fully understood, unstable lesions often associate with lipid-filled lesions with a thin fibrous cap, whereas stable plaques often have thick fibrous caps. Ruptures most often occur in the macrophage-rich shoulder region, where protease expression is high and extracellular matrix protein-producing SMCs are low. The local environment, therefore, may have more impact than total plaque numbers or sizes on the development of a rupture [51]. Mechanistic studies of plaque rupture have been difficult due to a lack of reliable animal models; the current available model is from the brachiocephalic artery in ApoE-deficient mice. Features of fibrous cap ruptures and buried fibrous caps in animals have been used to evaluate lesion stability [30], but whether these structures are comparable with ruptures in human lesions is still debatable. This model has been used to study the role of CatS in lesion stability. CatS and ApoE double-deficient mice display fewer plaque ruptures and buried fibrous caps than control mice [28]. CatK-deficient mice also displayed fewer buried fibrous caps than their wild-type counterparts [29].
Lipid Metabolism and Foam Cell Formation
Retention of LDL to the extracellular matrix, followed by aggregation and oxidation of these particles, are important events in the development of atherosclerosis. The binding of Apo B-100 on the surface of LDL to proteoglycans is well studied, though LDL can also bind to elastin and collagen [56]. Degradation of Apo B-100 can be mediated by cathepsins B, F, K, L, S, and V, inducing fusion of LDL particles that may lead to enhanced binding of LDL to proteoglycans [19, 57].
Macrophages engulf oxidized LDL particles, leading to foam cell formation. Larger foam cells were found in atherosclerotic lesions from both CatK-deficient mice and leukocyte CatS-deficient mice [24, 32]. In vitro studies showed that macrophages treated with a selective CatS inhibitor, CLIK60, doubled the accumulation of intracellular free cholesterol [32]. Similarly, incubation of CatK-deficient macrophages with oxidized LDL led to an increase in cholesterol ester accumulation, which may be explained by the upregulation of CD36 as induced by the absence of CatK [58]. The scavenger receptor CD36 mediates phagocytosis of oxidized LDL and thereby macrophage lipid load [59].
Cholesterol accumulated in macrophages can be transported out via a cholesterol acceptor (i.e., HDL), a process called reverse cholesterol transport. Cathepsins may participate in this process. Extracellular degradation of cholesterol acceptors by CatF, CatK, and CatS may reduce cholesterol efflux and thereby help to preserve foam cells in atherosclerosis [32, 60]. Intracellular CatK, however, may increase cholesterol efflux independent of cholesterol acceptor degradation [23], leading to a protective role of CatK in atherosclerosis.
Within atherosclerotic lesions, macrophages engulf oxidized LDL particles. This uptake can damage the lysosomal membrane, which may result in a flow of acidic components to the cytoplasm [61]. Oxidized lipids translocate CatB and CatL to the cytosol and nuclei, which is consistent with the findings that lysosomal CatB and CatL localize to the cytoplasm and nuclei of apoptotic macrophages/foam cells in human carotid atheroma [62]. Interestingly, cholesterol crystals induce acute inflammation by inducing translocation of lysosomal proteolytic content. This inflammation response is impaired in cells from CatB-deficient and CatL-deficient mice [63]. Thus, cathepsin translocation to the cytoplasm is important in apoptosis [62].
Apoptosis
Apoptosis may have a dual effect in atherosclerosis. In early plaques, apoptosis plays a protective role by limiting plaque progression, but in advanced lesions, apoptosis exaggerates inflammatory response and lesion instability [64]. In human atherosclerosis, CatB and CatL colocalize with apoptotic macrophages, and inhibitors of these cathepsins protected macrophages against oxysterol-induced apoptosis by reducing caspase-3 activation [65]. Animal studies indicate that CatS increases apoptosis in atherosclerotic lesions [32], although the exact mechanisms are not fully understood. In contrast to the apoptotic response caused by oxidized lipids or cholesterol-induced translocation of CatB and CatL [62, 63], inhibition of CatS did not alter the apoptotic response to these stimuli. But CatS inhibition protected macrophages cultured on fibronectin or type I collagen from apoptosis [32], suggesting that CatS has a different macrophage apoptosis-inducing mechanism than that of CatB or CatL.
Major Histocompatibility Complex Class II Antigen Presentation
Cathepsins are involved in major histocompatibility complex (MHC) class II antigen presentation and T-cell activation by antigen-presenting cells. The process of MHC class II molecules and the formation of MHC class II/peptide complexes have been reviewed elsewhere [66, 67]. Briefly, MHC class II molecules are assembled in the endoplasmic reticulum (ER) with the assistance of invariant chain (Ii) chaperone molecules. Ii protein is processed in endosomes and lysosomes by lysosomal enzymes, including cathepsins, to a 10-kDa class II-associated invariant-chain peptide (CLIP), which binds to the MHC class II molecule pocket, thereby preventing premature peptide loading. Further degradation of CLIP and replacement with an antigenic peptide are required for the formation and successful trafficking of peptide–MHC class II complexes to the antigen-presenting cell surface. Degradation of MHC class II-associated Ii has been described for CatF in macrophages [68], CatL in cortical thymic epithelial cells [69], and CatS in dendritic cells and B cells [66, 67, 70]. CatV also can convert Ii into CLIP [71]. CatK, in contrast, appears to have no effect on antigen presentation [72]. In atherosclerosis-prone LDLr-deficient mice, the absence of Ii reduced atherosclerotic lesion sizes, lesion CD4+ T cells, NK1.1+ T cells, and CD25+ active T cells; it also reduced IgG1, IgG2b, and IgG2c antibody titers [73]. These mice exhibited altered adaptive immunity and reduced exogenous antigen-induced atherosclerosis. These observations indirectly support a role of cathepsins in atherogenesis by regulating antigen presentation and associated immunity. The detailed role of cathepsins in antigen processing and presentation in the setting of atherosclerosis remains unknown and merits further investigation.
Cathepsins as Pharmacological Targets
The development of atherosclerosis is a gradual subclinical process that can span decades before finally manifestating clinically. The goal for pharmacological treatment is therefore the prevention of clinical events caused by lesion instability and plaque rupture, although atherosclerotic lesion regression or stabilization also is desired. Most AAA are asymptomatic, and patients are often undiagnosed [35]. In the United States, AAA screening is not routine, partly because in addition to general risk factor control, specific AAA therapy is only warranted in advanced lesions [74]. Pharmacological treatment to reduce inflammation and extracellular matrix degradation are therefore of interest for both of these vascular diseases. As discussed, cathepsins with high elastolytic and/or collagenolytic activities, which may affect the atherosclerotic and aortic aneurysm lesion development and stability [18, 35], therefore may be therapeutic targets.
Individual cathepsins play unique roles in physiological and pathophysiological processes, although redundancies may exist [66, 75]—favoring the application of pharmacological inactivation of each individual cathepsin using its selective inhibitors. Furthermore, cathepsins can localize to both acidic lysosomal compartments and extracellular space, presenting the possibility for cathepsin inhibitors that specifically target extracellular cathepsins, thereby reducing the risk for toxicity due to lysosomotropism [76]—a phenomenon in which drugs are stored and released from lysosomes over an extended period of time. Though this effect is common and often desired, it can lead to a local increased concentration of drugs targeting lysosomal enzymes, which in turn may cause unspecific binding to other enzymes [72, 77]. The development of selective cathepsin inhibitors has mainly focused on CatS and CatK due to their involvement in bone metastasis, osteoporosis, cancer, and on CatS because of its involvement in autoimmune diseases [76].
Cathepsin S Inhibitors
To our knowledge, the nitrile-based CatS inhibitor compound 6 [78] is the only selective CatS inhibitor used in vivo for the treatment of atherosclerosis in mice [33]. Atherosclerotic lesion size was reduced in compound 6-treated mice; elastin lamina breaks, plaque macrophages, and buried fibrous caps were also reduced. Mice treated with CatS inhibitor, however, exhibited a significant accumulation of Ii degradation products in spleen extracts [33], suggesting an intracellular role for this inhibitor. Because of its influence on CatS functions in HMC class II antigen presentation, this inhibitor may develop side effects regarding the immune defense in humans [33]. Other CatS inhibitors have been evaluated in clinical trials for rheumatoid arthritis and psoriasis [76], but no inhibitors have passed beyond phase II studies.
Cathepsin K Inhibitors
CatK long was considered a potent collagenase expressed solely in osteoclasts and therefore a natural target for inhibition of osteoclast-bone resorption. But recently a role for CatK was found in inflammation and atherosclerosis, and this protease might be a biological link between low bone density and cardiovascular disease—and therefore a possible common therapeutic target (reviewed in [72]). Treatment with CatK inhibitors for osteosclerosis also may affect atherosclerotic lesion development and stability by reducing ECM degradation. Such reduction may have a dual effect: On one side, CatK inhibition might acquire anti-atherogenic effects due to decreased degradation of collagen and elastin, as well as reduced apoptosis in vascular SMCs and macrophages [24, 29]. On the other side, CatK inhibition may reduce medial elastin degradation and thereby decrease intimal SMC accumulation, the main contributors to the formation of ECM in the fibrous cap, thereby indirectly causing lesion instability [31]. Furthermore, foam cell formation may be aggravated by CatK inhibition, which may have a negative effect on lesion development [24]. Cathepsin K inhibition may also affect vascular calcification process in atherosclerosis, a tightly regulated process similar to bone remodeling [72]. A role for cathepsins in the calcification process was demonstrated in chronic renal disease in ApoE-deficient mice, where CatS deficiency protected mice from atherosclerotic calcification [79]. One possible mechanism involves elastin degradation, which favors vascular calcification. The role for CatK in vascular calcification, however, is yet to be elucidated.
The earliest reported that CatK inhibitors bound irreversibly to CatK active sites; these inhibitors were used mainly in mechanistically studies due to serious safety concerns [80]. The difference between humans and rodents in key amino acid residues involved in substrate/inhibitor recognition [77] is an important issue in the development and evaluation of CatK inhibitors. Inhibitors designed toward the human enzyme might be less potent against rodent CatK. The most advanced and promising CatK inhibitor to date is odanacatib, which is well tolerated, increases bone mineral density, and decreases bone resorption [81, 82]. It is now being evaluated in phase III studies for bone mineral density in men (NCT01120600) and in post-menopausal women (NCT00729183) and for assessment of fracture risk in women with osteoporosis (NCT00529373). Although evidence exists for a role of CatK in atherosclerosis [24], no data are available regarding CatK inhibitors in cardiovascular diseases.
Future Perspective
Accumulating data from studies of atherosclerosis and AAA in animals support a role for cathepsins in these diseases, although observations in animals may not completely recapitulate those in humans. Selective and reversible cathepsin inhibitors are now being investigated in human trials for osteoporosis and rheumatoid arthritis. These inhibitors may also affect mechanisms underlying cardiovascular diseases. The natural next step, therefore, is examining the efficacy of these inhibitors in atherosclerosis and AAA. Furthermore, because the prevalence of osteoporosis and atherosclerosis increases and may coincide in the elderly, dual therapy targeting both diseases may be considered as a future therapeutic strategy [72]. New research will determine whether selective and reversible cathepsin inhibitors will be pharmacologically effective and physiologically safe in treating human cardiovascular diseases.
Acknowledgments
We thank Ms. Sara Karwacki for editorial assistance. This study is partially supported by grants from the Swedish Research Council (to SS), the American Heart Association (0840118 N to GPS), and the U.S. National Institutes of Health (HL60942, HL81090, and HL88547 to GPS).
Contributor Information
Sara Sjöberg, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Guo-Ping Shi, Email: gshi@rics.bwh.harvard.edu, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA. Department of Cardiovascular Medicine, Brigham and Women’s Hospital, 77 Avenue Louis Pasteur, NRB-7, Boston, MA 02115, USA.
References
- 1.Libby P, Lee RT. Matrix matters. Circulation. 2000;102(16):1874–6. doi: 10.1161/01.cir.102.16.1874. [DOI] [PubMed] [Google Scholar]
- 2.Robert L, Robert AM, Jacotot B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis. 1998;140(2):281–95. doi: 10.1016/s0021-9150(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA. 1995;92(9):3849–53. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102(3):576–83. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kafienah W, Bromme D, Buttle DJ, Croucher LJ, Hollander AP. Human cathepsin K cleaves native type I and II collagens at the N-terminal end of the triple helix. Biochem J. 1998;331(Pt 3):727–32. doi: 10.1042/bj3310727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi GP, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992;267(11):7258–62. [PubMed] [Google Scholar]
- 7.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115(15):2065–75. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9(8):970–7. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281(9):6020–9. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 10.Dubin G. Proteinaceous cysteine protease inhibitors. Cell Mol Life Sci. 2005;62(6):653–69. doi: 10.1007/s00018-004-4445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall A, Ekiel I, Mason RW, Kasprzykowski F, Grubb A, Abrahamson M. Structural basis for different inhibitory specificities of human cystatins C and D. Biochemistry. 1998;37(12):4071–9. doi: 10.1021/bi971197j. [DOI] [PubMed] [Google Scholar]
- 12.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson M, Boren J. Mechanism of lipoprotein retention by the extracellular matrix. Curr Opin Lipidol. 2004;15(5):505–14. doi: 10.1097/00041433-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111(6):897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jormsjo S, Wuttge DM, Sirsjo A, Whatling C, Hamsten A, Stemme S, et al. Differential expression of cysteine and aspartic proteases during progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2002;161(3):939–45. doi: 10.1016/S0002-9440(10)64254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–62. [PubMed] [Google Scholar]
- 18.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(8):1359–66. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 19.Oorni K, Sneck M, Bromme D, Pentikainen MO, Lindstedt KA, Mayranpaa M, et al. Cysteine protease cathepsin F is expressed in human atherosclerotic lesions, is secreted by cultured macrophages, and modifies low density lipoprotein particles in vitro. J Biol Chem. 2004;279(33):34776–84. doi: 10.1074/jbc.M310814200. [DOI] [PubMed] [Google Scholar]
- 20.Bando Y, Kominami E, Katunuma N. Purification and tissue distribution of rat cathepsin L. J Biochem. 1986;100(1):35–42. doi: 10.1093/oxfordjournals.jbchem.a121703. [DOI] [PubMed] [Google Scholar]
- 21.Joseph LJ, Chang LC, Stamenkovich D, Sukhatme VP. Complete nucleotide, deduced amino acid sequences of human, murine preprocathepsin L. An abundant transcript induced by transformation of fibroblasts. J Clin Invest. 1988;81(5):1621–9. doi: 10.1172/JCI113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184(2):302–11. doi: 10.1016/j.atherosclerosis.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007;21(12):3029–41. doi: 10.1096/fj.06-7924com. [DOI] [PubMed] [Google Scholar]
- 24.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113(1):98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 25.Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, et al. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26(8):1837–44. doi: 10.1161/01.ATV.0000229695.68416.76. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Bromme D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–70. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105(23):2766–71. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, et al. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26(4):851–6. doi: 10.1161/01.ATV.0000203526.75772.4b. [DOI] [PubMed] [Google Scholar]
- 29.Samokhin AO, Wong A, Saftig P, Bromme D. Role of cathepsin K in structural changes in brachiocephalic artery during progression of atherosclerosis in apoE-deficient mice. Atherosclerosis. 2008;200(1):58–68. doi: 10.1016/j.atherosclerosis.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 30.Jackson CL. Defining and defending murine models of plaque rupture. Arterioscler Thromb Vasc Biol. 2007;27(4):973–7. doi: 10.1161/01.ATV.0000261545.53586.f0. [DOI] [PubMed] [Google Scholar]
- 31.Guo J, Bot I, de Nooijer R, Hoffman SJ, Stroup GB, Biessen EA, et al. Leucocyte cathepsin K affects atherosclerotic lesion composition and bone mineral density in low-density lipoprotein receptor deficient mice. Cardiovasc Res. 2009;81(2):278–85. doi: 10.1093/cvr/cvn311. [DOI] [PubMed] [Google Scholar]
- 32.de Nooijer R, Bot I, von der Thusen JH, Leeuwenburgh MA, Overkleeft HS, Kraaijeveld AO, et al. Leukocyte cathepsin S is a potent regulator of both cell and matrix turnover in advanced atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(2):188–94. doi: 10.1161/ATVBAHA.108.181578. [DOI] [PubMed] [Google Scholar]
- 33.Samokhin AO, Lythgo PA, Gauthier JY, Percival MD, Bromme D. Pharmacological inhibition of cathepsin S decreases atherosclerotic lesions in Apoe−/− mice. J Cardiovasc Pharmacol. 2010;56(1):98–105. doi: 10.1097/FJC.0b013e3181e23e10. [DOI] [PubMed] [Google Scholar]
- 34.Anidjar S, Dobrin PB, Eichorst M, Graham GP, Chejfec G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J Vasc Surg. 1992;16(2):139–47. doi: 10.1067/mva.1992.35585. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–94. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 36.Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8(10):623–31. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99(1):96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117(11):3359–68. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobrin PB, Baker WH, Gley WC. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch Surg. 1984;119(4):405–9. doi: 10.1001/archsurg.1984.01390160041009. [DOI] [PubMed] [Google Scholar]
- 40.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115(13):1729–37. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 41.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104(9):1191–7. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul-Hussien H, Soekhoe RG, Weber E, von der Thusen JH, Kleemann R, Mulder A, et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170(3):809–17. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Cathepsin B, K, and S are expressed in cerebral aneurysms and promote the progression of cerebral aneurysms. Stroke. 2008;39(9):2603–10. doi: 10.1161/STROKEAHA.107.513648. [DOI] [PubMed] [Google Scholar]
- 44.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109(3):363–71. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, et al. Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg. 2006;43(5):1010–20. doi: 10.1016/j.jvs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA. 2007;104(8):2855–60. doi: 10.1073/pnas.0606091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai L, Beckers L, Wijnands E, Lutgens SP, Herias MV, Saftig P, et al. Cathepsin K gene disruption does not affect murine aneurysm formation. Atherosclerosis. 2010;209(1):96–103. doi: 10.1016/j.atherosclerosis.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, et al. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177(1):456–63. doi: 10.2353/ajpath.2010.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naghavi M, John R, Naguib S, Siadaty MS, Grasu R, Kurian KC, et al. pH Heterogeneity of human and rabbit atherosclerotic plaques; a new insight into detection of vulnerable plaque. Atherosclerosis. 2002;164(1):27–35. doi: 10.1016/s0021-9150(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 50.Moulton KS. Angiogenesis in atherosclerosis: gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17(5):548–55. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 51.van der Wal AC, Becker AE. Atherosclerotic plaque rupture–pathologic basis of plaque stability and instability. Cardiovasc Res. 1999;41(2):334–44. doi: 10.1016/s0008-6363(98)00276-4. [DOI] [PubMed] [Google Scholar]
- 52.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 53.Madri JA, Graesser D. Cell migration in the immune system: the evolving inter-related roles of adhesion molecules and proteinases. Dev Immunol. 2000;7(2–4):103–16. doi: 10.1155/2000/79045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengtsson E, Nilsson J, Jovinge S. Cystatin C and cathepsins in cardiovascular disease. Front Biosci. 2008;13:5780–6. doi: 10.2741/3115. [DOI] [PubMed] [Google Scholar]
- 55.Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, et al. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005;96(3):368–75. doi: 10.1161/01.RES.0000155964.34150.F7. [DOI] [PubMed] [Google Scholar]
- 56.Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med. 2005;258(5):395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 57.Linke M, Gordon RE, Brillard M, Lecaille F, Lalmanach G, Bromme D. Degradation of apolipoprotein B-100 by lysosomal cysteine cathepsins. Biol Chem. 2006;387(9):1295–303. doi: 10.1515/BC.2006.160. [DOI] [PubMed] [Google Scholar]
- 58.Lutgens SP, Kisters N, Lutgens E, van Haaften RI, Evelo CT, de Winther MP, et al. Gene profiling of cathepsin K deficiency in atherogenesis: profibrotic but lipogenic. J Pathol. 2006;210(3):334–43. doi: 10.1002/path.2054. [DOI] [PubMed] [Google Scholar]
- 59.Boullier A, Bird DA, Chang MK, Dennis EA, Friedman P, Gillotre-Taylor K, et al. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–22. doi: 10.1111/j.1749-6632.2001.tb03943.x. Discussion 22–3. [DOI] [PubMed] [Google Scholar]
- 60.Lindstedt L, Lee M, Oorni K, Bromme D, Kovanen PT. Cathepsins F and S block HDL3-induced cholesterol efflux from macrophage foam cells. Biochem Biophys Res Commun. 2003;312(4):1019–24. doi: 10.1016/j.bbrc.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Li W, Yuan XM, Olsson AG, Brunk UT. Uptake of oxidized LDL by macrophages results in partial lysosomal enzyme inactivation and relocation. Arterioscler Thromb Vasc Biol. 1998;18(2):177–84. doi: 10.1161/01.atv.18.2.177. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Yuan XM. Increased expression and translocation of lysosomal cathepsins contribute to macrophage apoptosis in atherogenesis. Ann N Y Acad Sci. 2004;1030:427–33. doi: 10.1196/annals.1329.053. [DOI] [PubMed] [Google Scholar]
- 63.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25(11):2255–64. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Dalen H, Eaton JW, Yuan XM. Apoptotic death of inflammatory cells in human atheroma. Arterioscler Thromb Vasc Biol. 2001;21(7):1124–30. doi: 10.1161/hq0701.092145. [DOI] [PubMed] [Google Scholar]
- 66.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3(6):472–82. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 67.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–41. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 68.Shi GP, Bryant RA, Riese R, Verhelst S, Driessen C, Li Z, et al. Role for cathepsin F in invariant chain processing and major histocompatibility complex class II peptide loading by macrophages. J Exp Med. 2000;191(7):1177–86. doi: 10.1084/jem.191.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280(5362):450–3. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 70.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10(2):197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 71.Tolosa E, Li W, Yasuda Y, Wienhold W, Denzin LK, Lautwein A, et al. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J Clin Invest. 2003;112(4):517–26. doi: 10.1172/JCI18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Podgorski I. Future of anticathepsin K drugs: dual therapy for skeletal disease and atherosclerosis? Future Med Chem. 2009;1(1):21–34. doi: 10.4155/fmc.09.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun J, Hartvigsen K, Chou MY, Zhang Y, Sukhova GK, Zhang J, et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122(8):808–20. doi: 10.1161/CIRCULATIONAHA.109.891887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 75.Reinheckel T, Deussing J, Roth W, Peters C. Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L. Biol Chem. 2001;382(5):735–41. doi: 10.1515/BC.2001.089. [DOI] [PubMed] [Google Scholar]
- 76.Palermo C, Joyce JA. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci. 2008;29(1):22–8. doi: 10.1016/j.tips.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Grabowskal U, Chambers TJ, Shiroo M. Recent developments in cathepsin K inhibitor design. Curr Opin Drug Discov Devel. 2005;8(5):619–30. [PubMed] [Google Scholar]
- 78.Gauthier JY, Black WC, Courchesne I, Cromlish W, Desmarais S, Houle R, et al. The identification of potent, selective, and bioavailable cathepsin S inhibitors. Bioorg Med Chem Lett. 2007;17(17):4929–33. doi: 10.1016/j.bmcl.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 79.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119(13):1785–94. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Black WC, Percival MD. The consequences of lysosomotropism on the design of selective cathepsin K inhibitors. Chembiochem. 2006;7(10):1525–35. doi: 10.1002/cbic.200600149. [DOI] [PubMed] [Google Scholar]
- 81.Lewiecki EM. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs. 2009;12(12):799–809. [PubMed] [Google Scholar]
- 82.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]