Abstract
Motor overflow is extraneous movement in a limb not involved in a motor action. Typically, overflow is observed in people with neurological impairments and in healthy children and adults during strenuous and attention-demanding tasks. In the current study, we found that young infants produce vast amounts of motor overflow, corroborating claims of symmetry being the default state of the motor system. While manipulating an object with one hand, all 27 of the typically-developing 4.5- to 7.5-month-old infants who we observed displayed overflow movements of the free hand (on 4/5 of unimanual actions). Mirror-image movements of the hands occurred on 1/8 of unimanual actions, and the hands and legs moved in synchrony on 1/3 of unimanual acts. Motor overflow was less frequent when infants were in a sitting posture and when infants watched their acting hand, suggesting that upright posture and visual examination may help to alleviate overflow and break obligatory symmetry in healthy infants.
Keywords: object manipulation, motor overflow, posture, symmetry, infants
Parents and researchers have noticed anecdotally that when infants explore an object with one hand by banging, squeezing, fingering, and rotating, the other hand—albeit empty—may also display banging, squeezing, and other extraneous movements (R. E. Keen, personal communication, August 19, 2010; J. J. Lockman, personal communication, March 3, 2009). Overflow movements have been well documented in elderly people, neurological patients, and older children during strenuous or attention demanding tasks (Addamo, Farrow, Hoy, Bradshaw, & Georgiou-Karistianis, 2007). The current study examined the prevalence and form of overflow movements in young infants during exploratory play with objects.
Symmetry in Infants’ Manual Actions
The development of skilled manual actions requires independent control of both limbs. In the first months of life, manual actions are clumsy and poorly controlled (Rochat, 1989; Thelen, Corbetta, & Spencer, 1996). By 6 months of age, unimanual exploration is more sophisticated and includes one-handed actions such as shaking, fingering, and rotating objects (Ruff, 1984, 1986). By the end of their first year, infants can coordinate and control independent actions of both arms (Corbetta & Thelen, 1996; Goldfield & Michel, 1986), allowing them to act on multiple objects at the same time and to perform role-differentiated bimanual actions (Fagard & Jacquet, 1989; Kimmerle, Mick, & Michel, 1995; Kotwica, Ferre, & Michel, 2008). Developmental changes in neural structures, including changes in interhemispheric communication in the corpus callosum (Fagard, Hardy-Léger, Kervella, & Marks, 2001; Michel, 1988), help to support the acquisition of finely tuned uni- and bi-manual actions in early infancy.
Some researchers have proposed an intrinsic spatiotemporal linkage between the limbs on the two sides of the body as the default state of the motor system (Dennis, 1976; Duque et al., 2007; Goldfield & Wolff, 2004). As a result, one challenge in acquiring skilled manual actions involves suppressing symmetry across the body so that each limb can be controlled separately (Fagard & Jacquet, 1989). For example, young infants overcome the tendency to reach with both hands rather than one hand in several positions: as they spontaneously orientate their heads to the left or right while lying supine on their backs (Michel & Harkins, 1986); as they learn to sit independently without the hands for support (Rochat, 1992; Rochat & Goubet, 1995); and as they start to swing one arm forward to initiate hand-and-knees crawling (Goldfield, 1989). Signals from one side of the body cannot interfere with the other side.
“Overflow movements” reflect a breakdown in the specificity of motor commands. Motor signals leak from one part of the body to other parts, producing extraneous movements, which accompany, but are incidental to, the voluntary action (Addamo et al., 2007). Sometimes overflow movements show such striking symmetry with the acting hand that they are termed “mirror movements.” For example, while one hand squeezes a ball, the other—empty—hand involuntarily makes squeezing motions in strict temporal synchrony with the acting hand (Kuhtz-Buschbeck, Sundholm, Eliasson, & Forssberg, 2000). Motor overflow can also manifest as “associated movements” where a remote part of the body moves in concert with the voluntary action, such as sticking the tongue out while performing a difficult motor task (e.g., Waber, Mann, & Merola, 1985) or with extraneous leg movements accompanying voluntary hand movements (Kline, Schmit, & Kamper, 2006).
Healthy children display overflow and mirror movements (Cohen, Taft, Mahadeviah, & Birch, 1967; Lazarus & Todor, 1987). However, motor overflow has only been assessed in children of preschool age and older and primarily during difficult motor tasks, such as performing complicated sequences of finger movements and patterned squeezing actions. The antecedents of childhood overflow movements should be present in infants. We reasoned that if symmetry is the default state of the motor system, then infants would show pervasive motor overflow during unimanual actions—including high rates of extraneous movements in the free hand and legs.
Neural Basis for Overflow Movements
Behavioral observations of infants’ overflow movements would give us some idea of how motor signals are being issued and controlled in young infants. Bouts of exploration when, for instance, the acting hand rotates an object and the free hand also rotates, serve as demonstrations of deficits in the specificity of motor commands. The free hand movement is completely incidental to the movement of the acting hand. The acting hand produced a meaningful exploratory action (turning the object over) while the free hand did not. Tight temporal synchrony between the movements of the two hands suggests an intrinsic linkage between the motor signals driving each hand movement.
In the elderly and neurologically impaired, changes in brain function produce these overflow movements. Inhibitory signals in the motor cortices are reduced or ineffective in controlling the specificity of commands going to each limb (Arányi & Rösler, 2002; Hoy, Fitzgerald, Bradshaw, Armatas, & Georgiou-Karistianis, 2004). Deficits in the corpus callosum (Duque et al., 2007; Geffen, Jones, & Geffen, 1994) and ipsilateral corticospinal tracts (Kanouchi, Yokota, Isa, Ishii, & Senda, 1997; Mayston, Harrison, & Stephens, 1999; Schott & Wyke, 1981) have been implicated in motor overflow. Overflow movements can also be elicited in neurologically intact adults. Effortful tasks can obstruct inhibitory signals in the motor cortices in a manner similar to the endogenous breakdowns seen in neurologically-impaired persons (see Arányi & Rösler, 2002).
Although, overflow movements across the lifespan may share some similar mechanisms, the pathways to their overt expression are likely to vary greatly across development. Children’s manual actions undergo substantial developments in fluency (Adolph & Berger, 2006; Bertenthal & Clifton, 1998) and in the neurological underpinnings (Garvey, 2003). Relatively little is known about what neurological factors underlie children’s motor overflow, except that overflow disappears around eleven years of age when the corpus callosum is approaching adult levels in size and myelination (Hasan et al., 2008; Keshavan et al., 2002). Communication between the two motor cortices seems to be a critical factor in producing well-controlled, differentiated manual actions (Fagard et al., 2001).
Current Study
Previous researchers have documented symmetry in infants’ manual actions through the spatiotemporal linkage of the two hands during bimanual actions (Corbetta & Thelen, 1996; Fagard, 1998; Goldfield & Michel, 1986). In these cases, both hands are involved in the goal-directed activity. The current study asked whether typically-developing infants exhibit overflow movements during unimanual actions—bilateral movement when one hand is acting on an object and the other hand is completely unengaged in producing a goal-directed action. The presence of overflow movements in this case would corroborate claims that symmetry is the default motor state (Dennis, 1976; Duque et al., 2007; Goldfield & Wolff, 2004).
We observed infants between 4.5 and 7.5 months of age, the period when coordinated object manipulation emerges (Eppler, 1995; Rochat, 1989). We recorded the movement patterns of infants’ hands and legs during unimanual object exploration to determine whether they exhibit overflow movements in their free, non-acting hand, and in their legs. Since infants are adept at using leg movements for exploration before they use hand movements (Galloway & Thelen, 2004), overflow leg movements would suggest that the whole body is implicated in motor overflow.
We focused on two potential regulators of infants’ motor overflow: posture and visual exploration. We observed infants in supported sitting and supine postures. Differences in posture affects infants’ propensity to reach (Savelsbergh & van der Kamp, 1993) and the quality of their object manipulation (Soska, Adolph, & Johnson, 2010). Reaching and object manipulation are relatively easy in a supported sitting posture because parents or pillows provide the necessary postural support and the arms must be lifted only to chest level (Rochat, 1992; Rochat & Goubet, 1995; Soska et al., 2010). Manual actions are more difficult while supine, because infants must fight against gravity to extend their arms upward to reach for objects or to hold and manipulate objects overhead (Bly, 1994; Savelsbergh & van der Kamp, 1994). We expected that the more difficult supine posture would increase the frequency of motor overflow in infants—similar to how effortful tasks do so in neurotypical adults. We also examined whether visual attention influences the frequency of motor overflow. Diverted attention increases the occurrence of overflow movements in adults (Baliz et al., 2005), and children who are more easily distracted produce higher levels of motor overflow (Waber et al., 1985), suggesting that increased looking at objects by infants may reduce the prevalence of overflow.
Method
Participants
We observed 27 infants (14 boys and 13 girls), ranging from 4.70 to 7.43 months of age (M = 5.98). All infants were born at term and had no known history of developmental pathology. All demonstrated functional bimanual activity: In a manual skill assessment (Soska et al., 2010), every infant transferred objects from hand to hand, fingered objects with one hand while the other hand supported the object, or rotated objects with both hands at least once. Parents reported the first day that infants were able to sit independently—sitting upright with legs outstretched in a “v” for 30 s without using hands for support—using baby books and calendars to augment their memories (see Adolph, 2002). The 10 infants who could sit independently had 15 to 60 days of sitting experience (M = 33.4 days, SD = 4.21). The remaining 17 infants could not sit independently and used their hands for balance in a tripod position (N = 3) or required caregiver support (N = 14). An experimenter verified each infant’s sitting abilities in a lab test. Parents also reported infants’ due dates for later calculation of gestational age (data from one infant were unavailable). Families were recruited from a commercial database and the maternity wards of nearby hospitals. Most families were middle class, White, and from the New York City metropolitan area. Data from an additional 11 infants were excluded due to fussiness (8 infants), experimenter error (2 infants), and equipment failure (1 infant). Infants received small toys or t-shirts as souvenirs for participation.
Procedure
To elicit different exploratory behaviors, we encouraged infants to play with 8 different toys while lying supine on their backs and while sitting on the floor supported by a caregiver, with 4 toys in each posture. Since some infants could not sit independently, parents held all of the infants in the sitting condition by supporting them around their hips and keeping their backs straight. Posture order was counterbalanced across the sample. Toys for each infant were chosen randomly from a set of twelve, including wooden discs and rods, plastic cylinders, and spongy squares. All toys were 7–10 cm in width, lightweight, and easily fit within one hand. The toys did not make noise and had colorful patterns on the fronts and backs. An experimenter presented toys one at a time to infants at midline. Infants played with each toy until they had accumulated 60 s of manipulation; if infants dropped the toy, the experimenter presented the toy again at midline. During both initial toy offers and offers following drops, infants were free to reach with whichever hand they chose. If infants did not grasp the toy after 5 s, the experimenter placed both of the infants’ hands around the toy. Although analyses focused on unimanual actions, infants were free to explore toys with either or both hands at any given time.
Across the 8 trials, infants accumulated 8 minutes of object exploration for off-line coding. This procedure was modeled after those used in previous studies on infant object exploration (Eppler, 1995; Rochat, 1989; Ruff, 1984; Ruff, Saltarelli, Capozzoli, & Dubiner, 1992), however, we used more objects and longer trials than in previous work. A video camera recorded (at 30 frames/s) infants’ hand and leg movements and eyes from a head-on view. During testing, the only items in infants’ line of sight were the toy and video camera. Parents remained behind infants and were told to silently look at the toy if infants attempted to interact with them. Times when infants were off-task were not counted toward the 60 s of accumulated play and were not coded. The experimenter and box of toys remained out of infants’ sight except for brief moments when the experimenter presented toys to infants at the start of each trial and after infants dropped toys.
Data Coding
A primary coder used OpenSHAPA (www.openshapa.org), a computerized video coding software (Sanderson et al., 1994), to score the type, frequency, and duration of infants’ unimanual actions, overflow hand and leg movements, and looking behaviors. The coder first identified every instance of three types of unimanual actions in the acting hand: rotating objects by turning them at least 90° to expose the back side of the toy; dropping objects by splaying the fingers or opening the palm so that they subsequently fell onto infants’ bodies or the floor; and shaking objects by moving them up, down, or to the side using the forearm or hand for at least 100 ms (3 continuous video frames). Instances were counted only if infants’ free hand (i.e., the hand not acting on the object) was empty and not engaging in any movement to assist in a bimanual action. The free hand could be in the air or resting on the body or floor.
In a series of subsequent coding passes, the coding software brought up the bits of video that included each unimanual action. In the first coding pass, the coder scored whether an overflow movement in the free hand began within a 100-ms window around the start of the manipulating hand’s movement—that is, if activation in the free hand began 1 video frame before, 1 frame after, or in the same frame relative to when the acting hand began moving. We considered motor overflow to be movements in the free hand that lasted at least 100 ms (3 video frames) but could last longer, including splaying the fingers, turning the whole hand, or bending the lower arm. For dropping, motor overflow was counted only for movements in the free hand that accompanied the dropping movements in the acting hand.
The coder also noted whether the movement of the free hand mirrored the movement of the acting hand. Mirror movements entailed the free hand performing the mirror-image movements of the acting hand, such as turning the wrist in the opposite direction during rotating, splaying the fingers in the same manner during dropping, or moving the lower arm/hand up and down in unison with the acting hand during shaking. Supporting Video 1 shows unimanual actions with accompanying motor overflow in a typical infant. Supporting Video 2 shows a typical infant performing unimanual actions with mirrored overflow.
In another coding pass, the coding software brought up each video segment containing a unimanual action, and the primary coder scored whether overflow leg movements occurred. We considered overflow leg movements to be continuous movements of the full leg, lower leg, or feet lasting at least 100 ms (3 video frames). The leg movement had to begin within a 100-ms window around the start of a unimanual action (1 frame before, 1 frame after, or in the same video frame). The coder also scored whether leg movements occurred on the same side, opposite side, or both sides of the body relative to the acting hand. Supporting Video 3 illustrates overflow leg movements (slowed to half speed to facilitate viewing) in a typical infant during unimanual object exploration.
In a final coding pass, the coding software again presented each video bit containing a unimanual action, and the primary coder scored infants’ looking toward the acting hand. As in Soska et al. (2010), looking was only coded when infants’ gaze was stable for at least 250 ms (8 video frames).
To determine inter-rater reliability for each of the coding passes, a second coder independently scored 25% of each infant’s data. The reliability coder agreed over 93% of the time on the codes (κs > .91, ps < .01). Both coders also scored a subset of data from each infant when the infant was not performing any movement with the acting hand (just holding the object). We wanted to see if scoring the movement of the free hand was affected by the presence of movement in the acting hand. Reliability between the coders on movements in the free hand was identical to when the acting hand did move. Coding disagreements were resolved through discussion.
Results
Preliminary analyses indicated no significant differences on any measure due to gender, condition order, or which toy infants were exploring. Every infant explored toys with the right and left hands during each trial. Across trials, infants produced more unimanual actions with the right hand (M = 35.48 actions, SD = 3.78) than the left (M = 25.11 actions, SD = 3.98), but right/left hand use was not related to motor overflow. Table 1 summarizes the dataset of unimanual actions used to assess infants’ motor overflow.
Table 1.
Dataset Used to Assess Infants’ Motor Overflow.
| M | SE | Total | |
|---|---|---|---|
| Rotating | 18.52 | 2.61 | 500 |
| Dropping | 8.23 | 1.21 | 214 |
| Shaking | 19.85 | 2.13 | 536 |
Note. Mean number of actions per infants, standard errors of the means, and total numbers of each action summed across all infants.
Since infants produced different numbers of unimanual actions, frequency of overflow and mirror movements were calculated as proportions. Using proportions meant that the data were not normally distributed. The data are bounded by 0 and 1, truncating the distribution on either side of the mean. To address these issues, we used generalized linear models, which allowed us to analyze the data similarly to an analysis of variance using a binomial distribution instead of a normal distribution. In each of the models, we used a probit link function to map our data onto a binomial distribution. Although the coded data are dichotomous (overflow or no overflow during each unimanual action), they are likely the outcome of a continuous underlying process; the motor signals giving rise to overt movements vary along a continuum rather than being all or none.
We used generalized estimating equations (GEEs) to analyze several repeated measures effects: whether type of unimanual action affects overflow and mirror movements; and whether overflow in the free hand affects overflow leg movements. We used a generalized linear mixed model (GLMM) to assess whether posture and visual exploration affected motor overflow. In addition to allowing non-normal distributions, both methods offer advantages over traditional analyses of variance: The models allow the impact of fixed-effects factors (including type of action, side of body, posture, and visual examination) to be examined without assuming independence among the observations. For example, the effects of posture and looking may covary across individual infants. The difference between the GEE and GLMM approach is that only fixed effects are used in the GEE, whereas random effects can also be accounted for in the GLMM. Specifically, in the GLMM that assessed effects of posture and visual exploration, infants’ intercepts (a proxy for the base rate of motor overflow) were included as a random effect across the different levels of the fixed effects. We chose to include individual intercepts as random effects based on examining the goodness of fit of the model (Hedeker & Gibbons, 2006). The structure of the covariance matrix was specified after examining the estimates of covariance parameters using an unstructured matrix. We used first-order autoregressive matrices for all of the analyses, meaning that the degree of covariance is progressively decreased across repeated observations. Follow-up tests were Sidak-corrected to control for multiple comparisons (Abdi, 2007).
Overflow Movements in the Hands
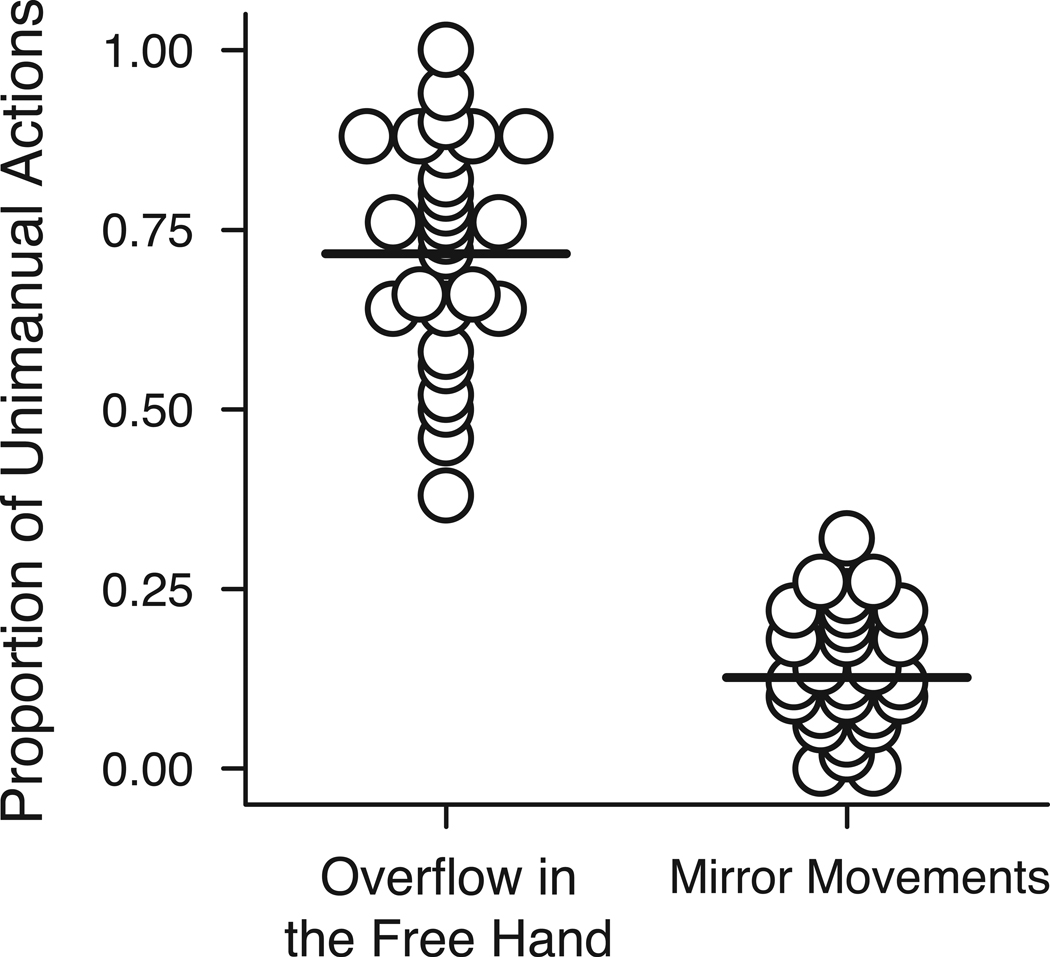
Most unimanual actions involved motor overflow in the free hand, and all infants displayed this phenomenon (M = 71.64% of unimanual actions, SD = 15.85; Figure 1). Overflow movements were ubiquitous across all three types of unimanual action (Ms = 69.48% of rotating, 69.49% of dropping, 74.05% of shaking), with a GEE confirming no main effect of action type (Wald χ2 (2) = 0.49, p > .05). Motor overflow took the form of wiggling, splaying, or clenching of the fingers on 28.17% of movements; flapping of the hand or twisting of the wrists on 34.49% of movements; and twisting or jerking of the forearms on 37.33% of movements. Almost 2/3 of infants’ motor overflow was in the form of movements of the hand alone. Overflow movements lasted 413 ms in duration on average (Range = 100–1630 ms). Overall, 33.8% of all overflow movements lasted 100–250 ms (mostly brief finger wiggles), 41.4% of overflow movements were 250–500 ms in length (largely finger clenching/splaying and wrist twisting), and 24.8% of overflow movements lasted 500–1630ms (mostly hand flapping and forearm bending).
Figure 1.
Proportion of overflow movements in the free hand and mirror movements for each infant out of total number of unimanual actions. Horizontal lines represent group averages.
All but two infants displayed mirror movements, where the fingers, hands, and forearms of both sides of the body moved in mirror image (M = 12.68% of actions, SD = 8.21; Figure 1). Mirror movements occurred most frequently during dropping (M = 25.80% of dropping actions, SD = 21.64), less frequently for rotating (M = 11.43% of rotating actions, SD = 12.93), and least frequently during shaking (M = 7.83% of shaking actions, SD = 10.32). A GEE confirmed that the proportion of mirror movements differed by type of unimanual action (Wald χ2 (2) = 31.43, p < .001). Follow-up tests revealed that the proportion of mirror movements was higher for dropping than rotating and shaking (ps < .01) and higher for rotating compared to shaking (p = .016).
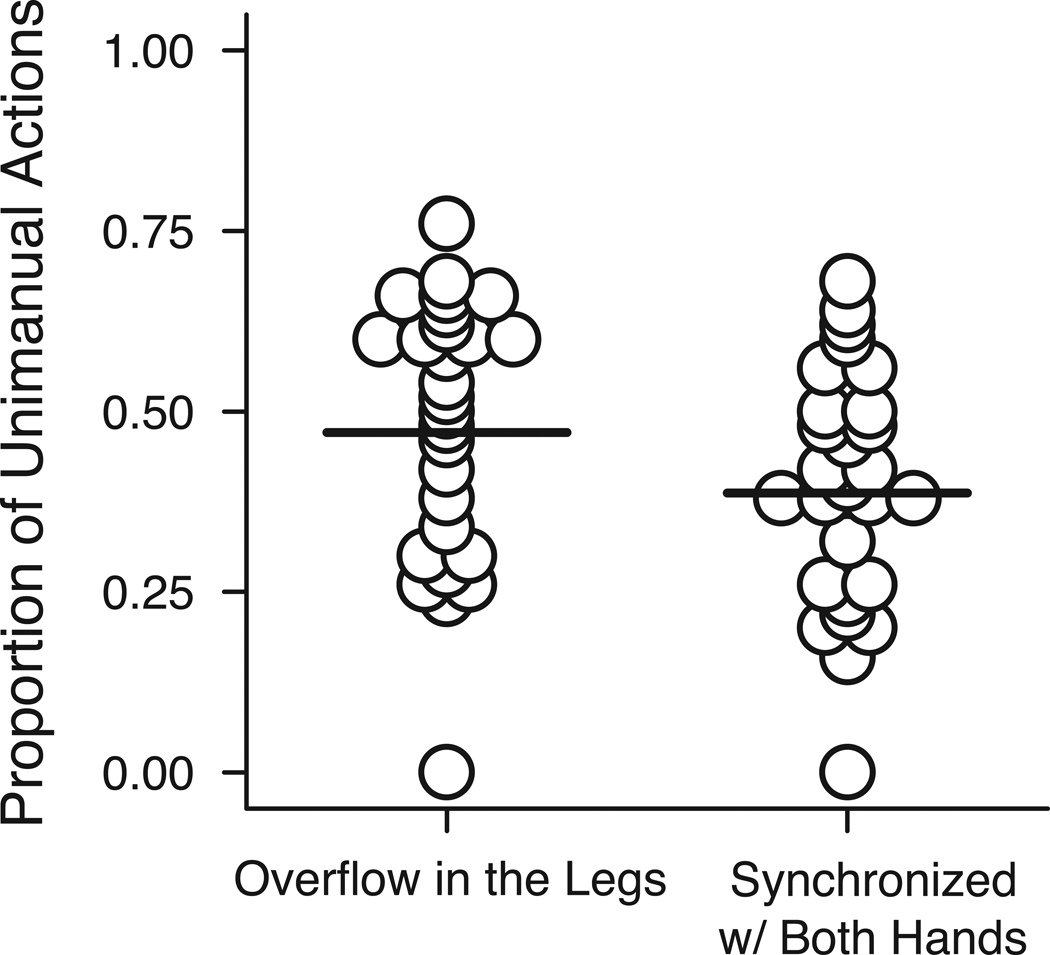
Overflow Movements in the Legs
Infants sometimes moved their legs in synchrony with unimanual actions (M = 47.10% of all unimanual actions, SD = 17.94; Figure 2). Most (2/3) of the leg movements involved pivoting at the knee, and/or flexion/extension and rotation at the ankles. Infants’ free hands, acting hands, and legs moved in temporal synchrony on over 1/3 of exploratory actions (M = 38.69% of unimanual actions, SD = 16.60; Figure 2).
Figure 2.
Proportion of overflow leg movements synchronized with the acting hand and proportion of overflow leg movements synchronized with both hands for each infant out of the total number of unimanual actions. Horizontal lines represent group averages.
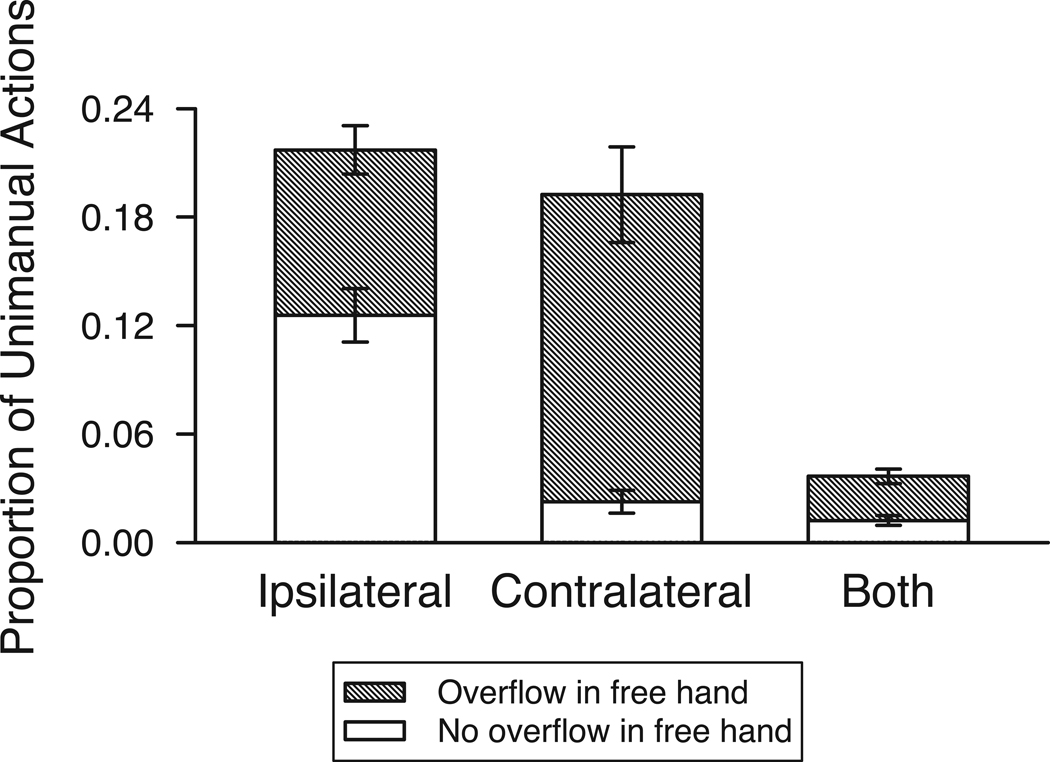
As shown in Figure 3, leg movements more often occurred ipsilateral or contralateral to the acting hand, rather than on both sides (note height of the left two bars). And whether the leg movement was ipsilateral or contralateral was affected by whether only the acting hand was moving or both the acting and free hands moved (see size of shaded regions within the left two bars). A GEE with side of leg movement (ipsilateral, contralateral, or both sides relative to the acting hand) and overflow in the free hand (present or absent) confirmed a significant interaction between leg movement side and overflow in the free hand (Wald χ2 (2) = 40.71, p < .001). Leg movements relegated to the same side (ipsilateral) as the acting hand were marginally more likely to occur when there was no overflow in the free hand (p = .054): only the acting hand and the leg on the same side moved. But overflow in the opposite (contralateral) leg or both legs occurred reliably more often (ps < .002) when there was overflow in the free hand. When the hand opposite the acting hand moved so did the leg opposite the acting hand.
Figure 3.
Proportion of unimanual actions with overflow movements in the legs that are ipsilateral (same side), contralateral (opposite side), and on both sides relative to the acting hand. Stacked bars represent leg overflow movements with and without overflow movements in the free hand. Motor overflow in the legs tended to be ipsilateral when there was no activity in the free hand but contralateral or in both legs when there was overflow in the free hand (see text for analyses). Error bars denote standard errors of the mean.
Effects of Posture, Looking, and Age on Motor Overflow
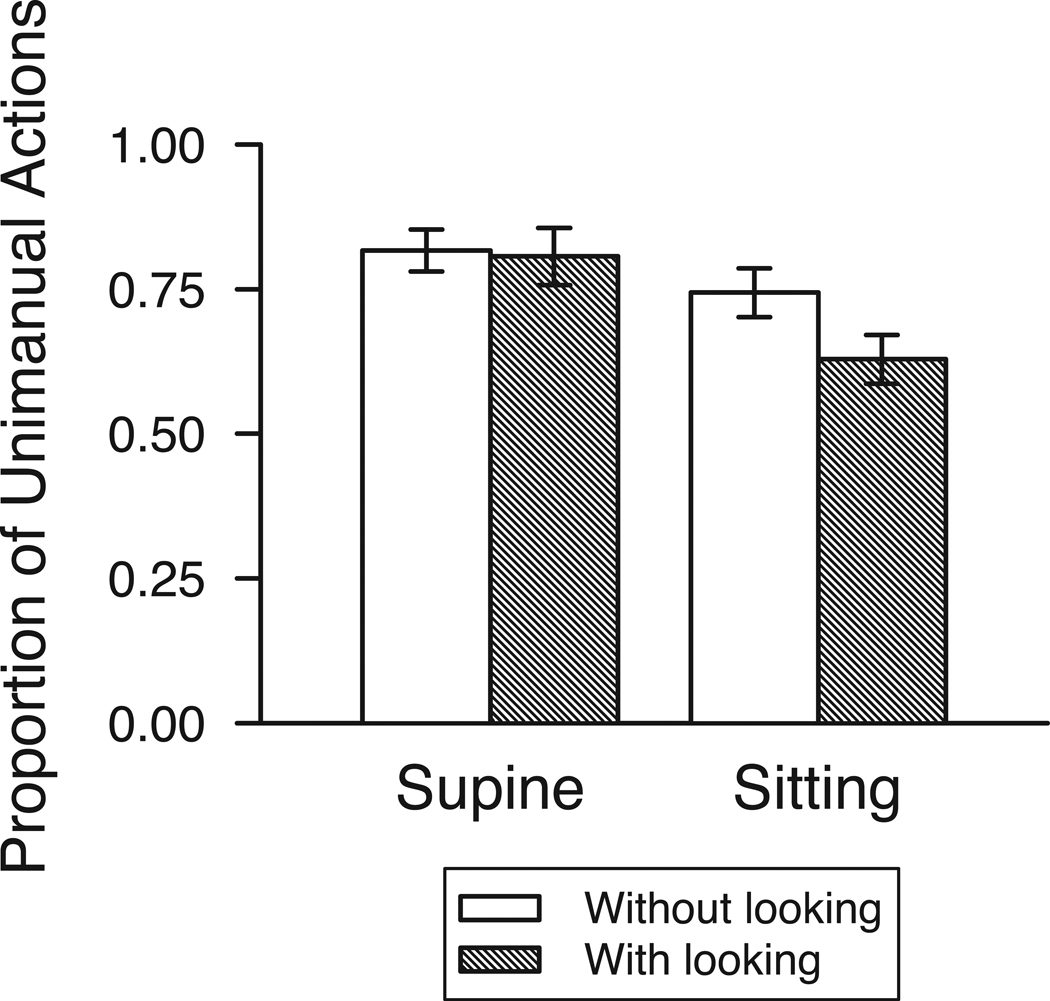
What factors influence the occurrence of motor overflow between the two hands? As shown in Figure 4, posture and visual attention affect motor overflow in young infants. Infants displayed more overflow with the free hand in a supine posture (M = 78.99% of actions while supine, SD = 18.99; M = 67.69% while sitting, SD = 17.32). And when infants directed visual attention to objects while manipulating them, motor overflow was reduced (M = 77.16% of actions without looking, SD = 17.80; M = 65.44% with looking, SD = 20.15). Although reduced, motor overflow still occurred on 2/3 of unimanual actions, even when infants looked at toys while manipulating them in the sitting posture. A GLMM with posture (supine or sitting), visual exploration (looking or no looking), and their interaction as repeated-measure factors confirmed significant effects of posture (F (1, 69) = 8.07, p = .006) and visual examination (F (1, 69) = 4.21, p = .04) but no significant interaction (F (1, 69) = .16, p > .05).
Figure 4.
Proportion of unimanual actions with overflow movements in supine and supported sitting with and without looking at the acting hand. Motor overflow was least frequent in an upright sitting posture and when infants directed visual examination toward the object being explored (see text for analyses). Error bars denote standard errors of the mean.
Although there was no interaction between posture and visual exploration and therefore no moderating effect, it is possible there was a mediating effect between posture and looking. That is, perhaps posture is only linked to reductions in motor overflow because posture induces changes in infants’ looking. Infants looked at objects while unimanually exploring them more often while sitting up (M = 53.37% of actions were accompanied by looking, SD = 19.24) compared to lying supine (M = 31.06% of actions, SD = 18.55), t (26) = 5.93, p < .001. Yet, both visual exploration (looking at the object) and posture (sitting upright) independently and uniquely predicted decreased frequency of motor overflow (Zs > 2.39, ps < .02) as confirmed by Probit analyses to assess a potential mediating effect (MacKinnon & Dwyer, 1993).
We also investigated the potential effects of infants’ chronological age at testing, gestational age, and independent sitting experience and ability. There was a significant correlation between infants’ age at testing and days of independent sitting experience (r (25) = .55, p = .003) and also infants’ gestational age and sitting experience (r (24) = .60, p = .001). However, there were no reliable correlations between infants’ age at testing, gestational age, or days of self-sitting experience and the frequency of motor overflow (rs < .17, ps > .05). When we performed a median split on age at testing and separated infants into older (from 6.08 to 7.4 months) and younger (from 4.7 to 6.07 months) groups there was still no age effect on motor overflow within our sample (t (25) = −.37, p > .05). Nor was there an effect of sitting ability on motor overflow when we grouped infants into sitters and non-sitters (t (25) = .64, p > .05).
Discussion
In the course of quiet object exploration with one hand, every infant in our sample of 4.5 to 7.5-month-olds displayed synchronous—and sometimes identical—movements in their free hand. Mirror movements were more frequent during rotating and dropping compared to shaking. All but one infant also exhibited extraneous movements in the legs. When overflow leg movements occurred coincident with just the acting hand, they were largely confined to the same side of the body. Overflow leg movements coincident with overflow in the free hand often happened on the opposite side of the body. The prevalence of extraneous movements decreased when infants were sitting upright rather than lying supine and when infants looked at the manipulating hand. As we discuss below, these findings may provide important clues about the mechanisms underlying the development of role-differentiated manual actions.
Are The Extraneous Movements Overflow?
Are extraneous movements in the free hand and legs really “motor overflow”? The movements co-occurred with movements in the acting hand and in some cases mirrored the movements in the acting hand, but are these criteria sufficient to consider them to be overflow movements? An alternative possibility is that the extraneous movements were simply spontaneous movements produced by a highly aroused motor system (e.g., Thelen, 1981). When infants are aroused, they often wave and shake their arms, rotate their wrists, flex and extend their fingers, kick and thump their legs, and rub their feet together (Thelen, 1979). On this alternative account, temporal co-occurrence was only accidental.
Several lines of evidence, however, argue against this explanation. Infants appeared to be calm rather than highly aroused, and the movements were relatively quiet and concerted. Extraneous movements were frequent during all types of manual actions coded (rotating, dropping, shaking), and even during episodes of shaking, both arms made only subtle up, down, and sideways movements. Other researchers have noticed similar overflow movements in infants’ arms and legs during more vigorous flailing, banging, and rubbing while playing with blocks and spoons (R. E. Keen, personal communication, August 19, 2010; J. J. Lockman, personal communication, March 3, 2009). Moreover, extraneous movements and unimanual actions were tightly synchronized. When we expanded our analysis to a 500-ms window around the movements of the acting hand (instead of 100 ms), 90% of free hand movements occurred within 100 ms of the movements of the acting hand. If movements of the free hand merely reflected spontaneous motor arousal, the timing of extraneous movements should have been spread evenly across the 500-ms window rather than tightly linked to the acting hand. Finally, 13% of extraneous movements exactly mirrored the movements of the acting hand.
Another possibility is that the extraneous movements were postural compensations to assist in balance or reverberations caused by movements of the torso. Again, several lines of evidence argue against this alternative characterization. In the sitting condition, parents supported their infants, thus mitigating postural demands and torso movements. Previous research indicates that postural stability in a supine posture does not affect manual actions after 4 months of age (Fallang, Saugstad, & Hadders-Algra, 2000). Large arm movements around the shoulder joint in the acting hand may disrupt posture, but we did not include such movements in our selection of unimanual object exploration. Although we did observe some extraneous hand movements that were small and short—lasting less than 250 ms—they were seldom spastic or jerky and were clearly visible to the naked eye (though were easier to code when played at slow speed). The vast majority of extraneous arm movements took the form of finger wriggles, hand flaps, and wrist twists and such movements are unlikely to be postural compensations. Similarly, extraneous leg movements were mostly small and subtle movements of the feet and lower legs that presumably contribute little to postural control. Finally, the fact that we observed extraneous movements when the free hand was lying on the floor or body argues against an explanation based on postural compensations or reverberations.
Is the motor overflow we observed in young infants during object play the same as motor overflow seen in elderly people and neurological patients? Or is it comparable to overflow movements in adults performing strenuous and attention-demanding tasks? On the one hand, the outward appearance of infants’ extraneous movements is strikingly similar to those in the adult work. But on the other hand, the movements we observed emerged in a context completely unlike those of these adult studies: Infants were playing quietly with no explicit task demands. In the current study, extraneous movements were closer in appearance to those of older children drawing pictures, squeezing toys, and inserting pegs into a pegboard (Lazarus & Todor, 1987; Licari, Larkin, & Miyahara, 2006; Noterdaeme, Mildenberger, Minow, & Amorosa, 2002). Infants’ overflow appeared to be a ramped up version of overflow in older children—infants’ movements were more frequent, robust, and visually striking.
Why Does Posture Affect Overflow?
The supine posture increased the frequency of overflow movements. Why might this be? Manipulating objects in a supine posture is more difficult for young infants. So, consistent with previous studies of overflow movements in children, adults, and elderly people (Waber et al., 1985), overflow movements in infants may increase with task difficulty.
What then makes manipulation more difficult in a supine posture than in supported sitting? The supine posture places additional gravitational constraints on infants’ exploration: They constantly need to keep the toy aloft (Bly, 1994). While sitting, however, infants can easily explore the toy at chest level (Bertenthal & von Hofsten, 1998; Soska et al., 2010). The ability to generate torque to counteract gravity is critical for executing manual actions and is generally easier in a sitting position compared to supine (Carvalho, Tudella, Caljouw, & Savelsbergh, 2008). In adults, difficult tasks increase the intensity of motor signals, which, in turn, results in increased motor overflow (Addamo, Farrow, Hoy, Bradshaw, & Georgiou-Karistianis, 2008). In infants, however, relations between motor difficulty and signaling in the motor system are unknown. In future work, effects of intensity of movements on overflow could be examined with electromyography and/or by comparing overflow during vigorous actions such as banging with the quieter sorts of actions observed in the current study.
Although posture had a real time affect on infants’ motor overflow, we did not find a developmental effect of postural experience. Caregivers supported infants during object play—minimizing balance control differences between sitters and non-sitters. However, if we had tested infants in both supported sitting and unsupported sitting postures, we likely would have found substantial differences in motor overflow. The added difficulty of keeping the torso upright while coordinating exploratory actions (e.g., Rochat & Goubet, 1995) should increase the frequency of motor overflow.
Acquiring self-sitting has other developmental effects on infants’ object exploration. Self-sitting helps infants coordinate trunk movements with eye and hand movements (Bertenthal & von Hofsten, 1998). In previous work when self-sitters and non-sitters were supported by caregivers, infants showed no differences in number of manual actions during free play (Soska et al., 2010). But for self-sitters (even when controlling for chronological age), looking increased when infants manipulated objects. Real time changes in posture affect the ease of exploration, and developmental changes in sitting ability drive changes in infants’ visual-manual exploration.
Why Does Looking Affect Overflow?
Looking at objects while manipulating them decreased the frequency of motor overflow in infants. Similarly, diverting attention away from a voluntary movement increased overflow movements in adults (Baliz et al., 2005). In the current study, looking could serve as a proxy for or an instigator of executive attention. Manual actions accompanied by looking might be more concerted and focused. Or looking might focus attention on the action at hand. Regardless, looking and attention are tightly coupled through early infancy (Colombo, 2001). In adults, diverting attention away from the motor task seems to obstruct the inhibitory mechanisms that keep overflow movements at bay (Addamo et al., 2007). In children, attention-related disorders are also connected with increased motor overflow (Licari & Larkin, 2008; Licari et al., 2006); children who are more easily distracted show more motor overflow than children who are frequently on task (Waber et al., 1985). Our results extend these findings, suggesting that attention plays a role in the extraneous movements of young infants as well.
What Changes Over Development?
Spontaneous overflow movements might occur because of the endogenous organization of synergies among muscle groups, such as those producing alternating leg movements during infant stepping (Thelen & Fisher, 1992) or bilateral hand movements (Kelso, Southard, & Goodman, 1979a, 1979b). Mirror movements in infants might be the result of decreased inhibition between the two motor cortices, similar to mirror movements in older children (Fagard et al., 2001). Young infants in our study, just acquiring one-handed exploratory actions, demonstrate deficits in motor control suggesting a lack of transcortical signals regulating motor commands (Tinazzi & Zanette, 1998). These motor signals may leak across the body to generate overflow leg movements. The links between infants’ extraneous hand and leg movements suggest that motor overflow is a whole body phenomenon. Given the importance of callosal fibers and interhemispheric transmission in the coordination of bimanual actions (Duque et al., 2007; Geffen et al., 1994), development of the corpus callosum may underlie changes in motor overflow and thus the emergence of the lateral specificity of manual actions in early infancy. At the same time, decreases in the number of ipsilateral corticospinal tracts over the first two years of life (Eyre, Taylor, Villagra, Smith, & Miller, 2001) may also contribute to the reduction of overflow.
Of course, our behavioral data do not directly inform us of the neurological bases for extraneous movements during motor overflow in infancy. Yet, the results suggest that overflow movements in infancy might be the earlier antecedents of overflow movements in older children. Our data are consistent with the hypothesis that symmetry is endogenous in the motor system, and that, in turn, transcortical inhibition may be a key factor in the development of fine-tuned manual actions (Michel, 1988). At 4.5 to 7 months of age, motor overflow is still quite frequent even when reduced through looking and sitting upright (roughly 2/3 of actions)—suggesting much more neural and behavioral development must take place before well-coordinated manual actions emerge. An important direction for future work would be testing infants longitudinally to track the prevalence of motor overflow as infants’ manual skills improve.
Perhaps most important, our data suggest that the developmental processes are not limited to the brain or the hands. Rather, neural, exploratory, and postural development are deeply entwined. Motor signals to the acting hand can leak beyond the free hand to the legs and feet. Posture may affect the prevalence of motor overflow because more difficult positions elicit stronger motor signals to the arms, which may, in turn, exceed infants’ weak transcortical inhibition. Moreover, acquisition of new postures in development affects coordinated visual-manual exploration, and visual attention mitigates overflow. The processes are likely bi-directional. Self-produced sensory-motor experiences can guide changes in the nervous system (Eyre et al., 2001; Gottlieb, 2007). In addition, it is possible that infants’ developing motor systems could exploit feedback from motor overflow, comparing signals from the hand hefting an object with the signals coming from the other, empty hand. Perceptual-motor systems must be considered at multiple levels to yield an accurate picture of early motor development.
Supplementary Material
Acknowledgments
This research was supported by NICHD Grant R37-HD33486 to K.E.A. We thank Sarah Sanclemente for her coding assistance and the Action Lab at NYU for their helpful comments on previous versions of this manuscript.
Footnotes
Portions of this work were presented at the 2009 meeting of the International Society for Developmental Psychobiology, Chicago, IL and the 2010 International Conference on Infant Studies, Baltimore, MD.
Contributor Information
Kasey C. Soska, Department of Psychology, New York University
Margaret A. Galeon, Department of Psychology, New York University
Karen E. Adolph, Departments of Psychology and Neural Science, New York University
References
- Abdi H. The Bonferonni and Šidák corrections for multiple comparisons. In: Salkind N, editor. Encyclopedia of measurement and statistics. Thousand Oaks, CA: Sage; 2007. pp. 103–107. [Google Scholar]
- Addamo PK, Farrow M, Hoy KE, Bradshaw JL, Georgiou-Karistianis N. The effects of age and attention on motor overflow production--A review. Brain Research Reviews. 2007;54:189–204. doi: 10.1016/j.brainresrev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Addamo PK, Farrow M, Hoy KE, Bradshaw JL, Georgiou-Karistianis N. The influence of task characteristics on younger and older adult motor overflow. Quarterly Journal of Experimental Psychology. 2008;62:239–247. doi: 10.1080/17470210802269217. [DOI] [PubMed] [Google Scholar]
- Adolph KE. Learning to keep balance. In: Kail R, editor. Advances in child development and behavior. Vol. 30. Amsterdam: Elsevier; 2002. pp. 1–40. [PubMed] [Google Scholar]
- Adolph KE, Berger S. Motor development. In: Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2 Cognition, perception, and language. New York: Wiley; 2006. pp. 161–213. Vol. 6th ed. [Google Scholar]
- Arányi Z, Rösler KM. Effort-induced mirror movements. Experimental Brain Research. 2002;145:76–82. doi: 10.1007/s00221-002-1101-1. [DOI] [PubMed] [Google Scholar]
- Baliz Y, Armatas CA, Farrow M, Hoy KE, Fitzgerald PB, Bradshaw JL, et al. The influence of attention and age on the occurrence of mirror movements. Journal of the International Neuropsychological Society : JINS. 2005;11:855–862. doi: 10.1017/s1355617705051003. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Clifton RK. Perception and action. In: Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2 Cognition, perception, and language. Vol. 2. New York: Wiley; 1998. pp. 51–102. [Google Scholar]
- Bertenthal BI, von Hofsten C. Eye, head and trunk control: The foundation for manual development. Neuroscience and Biobehavioral Reviews. 1998;22:515–520. doi: 10.1016/s0149-7634(97)00038-9. [DOI] [PubMed] [Google Scholar]
- Bly L. Motor skills acquisition in the first year. San Antonio, TX: Therapy Skill Builders; 1994. [Google Scholar]
- Carvalho RP, Tudella E, Caljouw SR, Savelsbergh GJP. Early control of reaching: Effects of experience and body orientation. Infant Behavior & Development. 2008;31:23–33. doi: 10.1016/j.infbeh.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Taft LT, Mahadeviah MS, Birch HG. Developmental changes in overflow in normal and aberrantly functioning children. Journal of Pediatrics. 1967;71:39–47. doi: 10.1016/s0022-3476(67)80228-2. [DOI] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Corbetta D, Thelen E. The developmental origins of bimanual coordination: A dynamic perspective. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:502–522. doi: 10.1037//0096-1523.22.2.502. [DOI] [PubMed] [Google Scholar]
- Dennis M. Impaired sensory and motor differentiation with corpus callosum agenesis: A lack of callosal inhibition during ontogeny? Neuropsychologia. 1976;14:455–469. doi: 10.1016/0028-3932(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, et al. Intermanual differences in movement-related interhemispheric inhibition. Journal of Cognitive Neuroscience. 2007;19:204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Eppler MA. Development of manipulatory skills and the deployment of attention. Infant Behavior & Development. 1995;18:391–405. [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Fagard J. Changes in grasping skills and the emergence of bimanual coordination during the first year of life. The psychobiology of the hand. 1998:123–143. [Google Scholar]
- Fagard J, Hardy-Láger I, Kervella C, Marks A. Changes in interhemispheric transfer rate and the development of bimanual coordination during childhood. Journal of Experimental Child Psychology. 2001;80:1–22. doi: 10.1006/jecp.2000.2623. [DOI] [PubMed] [Google Scholar]
- Fagard J, Jacquet A-Y. Onset of bimanual coordination and symmetry versus asymmetry of movement. Infant Behavior & Development. 1989;12:229–235. [Google Scholar]
- Fallang B, Saugstad OD, Hadders-Algra M. Goal directed reaching and postural control in supine position in healthy infants. Behavioural Brain Research. 2000;115:9–18. doi: 10.1016/s0166-4328(00)00231-x. [DOI] [PubMed] [Google Scholar]
- Galloway JC, Thelen E. Feet first: Object exploration in young infants. Infant Behavior & Development. 2004;27:107–112. [Google Scholar]
- Garvey MA. Cortical correlates of neuromotor development in healthy children. Clinical Neurophysiology. 2003;114:1662–1670. doi: 10.1016/s1388-2457(03)00130-5. [DOI] [PubMed] [Google Scholar]
- Geffen GM, Jones DL, Geffen LB. Interhemispheric control of manual motor activity. Behavioural Brain Research. 1994;64:131–140. doi: 10.1016/0166-4328(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Goldfield EC. Transition from rocking to crawling: Postural constraints on infant movement. Developmental Psychology. 1989;25:913–919. [Google Scholar]
- Goldfield EC, Michel GF. Spatiotemporal linkage in infant interlimb coordination. Developmental Psychobiology. 1986;19:259–264. doi: 10.1002/dev.420190311. [DOI] [PubMed] [Google Scholar]
- Goldfield EC, Wolff PH. A dynamical systems perspective on infant action and its development. In: Slater A, Bremner JG, editors. Theories of infant development. Malden, MA: Blackwell; 2004. pp. 1–29. [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10:1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Kramer LA, Papnicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Research. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Research Reviews. 2004;46:315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kanouchi T, Yokota T, Isa F, Ishii K, Senda M. Role of the ipsilateral motor cortex in mirror movements. Journal of Neurology, Neurosurgery & Psychiatry. 1997;62:629–632. doi: 10.1136/jnnp.62.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso JAS, Southard DL, Goodman D. On the coordination of two-handed movements. Journal of Experimental Psychology: Human Perception and Performance. 1979a;5:229–238. doi: 10.1037//0096-1523.5.2.229. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Southard DL, Goodman D. On the nature of human interlimb coordination. Science. 1979b;203:1029–1031. doi: 10.1126/science.424729. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, et al. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Kimmerle M, Mick LA, Michel GF. Bimanual role-differentiated toy play during infancy. Infant Behavior & Development. 1995;18:299–307. [Google Scholar]
- Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2006;130:159–169. doi: 10.1093/brain/awl278. [DOI] [PubMed] [Google Scholar]
- Kotwica KA, Ferre CL, Michel GF. Relation of stable hand-use preferences to the development of skill for managing multiple objects from 7 to 13 months of age. Developmental Psychobiology. 2008;50:519–529. doi: 10.1002/dev.20311. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Sundholm LK, Eliasson AC, Forssberg H. Quantitative assessment of mirror movements in children and adolescents with hemiplegic cerebral palsy. Developmental Medicine and Child Neurology. 2000;42:728–736. doi: 10.1017/s0012162200001353. [DOI] [PubMed] [Google Scholar]
- Lazarus J-AC, Todor JI. Age differences in the magnitude of associated movement. Developmental Medicine and Child Neurology. 1987;29:726–733. doi: 10.1111/j.1469-8749.1987.tb08817.x. [DOI] [PubMed] [Google Scholar]
- Licari M, Larkin D. Increased associated movements: Influence of attention deficits and movement difficulties. Human Movement Science. 2008;27:310–324. doi: 10.1016/j.humov.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Licari M, Larkin D, Miyahara M. The influence of developmental coordination disorder and attention deficits on associated movements in children. Human Movement Science. 2006;25:90–99. doi: 10.1016/j.humov.2005.10.012. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Annals of Neurology. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Michel GF. A neuropsychological perspective on infant sensorimotor development. In: Rovee-Collier C, Lipsitt LP, editors. Advances in infancy research. Vol. 5. NJ: Ablex; 1988. pp. 1–37. [Google Scholar]
- Michel GF, Harkins DA. Postural and lateral asymmetries in the ontogeny of handedness during infancy. Developmental Psychobiology. 1986;19:247–258. doi: 10.1002/dev.420190310. [DOI] [PubMed] [Google Scholar]
- Noterdaeme M, Mildenberger K, Minow F, Amorosa H. Quantitative and qualitative evaluation of neuromotor behavior in children with a specific speech and language disorder. Infant and Child Development. 2002;11:3–15. doi: 10.1007/s00787-002-0285-z. [DOI] [PubMed] [Google Scholar]
- Rochat P. Object manipulation and exploration in 2- to 5-month-old infants. Developmental Psychology. 1989;25:871–884. [Google Scholar]
- Rochat P. Self-sitting and reaching in 5- to 8-month-old infants: The impact of posture and its development on early eye-hand coordination. Journal of Motor Behavior. 1992;24:210–220. doi: 10.1080/00222895.1992.9941616. [DOI] [PubMed] [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5- to 6-month-old infants. Infant Behavior & Development. 1995;18:53–68. [Google Scholar]
- Ruff HA. Infants' manipulative exploration of objects: Effects of age and object characteristics. Developmental Psychology. 1984;20:9–20. [Google Scholar]
- Ruff HA. Components of attention during infants' manipulative exploration. Child Development. 1986;57:105–114. doi: 10.1111/j.1467-8624.1986.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Ruff HA, Saltarelli LM, Capozzoli M, Dubiner K. The differentiation of activity in infants’ exploration of objects. Developmental Psychology. 1992;28:851–861. [Google Scholar]
- Sanderson PM, Scott JJP, Johnston T, Mainzer J, Wantanbe LM, James J. MacSHAPA and the enterprise of Exploratory Sequential Data Analysis (ESDA) International Journal of Human-Computer Studies. 1994;41:633–681. [Google Scholar]
- Savelsbergh GJP, van der Kamp J. The coordination of infant's reaching, grasping, catching and posture: A natural physical approach. Advances in Psychology. 1993;97:289–317. [Google Scholar]
- Savelsbergh GJP, van der Kamp J. The effect of body orientation to gravity on early infant reaching. Journal of Experimental Child Psychology. 1994;58:510–528. doi: 10.1006/jecp.1994.1047. [DOI] [PubMed] [Google Scholar]
- Schott GD, Wyke MA. Congenital mirror movements. Journal of Neurology, Neurosurgery & Psychiatry. 1981;44:586–599. doi: 10.1136/jnnp.44.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, Adolph KE, Johnson SP. Systems in development: Motor skill acquisition facilitates three-dimensional object completion. Developmental Psychology. 2010;46:129–138. doi: 10.1037/a0014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Thelen E. Kicking, rocking, and waving: Contextual analysis of rhythmical stereotypies in normal human infants. Animal Behaviour. 1981;29:3–11. doi: 10.1016/s0003-3472(81)80146-7. [DOI] [PubMed] [Google Scholar]
- Thelen E, Corbetta D, Spencer JP. Development of reaching during the first year: Role of movement speed. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:1059–1076. doi: 10.1037//0096-1523.22.5.1059. [DOI] [PubMed] [Google Scholar]
- Thelen E, Fisher DM. Newborn stepping: An explanation for a "disappearing" reflex. Developmental Psychology. 1992;18:760–775. [Google Scholar]
- Tinazzi M, Zanette G. Modulation of ipsilateral motor cortex in man during unimanual finger movements of different complexities. Neuroscience Letters. 1998;244:121–124. doi: 10.1016/s0304-3940(98)00150-5. [DOI] [PubMed] [Google Scholar]
- Waber DP, Mann MB, Merola J. Motor overflow and attentional processes in normal school-age children. Developmental Medicine and Child Neurology. 1985;27:491–497. doi: 10.1111/j.1469-8749.1985.tb04573.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.