Abstract
Objective:
To report the phenotypic characterization of monozygotic twins with mutations encoding progranulin (PGRN).
Methods:
We studied a twin pair with an exon 4 gene deletion in the PGRN gene. Both twins had clinical and neuropsychological examinations as well as structural MRI and fluorodeoxyglucose PET (FDG-PET) scans. PGRN gene sequencing was performed followed by progranulin ELISA in plasma.
Results:
Both twins manifested symptoms within 3 years of each other, with early behavioral, language, dysexecutive, and memory problems. MRI and FDG-PET imaging demonstrated a strikingly similar topography of findings with clear left hemisphere predominance. Serum progranulin levels in both were well below those from a normal population sample.
Conclusions:
Compared with the heterogeneity seen in many families with PGRN mutations, these monozygotic twins demonstrated strong clinical, neuroimaging, and serum progranulin level similarities, demonstrating the importance of shared genetic profiles beyond environmental influences in the symptomatic expression of the disease.
Familial frontotemporal dementia (FTD) due to mutations in progranulin (PGRN) has been associated with a broad spectrum of phenotypic variability, including behavioral variant FTD, primary progressive aphasia, corticobasal syndrome, an anterograde amnesic disorder similar to Alzheimer disease dementia, and a syndrome resembling Lewy body dementia.1–7 There has been a wide range in age at onset, implying probable nongenetic as well as individual differences in disease expression along with additional modifying factors such as single nucleotide polymorphisms.8 Monozygotic twins provide a unique opportunity to better understand the manifestations of autosomal dominant inherited neurodegenerative diseases.
We report the clinical, neuropsychological, structural, and functional neuroimaging findings in a monozygotic twin pair with a PGRN mutation and a frontotemporal lobar degeneration spectrum phenotype.
METHODS
Subject recruitment.
Both subjects underwent clinical evaluations at other clinics and then at our institution and were then enrolled in the Mayo Alzheimer Disease Research Center.
Standard protocol approvals, registrations, and patient consents.
The Mayo Alzheimer Disease Research Center is a Mayo Foundation Institutional Review Board–approved program. The subjects and their spouses provided written consent for participation.
Clinical and neuropsychological evaluation.
Because of the differences in the subjects' age at onset and the timing of their evaluations, their neurologic, neuropsychological, and neuroimaging materials were analyzed to match data at similar points in their course to allow comparisons. For the neuropsychological data, their profiles were separated into 4 separate cognitive domains and index scores were converted to T scores (which have a mean of 50 and SD of 10) for comparison.
Neuroimaging.
Each twin underwent structural MRI and fluorodeoxyglucose PET (FDG-PET) imaging of the brain. MRI scans (GE Healthcare, Waukesha, WI) were obtained at 3 T with multiple sequence types including 3-dimensional T1-weighted magnetization-prepared rapid gradient echo, fluid-attenuation inversion recovery, and long echo time gradient echo. For PET images, FDG was injected IV; then after a 30-minute period, a helical CT image was obtained for attenuation correction and an 8-minute 3-dimensional scan immediately followed. Three-dimensional stereotactic surface projections were derived from the FDG-PET images, and these were compared with those of age-matched controls, yielding a 3-dimensional z score metabolic map (Cortex ID; GE Healthcare).9
Progranulin ELISA analyses in plasma.
Plasma progranulin levels were quantified using a commercially available ELISA (R&D Systems, Minneapolis MN) and were compared with data in neurologically normal control subjects.
RESULTS
Clinical and neuropsychological data.
Twin 1 (proband).
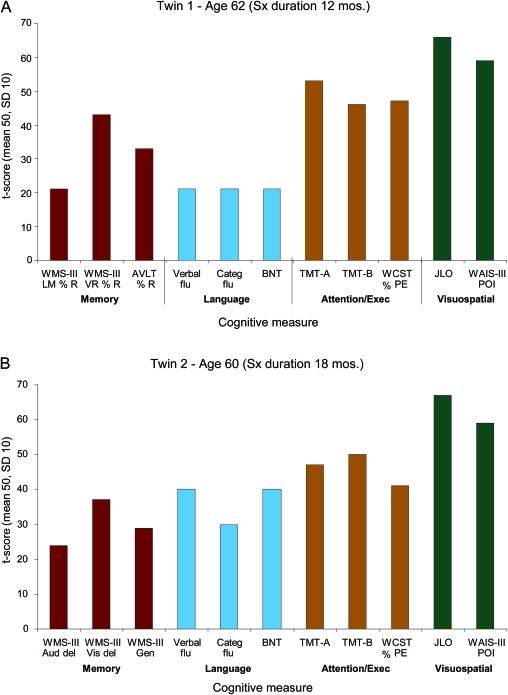
The proband, who has a PhD, was first encountered at the age of 62 with a 12-month history of paranoia, concerns about spousal infidelity, and impulsive spending habits, as well as short-term memory difficulties, language comprehension difficulties, mild apathy, and difficulty with work performance. At the time of his initial evaluation, he was no longer working because of cognitive decline, yet he reported no cognitive difficulties. In addition, his driver's license had been revoked because of a traffic infraction. He showed little concern for that event and his poor judgment. Instrumental activities of daily living were minimally affected. Initial evaluation revealed a mild fluent aphasia with dysnomia, circumlocution, and learning and memory deficits. There was no evidence of visuospatial difficulties nor prosopagnosia. Formal neuropsychological testing highlighted a large discrepancy in performance and verbal IQ (performance IQ > verbal IQ) along with severe verbal learning and memory deficits, moderate expressive language deficits, and average executive functions, but retention of visuospatial abilities (figure 1 and appendix e-1 on the Neurology® Web site at www.neurology.org). Genetic testing was performed, and a previously reported pathogenic 4-bp deletion (c.388_391delCAGT) in exon 4 of PGRN was identified.1
Figure 1. Profile of neuropsychological performance.
Scores on several neuropsychological measures categorized by cognitive domain are shown graphically, with increasing height of the bars representing increasingly better performance. Note the similar pattern of impairment, with weakest performances being present in the memory and language domains and strongest performance being present in visuospatial functioning. AVLT % R = Auditory Verbal Learning Test % Retention; BNT = Boston Naming Test; Categ flu = Category Fluency; Exec = Executive; JLO = Judgment of Line Orientation; Sx = symptom; TMT = Trail Making Test; Verbal flu = Verbal Fluency; WAIS-III POI = Wechsler Adult Intelligence Scale III Perceptual Organization Index; WCST % PE = Wisconsin Card Sorting Test % Perseverative Errors; WMS-III Aud Del = Wechsler Memory Scale III Auditory Delay Index; WMS-III Gen = Wechsler Memory Scale III General Memory Index; WMS-III LM % R = Wechsler Memory Scale III Logical Memory % Retention; WMS-III Vis Del = Wechsler Memory Scale III Visual Delay Index; WMS-III VR % R = Wechsler Memory Scale III Visual Reproduction % Retention.
Twin 2.
This right-handed man with a master's level education had a history of head trauma as a younger adult and may have experienced a brief period of anoxia during a cardiac ablation procedure at age 58. At the age of 59, he began to demonstrate personality changes with paranoia, concerns about spousal infidelity, impulsive spending habits, poor insight, and obsessional somatization regarding jaw and chest pain. At the age of 60 he underwent neuropsychological testing (figure 1 and appendix e-1). Although initial IQ indices for verbal and performance measures were high average and without discrepancies, more extensive testing demonstrated deficiencies in learning and memory and language and executive function with spared visuospatial functioning. He subsequently began to demonstrate disinhibition in his personal and work relationships, resulting in legal difficulties. Within 12 to 18 months of behavioral changes, the patient began to demonstrate memory problems such as forgetting to pay bills. His speech became more tangential. He gained 50 pounds from overeating and demonstrated persistently limited insight into his deficiencies. He was forced to retire from his professional activities at the age of 61 because of cognitive difficulties.
MRI and FDG-PET data.
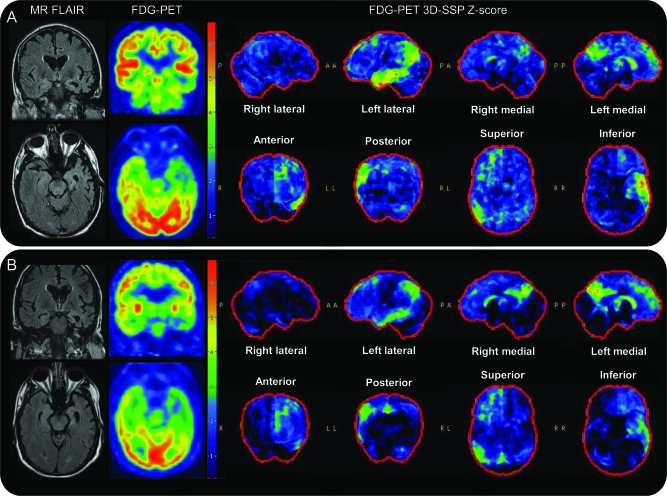
MRI highlighted the asymmetric left hemisphere atrophy with temporal lobe predominance, and FDG-PET demonstrated an asymmetric temporal-parietal distribution of hypometabolism also involving the precuneus and caudate. The imaging findings were strikingly similar between the 2 subjects (figure 2).
Figure 2. Magnetic resonance (MR) and fluorodeoxyglucose (FDG) PET imaging.
MR and FDG-PET imaging of twin 1 at age 62 (A) and twin 2 at age 60 (B). Fluid-attenuation inversion recovery (FLAIR) MR imaging demonstrates asymmetric cerebral volume loss in the left hemisphere compared with the right hemisphere, most notable in the anterior temporal lobe. FDG-PET images demonstrate moderately severe left ≫ right temporal and mild to moderate left ≫ right parietal and frontal hypometabolism. Three-dimensional stereotactic surface projection (3D SSP) z score images demonstrate left medial frontal, temporal and parietal, and bilateral (left worse than right) posterior cingulate hypometabolism. Note the strikingly similar topography of brain hypometabolism demonstrated on the z score images.
Progranulin ELISA analyses in plasma data.
Plasma progranulin analyses showed significantly reduced levels in both patients (twin 1: 11.6 ng/mL; twin 2: 15.6 ng/mL) compared with a series of 523 control individuals (average: 50.2 ng/mL; minimum 25.7 ng/mL).
DISCUSSION
In this monozygotic twin pair, there was general concordance in symptoms, age at onset, low plasma progranulin level, neuropsychological profile, and topography of findings on neuroimaging. Although there were some cognitive differences at the time of evaluation, these might reflect the differences between the time of onset and testing. The role of environmental influences is limited in some degree by the fact that both had pursued very similar occupational and cognitive pursuits in adulthood. This unique twin pair highlights the role of genetic determinism, even in the face of environmental differences, particularly in relation to the cortical asymmetry.
The findings in these twins also have intriguing implications for understanding the pathophysiology of PGRN mutations. The presumed mechanism for PGRN mutations is that haploinsufficiency results in a markedly reduced level of progranulin, yet how does a chronic low level of an important protein, which is presumably being produced and circulating in a generalized systemic manner including throughout the whole brain over decades, result in only a particular region in one side of the brain being affected? Interestingly, available studies with PGRN mutations suggest a greater asymmetry in atrophy and brain perfusion compared with sporadic behavioral variant FTD.5,7
Other affected members of a kindred with the same PGRN mutation typically exhibit various phenotypes ranging from Alzheimer-like dementia, FTD, and primary progressive aphasia, and therefore one could surmise that both genetic (the multitude of dissimilar polymorphisms across the genome) and environmental factors affect the topography of degeneration and hence the phenotype. As for the monozygotic twins with strikingly similar features, one could hypothesize that one or more of the identical non-PGRN genetic determinants could be driving the topography of degeneration, perhaps by making a particular set of neuronal networks more susceptible to neurodegeneration.3,5,7,10 Perhaps a similar genetic mechanism could explain the rare kindreds that have PGRN mutations with very similar phenotypes.3,7,10 The hemispheric homology in our case of monozygotic twins may represent an endophenotype that reflects a cortical network susceptibility in combination with additional genetic contributions beyond the PGRN null mutation; this hypothesis warrants further investigation.
Supplementary Material
ACKNOWLEDGMENT
We thank the subjects and their spouses/families for participating in aging and dementia research.
GLOSSARY
- FDG-PET
fluorodeoxyglucose PET
- FTD
frontotemporal dementia
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. McDade: primary author, data analysis. Dr. Boeve: corresponding author, author of manuscript, data analysis. Dr. Burrus: revision of manuscript. Dr. Boot: revision of manuscript, data analysis. Dr. Kantarci: MRI data analysis, revision of manuscript. Dr. Fields: revision of manuscript, neuropsychological data analysis. Dr. Lowe: revision of manuscript, PET data analysis. Dr. Peller: revision of manuscript, PET data acquisition and analysis. Dr. Knopman: revision of manuscript. M. Baker: revision of manuscript, genetic analysis, progranulin protein analysis. N. Finch: revision of manuscript, genetic analysis, progranulin protein analysis. Dr. Rademakers: revision of manuscript, genetic analysis, progranulin protein analysis. Dr. Petersen: revision of manuscript, administrative and funding support.
DISCLOSURE
Dr. McDade reports no disclosures. Dr. Boeve receives publishing royalties for Behavioral Neurology of Dementia (Cambridge Medicine, 2009) and receives research support from Cephalon, Inc., Allon Pharmaceuticals, the NIH (NIA, CIDR), and the Mangurian Foundation. Dr. Burrus and Dr. Boot report no disclosures. Dr. Kantarci receives research support from the NIH. Dr. Fields serves on the editorial advisory board of International Journal of Neuroscience and has received NIH funding through the Building Interdisciplinary Research Careers in Women's Health program. Dr. Lowe serves on a scientific advisory board for Bayer Schering Pharma and receives/has received research support from GE Healthcare, Siemens Molecular Imaging, Avid Radiopharmaceuticals, Inc., the NIH/NIA, the MN Partnership for Biotechnology and Medical Genomics, and The Leukemia & Lymphoma Society. Dr. Peller has received research support from GE Healthcare. Dr. Knopman serves as Deputy Editor for Neurology®; serves on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc. and Forest Laboratories, Inc.; and receives research support from the NIH. M. Baker holds patents re: Methods and materials for detecting and treating dementia. N. Finch reports no disclosures. Dr. Rademakers holds patents re: Methods and materials for detecting and treating dementia and receives research support from the NIH, the Pacific Alzheimer Research Foundation (Canada), the Association for Frontotemporal Dementia, the Amyotrophic Lateral Sclerosis Association, CurePSP, and the Consortium for Frontotemporal Dementia. Dr. Petersen serves on scientific advisory boards for Pfizer Inc, the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA.
REFERENCES
- 1. Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916–919 [DOI] [PubMed] [Google Scholar]
- 2. Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006;442:920–924 [DOI] [PubMed] [Google Scholar]
- 3. Boeve BF, Maraganore DM, Parisi JE, et al. Corticobasal degeneration and frontotemporal dementia presentations in a kindred with nonspecific histopathology. Dement Geriatr Cogn Disord 2002;13:80–90 [DOI] [PubMed] [Google Scholar]
- 4. Gliebus G, Bigio EH, Gasho K, et al. Asymmetric TDP-43 distribution in primary progressive aphasia with progranulin mutation. Neurology 2010;74:1607–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelley BJ, Haidar W, Boeve BF, et al. Prominent phenotypic variability associated with mutations in progranulin. Neurobiol Aging 2009;30:739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol 2010;67:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Ber I, Camuzat A, Hannequin D, et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 2008;131:732–746 [DOI] [PubMed] [Google Scholar]
- 8. Finch N, Carrasquillo MM, Baker M, et al. TMEM106B regulates progranulin levels and penetrance of FTLD in GRN mutation carriers. Neurology 2011;76:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238–1248 [PubMed] [Google Scholar]
- 10. Mesulam M, Johnson N, Krefft TA, et al. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol 2007;64:43–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.