Abstract
Objective:
To examine national time trends of resective surgery for the treatment of medically refractory epilepsy before and after Class I evidence demonstrating its efficacy and subsequent practice guidelines recommending early surgical evaluation.
Methods:
We performed a population-based cohort study with time trends of patients admitted to US hospitals for medically refractory focal epilepsy between 1990 and 2008 who did or did not undergo lobectomy, as reported in the Nationwide Inpatient Sample.
Results:
Weighted data revealed 112,026 hospitalizations for medically refractory focal epilepsy and 6,653 resective surgeries (lobectomies and partial lobectomies) from 1990 to 2008. A trend of increasing hospitalizations over time was not accompanied by an increase in surgeries, producing an overall trend of decreasing surgery rates (F = 13.6, p < 0.01). Factors associated with this trend included a decrease in epilepsy hospitalizations at the highest-volume epilepsy centers, and increased hospitalizations to lower-volume hospitals that were found to be less likely to perform surgery. White patients were more likely to have surgery than racial minorities (relative risk [RR], 1.13; 95% confidence interval [CI], 1.10–1.17), and privately insured individuals were more likely to receive lobectomy than those with Medicaid or Medicare (RR, 1.28; 95% CI, 1.25–1.30).
Conclusion:
Despite Class I evidence and subsequent practice guidelines, the utilization of lobectomy has not increased from 1990 to 2008. Surgery continues to be heavily underutilized as a treatment for epilepsy, with significant disparities by race and insurance coverage. Patients who are medically refractory after failing 2 antiepileptic medications should be referred to a comprehensive epilepsy center for surgical evaluation.
Epilepsy is a debilitating neurologic disorder affecting nearly 1% of the world's population.1 Medically refractory focal epilepsies are potentially surgically remediable. The most common localized epileptic disorder is temporal lobe epilepsy (TLE).2 Anterior temporal lobectomy is a safe, effective, and well-established surgical treatment resulting in seizure freedom in two-thirds of patients with intractable TLE, and targeted resections can eliminate seizures in one-third to one-half of patients with frontal lobe epilepsy.3,4 However, surgery is thought to be underutilized in the treatment of epilepsy.5 Patients with intractable focal epilepsy often fail to receive surgical evaluation, despite evidence suggesting that individuals who fail just 2 antiepileptic drugs (AEDs) are unlikely to respond completely to further drug combinations.6,7 A survey in 1990 found that while 100,000 to 300,000 patients with intractable epilepsy in the United States were potential candidates for surgical treatment, only 1,500 underwent surgery that year.8,9 Underscoring this missed opportunity, a recent analysis using conservative assumptions estimated that temporal lobectomy in a 35-year-old patient with TLE would, on average, increase survival by 5 years.10
In 2001, the first randomized, controlled trial of anterior temporal lobectomy for patients with medically refractory epilepsy showed that 58% of patients with TLE who received temporal lobectomy were seizure-free 1 year after treatment, but only 8% of those randomized to continued medical treatment achieved this outcome.11 These results led to a recommendation by the American Academy of Neurology that patients with intractable epilepsy be referred to a comprehensive epilepsy center for surgical evaluation.12 However, it remains unknown whether the utilization of epilepsy surgery has changed accordingly along new evidence-based guidelines.
METHODS
Standard protocol approvals, registrations, and patient consents.
No human subjects or identifiable patient data were used in this study. All aspects of this study are in compliance with UCSF Clinical and Translational Science Institute policies.
Data source.
Data were obtained from the Nationwide Inpatient Sample (NIS) hospital discharge database (online at http://www.hcup-us.ahrq.gov/nisoverview.jsp). This database is part of the Healthcare Cost and Utilization Project (HCUP), federal-state-industry partnership sponsored, and is the largest database of US inpatient hospital stays that incorporates data from all payers.13 Data include 100% of inpatient hospitalizations from a stratified random sample of approximately 20% of nonfederal hospitals in the United States. Veterans Hospitals and other federal facilities are excluded. Each NIS entry includes all diagnosis and procedure codes recorded for the patient's hospitalization at the time of discharge. Patient-level observations in the NIS datasets are weighted to account for the complex sampling scheme of the database, thus providing estimates for the entire US population.
Data extraction and plotting.
All available data from 1990 through 2008 were queried. Patients admitted for medically refractory localized epilepsy were identified (primary or secondary diagnosis code 345.41 or 345.51), including those who did or did not receive lobectomy/partial lobectomy (procedure code 01.53) during their hospitalization. These diagnosis and procedure codes have been utilized in other NIS-based studies of lobectomy for epilepsy.14,15 The total number of hospitalizations for intractable localized epilepsy, lobectomy procedures, and the percentage of intractable localized epilepsy patients receiving lobectomy were plotted annually from 1990 to 2008, and were also measured for each individual hospital in the database. Hospital-level data during 1990–1994 and 2004–2008 were stratified by hospital caseload of lobectomy for epilepsy to compare the 20 hospitals performing the most procedures to all other hospitals performing the procedure. Patient age, gender, race, and primary payer was extracted for all hospitalizations and stratified by whether or not hospitalization included lobectomy. Both adults and pediatric patients were included. Hospital-related data, including hospital size by number of beds, teaching vs nonteaching institution, urban vs nonurban location, and geographical region and state were similarly recorded for each hospitalization. To isolate hospital size (by number of beds) as a solitary factor of interest, the NIS takes into account hospital location and teaching status as potential confounding variables.
Statistical analysis.
We evaluated trends in the number of hospitalizations for intractable localized epilepsy, lobectomies performed in this population, and the percentage of hospitalizations including lobectomy from 1990 to 2008 using negative binomial regression. Hospitalization count, procedure count, or proportional procedure rate were the dependent variables and calendar year was the key independent variable. Data stratified by white race vs racial minority patients and privately insured vs Medicare/Medicaid patients were similarly tracked from 1990 to 2008 and analyzed. Total hospitalizations and procedures during 1990–1994 vs 2004–2008, and between the top 20 hospitals by caseload vs all other hospitals performing lobectomy for epilepsy, were compared using a χ2 test. Overall hospital caseload during 1990–1994 vs 2004–2008 was compared using Wilcoxon rank sum test. Patient and hospital characteristics were compared between intractable focal epilepsy patients who did or did not receive lobectomy using χ2 tests for categorical variables and a t test for age. Patient-level variables were entered into a multivariate analysis logistic regression model in a stepwise backward fashion, controlling for hospital-level variables. Within this statistical model, potential interactions between patient-level variables (e.g., race) and hospital-level variables (e.g., size) were also specifically tested. Relative risks for all categorical analyses were calculated with a 95% confidence interval (CI). Probability values were 2-sided and statistical significance was assessed at p < 0.05. All analyses were performed using SPSS version 17 (IBM, Somers, NY).
RESULTS
We identified 20,808 hospitalizations to US hospitals for medically refractory localized epilepsy from 1990 to 2008, with patient age ranging from 0 to 101 years. Among these, 1,326 (6.4%) hospitalizations included surgical lobectomy or partial lobectomy. Data were provided by approximately 20% of registered nonfederal hospitals in the United States at any given time. Weighted data, extrapolated to the entire US population, revealed 112,026 estimated hospitalizations for intractable focal epilepsy from 1990 to 2008, with 6,653 (5.9%) including lobectomy.
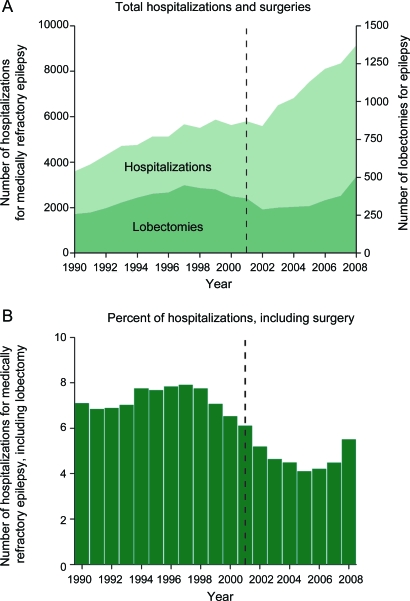
Hospitalizations for medically refractory focal epilepsy increased by approximately 100% from ∼4,000 to 8,000 hospitalizations between 1990 and 2008 (figure 1A), resulting in a positive trend (F = 37.5, p < 0.001). No significant trend was observed in the annual number of lobectomies (∼300–450 per year) performed on these patients over the same period (figure 1A; F = 0.4, p = 0.56). However, the percentage of intractable epilepsy hospitalizations including lobectomy showed a downward trend over time (figure 1B; F = 13.6, p < 0.01), as the procedure was performed during only 4.3% of hospitalizations during 2004–2008 compared to 6.9% of hospitalizations during 1990–1994. These data suggest that while an increasing number of patients were hospitalized for uncontrolled focal epilepsy between 1990 and 2008, the yearly number of lobectomies performed on these patients did not change appreciably, and the rate of surgery during epilepsy hospitalization actually decreased during this time.
Figure 1. Trends of hospitalizations for medically refractory focal epilepsy and lobectomy procedure rates, 1990–2008.
(A) Hospitalizations for medically refractory focal epilepsy (left y-axis) increased from 1990 to 2008 (F = 37.5, p < 0.001). No significant trend was observed in the annual number of lobectomies (right y-axis) performed on this these patients over the same period (F = 0.4, p = 0.56). (B) The percent of intractable epilepsy hospitalizations including lobectomy showed a downward trend over time (F = 13.6, p < 0.01). Dashed line represents publication year (2001) of a randomized, controlled trial examining surgical lobectomy for uncontrolled epilepsy.
To determine if lobectomy trends differed between high-volume epilepsy centers and lower-volume hospitals, we stratified data by hospital caseload and compared 2 5-year periods: 1990–1994 and 2004–2008 (table 1). Of the 20 US hospitals performing the highest yearly number of lobectomies for epilepsy from 1990 to 2008, 90% were teaching institutions and all were located in urban regions. The total number of institutions performing the procedure grew by 131% from 1990–1994 (table 1, top) to 2004–2008 (table 1, bottom), but the average annual caseload per hospital performing lobectomy was dramatically lower during 2004–2008 (4.4 ± 4.6, mean cases ± SD) compared to 1990–1994 (9.0 ± 10.6; p < 0.001). At the 20 highest-volume centers, uncontrolled focal epilepsy hospitalizations decreased by 32% between 1990–1994 and 2004–2008, but the rate of lobectomy (9.5%–9.6%) did not change (relative risk, 1.01; 95% CI, 0.90–1.13). However, among lower-volume hospitals (all other institutions performing any lobectomies for epilepsy), epilepsy hospitalizations quadrupled while the rate of surgery decreased considerably from 9.4% during 1990–1994 to 4.5% during 2004–2008 (relative risk, 0.38; 95% CI, 0.35–0.41). These results suggest that the overall decreased utilization of lobectomy for epilepsy from 1990 to 2008 is associated with a decreased number of patients being admitted to the highest-volume epilepsy centers, as well as increased hospitalizations to lower-volume hospitals that are significantly less likely to perform the procedure.
Table 1.
Number of hospitals, hospitalizations for medically refractory epilepsy, and lobectomies stratified by hospital caseload: 1990–1994 vs 2004–2008
Abbreviation: CI = confidence interval.
Statistically significant value (p < 0.05).
Relative risk comparing likelihood of lobectomy during hospitalization from 1990–1994 vs 2004–2008.
We next compared demographics of patients hospitalized for medically refractory localized epilepsy who did or did not undergo lobectomy (table 2, top). Both adults and pediatric patients were included, and individuals who underwent lobectomy were slightly younger (30.6 ± 14.3 years, mean age ± SD) than those who did not have surgery (31.9 ± 19.3 years; p < 0.001), but males and females received the procedure at similar rates (5.9%–6.0%; relative risk, 1.02; 95% CI, 0.97–1.07). While 6.5% of white patients received lobectomy during hospitalization, surgery rates were lower in racial minorities, including black, Hispanic, Asian/Pacific Islander, and Native American patients (2.4%–5.9% each; all relative risks and 95% CI < 1). We also found differences by insurance status, as while 7.4% of privately insured patients received lobectomy, surgical rates were lower among Medicaid (4.8%; relative risk, 0.65; 95% CI, 0.61–0.69) and Medicare patients (3.9%; relative risk, 0.52; 95% CI, 0.49–0.56). Procedure rates in white vs minority patients and privately insured vs Medicare/Medicaid patients between 1990 and 2008 did not reveal significant trends over time (data not shown).
Table 2.
Patient and hospital characteristics of hospitalizations for medically refractory epilepsy with or without lobectomya
Abbreviation: CI = confidence interval.
Data are cumulative, 1990–2008.
Statistically significant value (p < 0.05).
Hospital size classification is dependent on number of beds and hospital type. For example, for urban, teaching hospitals, “small” signifies <300 beds and “large” signifies >500 beds.
Differences in hospital-related factors were also examined among all patients with medically refractory focal epilepsy (table 2, bottom). While 7.4% of hospitalizations at small hospitals included surgery, the rates of lobectomy at medium and large hospitals were lower (5.3%–6.0%; all relative risks and 95% CI < 1). The designation of hospital size takes into account hospital location (urban vs nonurban) and teaching status as potential confounding variables (see table 2, footnote c). Surgery rates were also lower at nonteaching hospitals (3.6%) vs teaching hospitals (6.5%; relative risk, 0.54; 95% CI, 0.50–0.58) and at hospitals located in a nonurban (3.1%) vs urban location (6.1%; relative risk, 0.50; 95% CI 0.43–0.59). Differences were observed by geographic region within the United States, as 6.8%–7.0% of hospitalizations in the South and West included lobectomy, but only 4.4%–5.4% of hospitalizations in the Northeast and Midwest did so (see table 2 for relative risks). By US state, while New York and California had the largest number of hospitalizations for intractable focal epilepsy over the study period, the highest percentages of patients receiving surgery were found in Rhode Island and Iowa, followed by Maryland and Connecticut (figure e-1 on the Neurology® Web site at www.neurology.org).
Multivariate analysis was performed to identify potential patient-level predictors of lobectomy while controlling for hospital-level variables. Statistically significant predictors of receiving surgery during hospitalization included age <30 years (relative risk, 1.10; 95% CI, 1.07–1.17), white race (relative risk, 1.13; 95% CI, 1.10–1.17), and private insurance (relative risk, 1.28; 95% CI, 1.25–1.30). Furthermore, an interaction between patient race and insurance payer was observed, as white patients were more likely to have private insurance than nonwhite individuals (relative risk, 1.31; 95% CI, 1.28–1.34).
DISCUSSION
Between 1990 and 2008, the number of lobectomies and partial lobectomies for medically refractory focal epilepsy in the United States did not change appreciably despite Class I evidence and new clinical practice guidelines. When adjusted for hospitalizations for epilepsy, the overall rate of lobectomy during hospitalization actually decreased during this time. While surgery is the standard of care for candidate patients with intractable localized epilepsy,5,7,16 it remains significantly underutilized.
One possible interpretation of these findings is that patient referrals to epilepsy centers did increase over time, but these patients were found to be unsuitable candidates for surgery. However, we found that the rate of lobectomy at the 20 highest-volume epilepsy centers remained constant over the study period. Instead, the overall decrease in surgical rates between 1990 and 2008 was associated with 2 concurrent trends: decreased hospitalizations of uncontrolled focal epilepsy patients to high-volume epilepsy centers, and increased hospitalizations to low-volume hospitals that were considerably less likely to perform the procedure.
Another possible explanation is that fewer patients were candidates for surgery in 2008 vs 1990, perhaps as a result of novel pharmacologic therapies. While several new AEDs have become available since 1990,17 these newer and heavily marketed agents have had only a modest impact on the rate of intractable epilepsy, and have not altered the proportion of patients who are surgical candidates.18–20 In fact, the opposite trend was observed in the present study: an increasing number of intractable epilepsy hospitalizations from 1990 to 2008, suggesting a persistent and significant disease burden. While the reasons for increasing hospitalizations for epilepsy are not fully known, our data suggest that practitioners may be referring more patients to smaller, local institutions. This may result in a larger number of epilepsy patients being evaluated in the inpatient setting, but fewer ultimately receiving definitive resective therapy. It is also possible that more patients are receiving palliative surgical therapy, such as vagus nerve stimulation (VNS), instead of resection. VNS implantation is technically less challenging for surgeons, and may reduce seizure burden in certain patients, but it rarely results in complete seizure freedom, making it a less optimal treatment choice for patients who are good candidates for resection.21 Finally, refinements of the temporal lobectomy technique, such as the selective amygdalohippocampectomy, have become increasingly utilized in TLE patients with encouraging results.22 However, these techniques remain classified as surgical resection for epilepsy in the NIS database, and are thus captured in our study. Further study comparing treatment trends of VNS, amygdalohippocampectomy, partial lobectomy, and lobectomy for epilepsy are warranted to further clarify these issues.
Why has utilization of lobectomy for intractable localized epilepsy not increased despite Class I clinical evidence supporting its efficacy? The answer is not fully known, but there are several issues to consider. It is known that changes in practice patterns can often lag considerably after evidence-based guidelines,23 and one author recently proposed that epilepsy referral guidelines may be seen by some practitioners as impractical and autonomy-limiting.24 Other reasons are the perceived morbidity of surgery, lack of awareness of epilepsy-related morbidity and mortality, or perceived advantages of pharmacologic management held by some patients, primary care practitioners, and neurologists/neurosurgeons.
In TLE patients who have failed 2 AED regimens, lobectomy produces seizure freedom in two-thirds of individuals; furthermore, it abolishes seizures in one-third to one-half of patients with less common focal epilepsies such as frontal lobe epilepsy.3,4,7 In contrast, less than 5% of patients who do not undergo surgery (but continue receiving aggressive anticonvulsant management) enter remission each year.6,11,16,19,20,25 Also, uncontrolled epilepsy is associated with cognitive and neuropsychological deficits and diminished quality of life,5,26,27 a 0.5%–1% annual mortality rate, and a lifetime standardized mortality ratio of 2–3 times the general population.3,16,28 In contrast, lobectomy is associated with only 2% significant morbidity and 0.24% total surgical mortality,3,16,28 as well as improvement in overall life span, neuropsychological profile,29,30 and quality-adjusted life-years.10 Thus, while the risks of surgery must be carefully considered, they are small compared to the cumulative lifetime risk of uncontrolled epilepsy.
In the present study, we observed fewer hospitalizations to the most active surgical epilepsy centers during 2004–2008 compared to 1990–1994, and a dramatic increase in hospitalizations to lower-volume centers that were significantly less likely to perform surgery. We recommend that patients with medically refractory epilepsy be referred early to an epilepsy center with the capacity for comprehensive surgical treatment. This recommendation is consistent with guidelines by the National Association of Epilepsy Centers and the American Academy of Neurology,12,31 as well as International League Against Epilepsy guidelines regarding pediatric epilepsy surgery.32 At high-volume epilepsy centers, evaluation is performed by a comprehensive treatment team consisting of epileptologists, neuropsychologists, neurosurgeons, and neuroradiologists, and many of these centers have one or more neurosurgeons specialized in the surgical treatment of epilepsy. These specialized and experienced providers help ensure thorough patient evaluation and the delivery of appropriate treatment, whether it be medical optimization, surgical resection, or other surgical therapy.31 It is also potentially concerning that the average annual caseload of hospitals performing lobectomy in our study was approximately 50% lower during 2004–2008 compared to 1990–1994. Lower hospital volume and experience has been associated with worse outcomes and increased morbidity in neurosurgery33,34 and other surgical fields.35,36
In addition to an overall underutilization of epilepsy surgery, our results suggest significant disparities in treatment by both race and insurance coverage. We found that racial minorities were significantly less likely to receive surgical treatment than white individuals, and patients with Medicare or Medicaid had surgery at notably lower rates than those with private insurance. Previous studies have also found similar inequalities in the epilepsy surgery, noting in particular underutilization among black patients,14,37 as well as significant treatment disparities by socioeconomic status.14,38,39 While the reasons for this disparity are not fully understood, we did observe that white patients were more likely to have private insurance than nonwhite individuals. Therefore, it is possible that lower rate of surgery among racial minorities is influenced by access to care or financial considerations of treating institutions.
There are important limitations to the present study. First, while the overall number of lobectomies and the percentage of hospitalizations for intractable focal epilepsy including lobectomy were known, it was not known whether patients who did not receive surgery were surgical candidates, and readmission for the procedure to another hospital cannot be excluded. Also, current diagnosis codes do not specify brain lobe in hospitalizations for intractable focal epilepsies, but it is known that in the past, the majority of epilepsy resections have been temporal lobectomy for TLE.3,7,28 Next, examining all lobectomies for intractable epilepsy provides a useful mechanism to 1) estimate overall epilepsy surgery utilization over time and 2) compare surgical rates between patient subgroups. Finally, as the NIS only includes hospitalizations from a stratified random subset (20%) of nonfederal hospitals in the United States, our results may reflect bias due to exclusion of potentially useful data.
Medically refractory epilepsy is a devastating neurologic disorder. Lobectomy is a safe and effective procedure for patients with intractable localized epilepsy, but it is significantly underutilized, particularly among racial minorities and the underinsured. Patients with medically refractory epilepsy should be referred to a comprehensive epilepsy center for surgical evaluation by an experienced epilepsy treatment team. Early referral is important, given the significantly deleterious effects of persistent seizures on patient quality of life and survival.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Boudourakis, Soni, Wang, and Rolston for comments on the manuscript.
GLOSSARY
- AED
antiepileptic drug
- CI
confidence interval
- HCUP
Healthcare Cost and Utilization Project
- NIS
Nationwide Inpatient Sample
- RR
relative risk
- TLE
temporal lobe epilepsy
- VNS
vagus nerve stimulation
Footnotes
Editorial, page 1194
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: Drs. Englot and Chang. Acquisition of data: Dr. Englot, D. Ouyang, and Dr. Chang. Analysis and interpretation of data: Dr. Englot, D. Ouyang, and Drs. Garcia, Barbaro, and Chang. Drafting of the manuscript: Drs. Englot and Chang. Critical revision of the manuscript for important intellectual content: Dr. Englot, D. Ouyang, and Drs. Garcia, Barbaro, and Chang. Statistical analysis: Dr. Englot and D. Ouyang. Obtained funding: Drs. Englot and Chang. Study supervision: Dr. Chang.
DISCLOSURE
Dr. Englot and D. Ouyang report no disclosures. Dr. Barbaro serves on a data safety monitoring board for the NIH/NINDS; has received funding for travel from Elekta AB; and serves on the editorial board of Journal of Neurosurgery. Dr. Garcia serves on a Special Emphasis Panel for the Centers for Disease Control; serves on the editorial board of Epilepsia; receives research support from UCB, Medtronics, Inc., and the NIH; and has served as an expert witness in medico-legal cases. Dr. Chang receives research support from the NIH/NINDS.
REFERENCES
- 1. Devinsky O. Diagnosis and treatment of temporal lobe epilepsy. Rev Neurol Dis 2004;1:2–9 [PubMed] [Google Scholar]
- 2. Engel J., Jr Outcome with respect to epileptic seizures. In: Engel J., Jr ed. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987: 553–571 [Google Scholar]
- 3. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537 [DOI] [PubMed] [Google Scholar]
- 4. Jeha L, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Ludders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 2007;130:574–584 [DOI] [PubMed] [Google Scholar]
- 5. Engel J., Jr Surgical treatment for epilepsy: too little, too late? JAMA 2008;300:2548–2550 [DOI] [PubMed] [Google Scholar]
- 6. Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia 2009;50(suppl); 8:57–62 [DOI] [PubMed] [Google Scholar]
- 7. Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engel J., Jr Finally, a randomized, controlled trial of epilepsy surgery. N Engl J Med 2001;345:365–367 [DOI] [PubMed] [Google Scholar]
- 9. Engel J, Jr, Shewman D. Overview: who should be considered a surgical candidate? In: Engel J., Jr ed. Surgical Treatment of the Epilepsies, 2nd ed. New York: Raven Press; 1993: 23–34 [Google Scholar]
- 10. Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA 2008;300:2497–2505 [DOI] [PubMed] [Google Scholar]
- 11. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318 [DOI] [PubMed] [Google Scholar]
- 12. Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology 2003;60:538–547 [DOI] [PubMed] [Google Scholar]
- 13. Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract 2002;5:143–151 [PubMed] [Google Scholar]
- 14. McClelland S, 3rd, Guo H, Okuyemi KS. Racial disparities in the surgical management of intractable temporal lobe epilepsy in the United States: a population-based analysis. Arch Neurol 2010;67:577–583 [DOI] [PubMed] [Google Scholar]
- 15. McClelland S, 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Arch Neurol 2011;68:725–729 [DOI] [PubMed] [Google Scholar]
- 16. Thom M, Mathern GW, Cross JH, Bertram EH. Mesial temporal lobe epilepsy: How do we improve surgical outcome? Ann Neurol 2010;68:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arzimanoglou A, Ben-Menachem E, Cramer J, Glauser T, Seeruthun R, Harrison M. The evolution of antiepileptic drug development and regulation. Epileptic Disord 2010;12:3–15 [DOI] [PubMed] [Google Scholar]
- 18. Prunetti P, Perucca E. New and forthcoming anti-epileptic drugs. Curr Opin Neurol 2011;24:159–164 [DOI] [PubMed] [Google Scholar]
- 19. Choi H, Heiman GA, Munger Clary H, Etienne M, Resor SR, Hauser WA. Seizure remission in adults with long-standing intractable epilepsy: an extended follow-up. Epilepsy Res 2011;93:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia 2007;48:1303–1307 [DOI] [PubMed] [Google Scholar]
- 21. Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a systematic review of efficacy and predictors of response. J Neurosurg 2011;115:1248–1255 [DOI] [PubMed] [Google Scholar]
- 22. Yasargil MG, Krayenbuhl N, Roth P, Hsu SP, Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg 2010;112:168–185 [DOI] [PubMed] [Google Scholar]
- 23. Committee of Quality of Health Care in America IoM Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001 [PubMed] [Google Scholar]
- 24. Wiebe S. Still an elusive target: guiding practice for epilepsy surgery. Neurology 2010;75:678–679 [DOI] [PubMed] [Google Scholar]
- 25. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319 [DOI] [PubMed] [Google Scholar]
- 26. Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res 2009;177:147–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia 2006;47(suppl 2): 96–98 [DOI] [PubMed] [Google Scholar]
- 28. Wiebe S. Effectiveness and safety of epilepsy surgery: what is the evidence? CNS Spectr 2004;9:120–122, 126–132 [DOI] [PubMed] [Google Scholar]
- 29. Wachi M, Tomikawa M, Fukuda M, et al. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia 2001;42(suppl 6): 4–8 [PubMed] [Google Scholar]
- 30. Westerveld M, Sass KJ, Chelune GJ, et al. Temporal lobectomy in children: cognitive outcome. J Neurosurg 2000;92:24–30 [DOI] [PubMed] [Google Scholar]
- 31. Labiner DM, Bagic AI, Herman ST, et al. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010;51:2322–2333 [DOI] [PubMed] [Google Scholar]
- 32. Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006;47:952–959 [DOI] [PubMed] [Google Scholar]
- 33. Smith ER, Butler WE, Barker FG., 2nd In-hospital mortality rates after ventriculoperitoneal shunt procedures in the United States, 1998 to 2000: relation to hospital and surgeon volume of care. J Neurosurg 2004;100:90–97 [DOI] [PubMed] [Google Scholar]
- 34. Kalkanis SN, Eskandar EN, Carter BS, Barker FG., 2nd Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery 2003;52:1251–1261; discussion 1261–1252 [DOI] [PubMed] [Google Scholar]
- 35. Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA 2010;304:53–60 [DOI] [PubMed] [Google Scholar]
- 36. Boudourakis LD, Wang TS, Roman SA, Desai R, Sosa JA. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg 2009;250:159–165 [DOI] [PubMed] [Google Scholar]
- 37. Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy Behav 2006;9:243–264 [DOI] [PubMed] [Google Scholar]
- 38. Burneo JG, Jette N, Theodore W, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia 2009;50:2285–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Begley CE, Basu R, Reynolds T, et al. Sociodemographic disparities in epilepsy care: Results from the Houston/New York City health care use and outcomes study. Epilepsia 2009;50:1040–1050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.