Abstract
Objectives:
More than 30 different rare mutations, including copy number variants (CNVs), in the amyloid precursor protein gene (APP) cause early-onset familial Alzheimer disease (EOFAD), whereas the contribution of common APP variants to disease risk remains controversial. In this study we systematically assessed the role of both rare and common APP DNA variants in Alzheimer disease (AD) families.
Methods:
Families with EOFAD genetically linked to the APP region were screened for missense mutations and locus duplications of APP. Further, using genome-wide DNA microarray data, we examined the APP locus for CNVs in a total of 797 additional early- and late-onset AD pedigrees. Finally, 423 single nucleotide polymorphisms (SNPs) in the APP locus, including 2 promoter polymorphisms previously associated with AD risk, were tested in up to 4,200 individuals from multiplex AD families.
Results:
Analyses of 8 21q21-linked families revealed one family carrying a nonsynonymous mutation in exon 17 (Val717Leu) and another family with a partially penetrant 3.5-Mb locus duplication encompassing APP. CNV analysis in the APP locus revealed an additional family carrying a fully penetrant 380-kb duplication, merely spanning APP. Last, contrary to previous reports, association analyses of more than 400 different SNPs in or near APP failed to show significant effects on AD risk.
Conclusion:
Our study shows that APP mutations and locus duplications are a very rare cause of EOFAD and that the contribution of common APP variants to AD susceptibility is insignificant. Furthermore, duplications of APP may not be fully penetrant, possibly indicating the existence of hitherto unknown protective genetic factors.
Highly penetrant mutations in the gene encoding amyloid precursor protein (APP) (21q21.2) were the first reported genetic causes of early-onset familial Alzheimer disease (EOFAD)1 (see also Alzheimer Disease & Frontotemporal Dementia Mutation Database, www.molgen.ua.ac.be/ADMutations/2). Most of the currently known AD-causing mutations in APP lead to an increase in the ratio of the amyloid-β42 (Aβ42) to Aβ40 peptide3,4 and synaptic Aβ levels.5 AD pathology is also found in patients with Down syndrome, i.e., trisomy of chromosome 21, indicating that extra copies of APP alone may lead to neurotoxic Aβ production in the absence of any missense mutations. Furthermore, several reports have shown that the presence of APP locus duplications cause EOFAD.6–10 Finally, recent candidate gene studies have also implicated the existence of rare variants in the APP promoter in EOFAD by increasing APP expression,11,12 although these findings have been refuted elsewhere.13–19 In contrast, the contribution of common APP variants to Alzheimer disease (AD) risk remains unclear (see also AlzGene database, www.alzgene.org20). In this study, we thoroughly investigated the role of both rare and common APP DNA sequence variants in several large collections of both EOFAD and late-onset AD (LOAD) families. Our results suggest that missense mutations in APP and locus duplications are a rare cause of AD, whereas common variants in APP probably play no major role, if any, in modulating AD risk. In addition, we observe evidence that some APP locus duplications may only display reduced penetrance.
METHODS
Participants.
National Institute of Mental Health families.
In total, this sample includes 1,536 individuals from 457 multiplex AD families.21 Of these, 131 pedigrees (517 subjects [316 affected subjects, onset age 64.5 + 9.5 years]) are from families with an “early/mixed” onset age, i.e., at least one sampled affected subject showed an onset age of <65 years), whereas in the remaining pedigrees all sampled affected subjects showed an onset age of ≥65 years. Age at onset for all cases of AD was determined by a clinician based on an interview with a knowledgeable informant and review of any available records. From our earlier whole-genome linkage screen on these families,22 we identified 8 families in the early/mixed onset-age stratum that showed evidence of genetic linkage to the region encompassing APP at ∼26 Mb (i.e., between markers D21S1437 at ∼20 Mb and D21S1440 at ∼38 Mb) (table 1).
Table 1.
Genetic association results of 2 APP promoter polymorphisms previously associated with AD riska
Abbreviations: AD = Alzheimer disease; APP = amyloid precursor protein; CAG = Consortium on Alzheimer's Genetics; NCRAD = National Cell Repository for Alzheimer's Disease; NIA = National Institute on Aging; NIMH = National Institute of Mental Health; SNP = single nucleotide polymorphism.
p Value represents p values calculated using PBAT with additive model and affection status, restricted to Caucasian-only families (negative p values indicate undertransmission to affected family members). Fams represents the number of informative families. Combined represents meta-analysis of results across all 4 samples using METAL. See table e-2 for meta-analysis results on 421 genotyped and imputed SNPs in the APP region determined in the NIMH and NCRAD samples.
Additional independent family samples.
In addition to the National Institute of Mental Health (NIMH) families, we analyzed members of 3 independent AD family collections. Two of these were obtained from the National Cell Repository for Alzheimer Disease (NCRAD), and ascertainment and collection details can be found at the NCRAD Web site (www.ncrad.org). The collection of families labeled here as NIA (National Institute on Aging) comprised 1,111 samples from 351 pedigrees (Caucasian: 1,040 samples from 329 pedigrees). The collection of families labeled here as NCRAD comprised 1,260 samples from 368 pedigrees (Caucasian: 1,106 samples from 330 pedigrees). Finally, the collection of families labeled CAG (Consortium on Alzheimer's Genetics) originated from multiple NIA-funded Alzheimer Disease Research Centers under the auspices of the Consortium on Alzheimer's Genetics. Probands were included only if they had at least one unaffected living sibling willing to participate in this study. For all non-NIMH families we only included pedigrees in which all sampled affected individuals had onset ages of at least 50 years.
Note that different combinations of these family samples were used in different parts of our study. APP sequencing was performed in chromosome 21–linked NIMH families only. APP copy number variant (CNV) and common marker association analyses were performed on all remaining NIMH and all the NCRAD families. Last, members from all 4 family samples (i.e., 4,180 individuals) were genotyped for the 2 previously associated APP promoter single nucleotide polymorphisms (SNPs) (rs459543 [+37c/g] and rs463946 [−3102G/C]).
Standard protocol approvals, registrations, and patient consents.
Written informed consent for participation was provided by all subjects or, for those with substantial cognitive impairment, by a caregiver, legal guardian, or other proxy by the clinical sites responsible for subject recruitment. The study protocols for all populations were reviewed and approved by the institutional review boards of the respective recruitment sites. Genetic experiments were reviewed and approved by the institutional review board of Massachusetts General Hospital.
Experimental procedures.
Fluorescent in situ hybridization.
Bacterial artificial chromosome clones containing both ends of the APP gene (RP11-15D13 for the 5′ end and RP11-410J1 for the 3′ end) and CTB-63H24 mapping to 21q22.3 (control probe) were used for fluorescent in situ hybridization (FISH). RP11-15D13 and RP11-410J1 were labeled with Cy3-dUTP, and CTB-63H24 was labeled with fluorescein isothiocyanate-dUTP by nick translation. FISH was performed according to the protocol described in Mohapatra et al.23
SNP genotyping.
Genome-wide association study (GWAS) SNPs were generated in a separate project (L. Bertram, A.R. Parrado, B. Hooli, C. Lange, R.E. Tanzi, 2012, unpublished) on the Affymetrix Genome-Wide Human SNP Array 6.0, using individually optimized genotyping and allele-calling procedures. Before statistical analyses, the number of SNPs was augmented by genotype imputation using IMPUTE2.0 software. Details on the methods used for genotype imputation and statistical analyses can be found in appendix e-1 on the Neurology® Web site at www.neurology.org.24–27 The 2 promoter SNPs (rs459543 [+37c/g] and rs463946 [−3102G/C]) were assayed separately via high-efficiency fluorescence polarization-detected single base extension on a Criterion Analyst AD high-throughput fluorescence detection system (Molecular Devices), using customized PCR primers and cycling conditions.28 Genotyping efficiency on the 2 SNPs tested here was >95%, whereas the error rate was <1% (based on ∼10% duplicated samples). Both SNPs were in Hardy-Weinberg equilibrium (p > 0.05) in all 4 samples.
CNV analysis.
This was done for individuals from the NIMH and NCRAD datasets for whom we used the Affymetrix Genome-Wide Human SNP Array 6.0 as part of an ongoing GWAS. Raw probe intensities from each sample were normalized against the HapMap CEU reference intensity dataset. For the purpose of CNV analysis, we excluded samples showing chromosomal abnormalities and high CNV count. All samples included were subject to waviness factor adjustment.29,30 CNV calling and segmentation were performed with PennCNV (www.openbioinformatics.org/penncnv/30) using default criteria.
Fluidigm Digital Array protocol.
We used this method as a validation experiment for the APP duplication observed in the NCRAD family. In brief, 16 ng of DNA from all subjects was mixed with 1× TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA), 1× FAM-labeled APP copy number probe (Hs05532959_cn, Applied Biosystems), 1× VIC-labeled RNase P TaqMan assay, and 1× sample loading reagent (Fluidigm Inc., South San Francisco, CA). DNA samples from individuals of NIMH family VI carrying an APP duplication were used as positive controls, and all samples were run on a 48.776 array. The experimental protocol and data analysis procedures are described in detail elsewhere.31,32
RESULTS
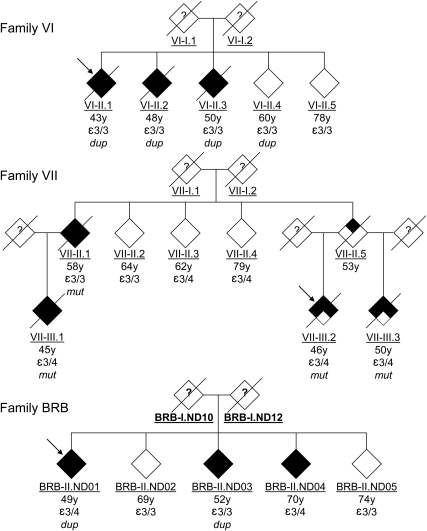
The 8 EOFAD (table 1) families showing linkage at or near the APP region were resequenced for mutations in APP exons 16 and 17, the promoter region (−1 to −676 kb), and the 3′ untranslated region (1–1,221 bp). In addition, these families were also tested for APP locus duplications using semiquantitative multiplex PCR and subsequently confirmed by FISH (figure e-1). One family (VII) carried a previously reported and fully penetrant missense mutation in exon 17 (Val717Leu, rs63750264 G>C, “Indiana-2”33) (figure 1). The age of onset of AD in this family was between 45 and 58 years, whereas the 3 unaffected individuals were between 62 and 79 years of age at last examination. The clinical diagnosis of AD was confirmed neuropathologically in 2 affected individuals of this pedigree (VII-II.3 and VII-II.1), whereas the other 2 received a diagnosis of probable AD (VII-II.2 and VII-II.4). A second chromosome 21–linked family (VI) (figure 1) was found to carry APP locus duplication which, in contrast to previous reports, only showed partial penetrance. Using genome-wide microarray data we were able to delineate the size of the duplicated segment to ∼3.4 Mb (figure 2). In this family, the duplication was present in all 3 affected individuals (onset ages: 43–50 years; all AD diagnoses confirmed by neuropathologic examination), but also in one unaffected individual (VI-II.4, last age at examination 60 years), whereas no duplication was found in the remaining unaffected sibling (VI-II.5, 78 years) (figure 1). Tests for expression level differences of APP mRNA and protein, as well as Aβ levels, in Epstein-Barr virus–transformed lymphoblastoid cell lines of all members of this family did not show significant differences between carriers and noncarriers of the APP duplication, regardless of affection status (data not shown). This finding is in line with earlier reports indicating that pathologically relevant increases in APP/Aβ expression may be restricted to the brain and are not detectable in peripheral cells.12 Unfortunately, brain samples were not available for any member of this family.
Figure 1. Pedigree charts of families found to carry disease-causing APP mutations and locus duplications.
Information for each individual is (from top to bottom): age at onset (in affected individuals) or age at last examination (unaffected individuals); APOE genotype; and APP mutation finding. Probands are indicated by arrows. No DNA or clinical information was available from the founders (?). dup = carriers of APP duplication; mut = carriers of Val717Leu mutation.
Figure 2. Delineation of the APP duplicated region identified here and those in previous studies.
Approximate locations of the duplicated intervals across studies are shown. Solid arrows indicate minimal size of the duplicated interval; dotted lines indicate maximal boundaries. Note that our study is the only to use high-density genome-wide association study data, allowing a much more precise delineation of the duplicated interval than the lower-resolution microsatellite-based mapping. Physical location of duplicated segments from microarray data mapped to hg18 assembly are family (Fam.) VI (chr21: 23984747–27466529) and family BRB (chr21: 26125668–26505191).
Analysis for CNVs in APP in the microarray data from the remaining 429 NIMH families and 368 NCRAD families revealed one family in the NCRAD dataset (BRB, figure 1) carrying APP locus duplication, which was subsequently confirmed using the Fluidigm Digital Array (table e-1). Although fully penetrant, this latter duplication is interesting for 2 reasons. First, the duplicated segment (∼0.38 Mb) is approximately 10-fold smaller than the duplicated segment identified in the NIMH family. Barely encompassing the entire genomic interval of APP, this segment represents the shortest APP duplication identified to date (figure 2). Second, the duplication was carried by only 2 of 3 affected siblings in this family (onset ages 49 and 52 years), whereas a third affected individual (onset age 70 years) showed a diploid, i.e., normal, copy status in this region. Similarly, the 2 unaffected siblings (ages at last examination 69 and 74 years) also showed no evidence for duplication of the APP region. Thus, this family coalesces the sort of genetic heterogeneity that is typical of AD (and several other neurodegenerative disorders), i.e., the presence of likely disease-causing and susceptibility-increasing factors.
Finally, association analyses of 421 common SNPs located within a 340-kb interval encompassing APP was undertaken based on observed and imputed GWAS microarray SNP data probing for effects on disease risk. However, the strongest association with AD risk in these analyses only showed a nominal p value of ∼0.008 (rs117650267) (table e-2), which would not even approach statistical significance if corrected for multiple testing. Given our relatively liberal choice of including imputed genotypes (based on a posterior call probability of 0.5), it is unlikely, albeit not impossible, that the use of overstringent quality control procedures precluded us from detecting any genuine association results, e.g., by excluding certain informative SNPs or families. We did fail to detect evidence for association with 2 SNPs in the APP promoter region (rs459543 [+37C/G] and rs463946 [−3102G/C]) (table 1) previously reported to be associated with AD risk.13,14 Because these SNPs were not observed or imputed by the Affymetrix microarray, they were manually genotyped in nearly 4,200 individuals originating from 4 independent family datasets, yielding >70% power to detect the previously reported effect sizes. The absence of significant association using common polymorphisms is in line with recent GWASs reporting no evidence of association with markers near the APP region (see the AlzGene database for a list of all GWASs performed in AD).
DISCUSSION
We undertook a systematic assessment of the contribution of rare and common APP DNA sequence variants across large collections of independent AD family samples. Mutational screening of EOFAD families linked to the APP-encompassing region on chromosome 21 revealed one family carrying a previously reported missense mutation and one family carrying a duplication of the APP locus. Although the missense mutation showed complete penetrance in the affected family, the occurrence of one unaffected individual in the family VI carrying the APP duplication at >3 SD from the average familial onset age is strongly indicative of incomplete penetrance, implying existence of yet unidentified protective factors. Although the possibility that this individual will also develop AD at some later time cannot be definitely excluded, our findings already suggest that other genetic or nongenetic factors can mitigate the effects of APP locus duplications and either confer complete protection against AD or at least substantially delay its onset age. In the currently available literature there is one other report in which an unaffected individual was also found to carry an APP duplication (individual III.21 in family 11049). However, the last age of examination of this individual is still within 1 SD of the average familial onset age, whereas the difference here is greater than 3 SD in the unaffected sibling in family VI. The second, independent APP duplication observed in our study (family BRB in the NCRAD dataset) represents the smallest reported duplicated interval on chromosome 21, effectively reducing the obligate AD-causing region to APP (chr21: 26122781–26521135, NCBI36/hg18 assembly). To date, this is also the first reported case of an APP duplication co-occurring with another cause of AD within the same pedigree. Aggregating the CNV data across different studies published to date (figure 2) suggests that most (if not all) instances of locus duplications in this chromosomal interval are not linked to the same founder individual, but rather have occurred independently of one another. Overall, these results suggest that APP duplications are a rare cause of EOFAD and extremely rare (if not absent) in LOAD.
Contrary to these findings confirming and extending prior evidence, we were unable to corroborate the presence of sequence variants in the APP promoter, neither as causative nor as risk factors for AD. This includes variants 534G→A, 479C→T, 369C→G, and 118C→A, which were previously shown to cause AD by increasing expression levels of APP.12 None of the NIMH chromosome 21–linked families carried any mutations in the APP promoter region, including the variants described above. Our failure to detect mutations at these sites are in agreement with a prior study14 reporting a higher frequency of the presumed disease-causing alleles in healthy controls compared with individuals with AD. Taken together, these data suggest that these APP promoter sequence variants do not have a role in AD pathogenesis. This raises the possibility that other, hitherto elusive, DNA sequence variants in or near APP may account for the onset of AD in the 6 families linked to chromosome 21q but not found to carry any disease-causing APP mutations (table e-3). Given the current lack of evidence implying AD-causing mutations in regions beyond those investigated here (see Alzheimer Disease & Frontotemporal Dementia Mutation Database), this alternative appears unlikely.
Finally, genetic association analyses of common variants, including 2 APP promoter polymorphisms previously reported to show association with LOAD risk, did not reveal any significant evidence for association with either risk for AD or onset-age variation. Whereas some equipoise from earlier and often smaller studies still exists, our results are in line with, and substantially extend, those of a recent study investigating 44 SNPs in almost 1,200 case patients and controls from the United States,34 although that study did not directly test the 2 previously associated promoter SNPs (rs459543 and rs463946) that were investigated here. In addition, none of the currently published GWASs in AD (see www.alzgene.org for details) have thus far reported significant association between risk for AD and common sequence variants in or near APP, providing further evidence against the notion that common sequence variation in this gene contributes to risk for LOAD. Although this is similar to the lack of risk associated with common variants in the other 2 EOFAD genes, PSEN1 and PSEN2 (presenilin 1 and 2), it is in contrast to other neurodegenerative disorders, e.g., Parkinson disease or frontotemporal dementia, for which genes known to contain rare, disease-causing variants giving rise to disease forms transmitted in a Mendelian fashion are also among the lead GWAS findings based on common polymorphisms.35 It remains to be seen whether the investigation of subjects drawn from genetic backgrounds other than Caucasian will reveal different patterns.
Our comprehensive and systematic analyses investigating the role of APP in AD genetics in subjects of Caucasian descent suggest that missense mutations in APP and locus duplications are a rare cause of AD, whereas common variants in APP probably play no major role, if any, in contributing to risk for AD. In addition, the incomplete penetrance of the APP locus duplication observed in family VI emphasizes the need to more systematically search for protective variables. A better understanding of these risk-reducing factors may be essential for developing better and more effective early prevention and treatment strategies against this devastating disorder.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all families for participating in this study. Data and biomaterials were collected in 3 projects that participated in the NIMH Alzheimer Disease Genetics Initiative. From 1991 through 1998, the principal investigators and co-investigators were the following: Massachusetts General Hospital, Boston, MA (U01-MH46281), Marilyn S. Albert, PhD, and Deborah Blacker, MD, ScD; Johns Hopkins University, Baltimore, MD (U01-MH46290), Susan S. Bassett, PhD, Gary A. Chase, PhD, and Marshal F. Folstein, MD; and University of Alabama, Birmingham, AL (U01-MH46373), Rodney C.P. Go, PhD, and Lindy E. Harrell, MD.
GLOSSARY
- Aβ
amyloid-β
- AD
Alzheimer disease
- APP
amyloid precursor protein
- CNV
copy number variant
- EOFAD
early-onset familial Alzheimer disease
- FISH
fluorescent in situ hybridization
- GWAS
genome-wide association study
- LOAD
late-onset Alzheimer disease
- NCRAD
National Cell Repository for Alzheimer's Disease
- NIA
National Institute on Aging
- NIMH
National Institute of Mental Health
- SNP
single nucleotide polymorphism
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Hooli: acquisition of data, analysis of data, study coordination, drafting and revision. Dr. Mohapatra: acquisition of data, analysis of data and revision. Dr. Mattheisen: statistical analyses. Dr. Parrado: analysis of data. J.T. Roehr: acquisition of data and analysis of data. Dr. Shen: acquisition of data. Dr. Gusella: study supervision and revision. Dr. Moir: study supervision and revision. Dr. Saunders: study supervision and revision. Dr. Lange: study design, revision. Dr. Tanzi: study supervision and coordination, revision. Dr. Bertram: study concept and design, analysis of data, statistical analysis, study supervision and coordination, drafting and revision.
DISCLOSURE
Dr. Hooli, Dr. Mohapatra, Dr. Mattheisen, Dr. Parrado, and J. Roehr report no disclosures. Dr. Shen receives research support from Children's Tumor Foundation. Dr. Gusella serves on a scientific advisory board and as a consultant for Quest Diagnostics, Inc.; serves on editorial advisory boards for DNA and Cell Biology, Neurobiology of Disease, Neurogenetics, Biomed Central Neuroscience, Biomed Central Biology, and Molecular Autism; holds patents re: Methods for altering mRNA splicing and treating familial dysautonomia and other mechanistically related disorders; Methods for detecting mutations associated with familial dysautonomia; Gene for identifying individuals with familial dysautonomia; Vectors for delivering viral and oncogenic inhibitors; Kits for detecting polymorphisms associated with familial dysautonomia; Tumor suppressor merlin and antibodies thereof; Use of genetic markers to diagnose familial dysautonomia; TPR-containing genes; Tumor suppressor gene merlin; Huntingtin DNA, protein and uses thereof; Transport protein gene from the Huntington's disease region; Flexible scheme for admission control of multimedia streams on integrated networks; Use of genetic markers to diagnose familial dysautonomia; DNA encoding a protein-coupled receptor kinase; Test for Huntington's disease; and Isolation and localization of DNA segments; and receives/has received research support from the NIH (NINDS, NIGMS, NICHD), the Simons Foundation Autism Research Initiative, CHDI Foundation Inc., Autism Speaks, and the Huntington's Disease Society of America Coalition for the Cure. Dr. Moir serves as an Assistant Editor for the International Journal of Genetic and Medical Nanotechnology; holds a patent re: A novel method to diagnose and/or treat Alzheimer's disease; and receives research support from the Helmsley Foundation, the NIH, and the Cure Alzheimer's Fund. Dr. Saunders receives research support from the NIH/NINDS and the Cure Alzheimer's Fund. Dr. Lange reports no disclosures. Dr. Tanzi serves as a consultant for and has received funding for travel and speaker honoraria from Pfizer Inc, Eisai Inc., Prana Biotechnology, and Probiodrug AG; serves on the editorial board of Neuron editorial board; and receives research support from the NIH/NIMH and the Cure Alzheimer's Fund. Dr. Bertram serves on editorial advisor boards for Neurogenetics, European Journal of Clinical Investigation, and International Journal of Molecular Epidemiology and Genetics.
REFERENCES
- 1. Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 1991;349:704–706 [DOI] [PubMed] [Google Scholar]
- 2. Cruts M, Van Broeckhoven C. Molecular genetics of Alzheimer's disease. Ann Med 1998;30:560–565 [DOI] [PubMed] [Google Scholar]
- 3. Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 2005;120:545–555 [DOI] [PubMed] [Google Scholar]
- 4. Wolfe MS. When loss is gain: reduced presenilin proteolytic function leads to increased Aβ42/Aβ40: talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep 2007;8:136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hooli BV, Tanzi RE. A current view of Alzheimer's disease. F1000 Biol Rep 2009;1:54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kasuga K, Shimohata T, Nishimura A, et al. Identification of independent APP locus duplication in Japanese patients with early-onset Alzheimer disease. J Neurol Neurosurg Psychiatry 2009;80:1050–1052 [DOI] [PubMed] [Google Scholar]
- 7. McNaughton D, Knight W, Guerreiro R, et al. Duplication of amyloid precursor protein (APP), but not prion protein (PRNP) gene is a significant cause of early onset dementia in a large UK series. Neurobiol Aging 2012;33:426.e13–426.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rovelet-Lecrux A, Hannequin D, Raux G, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 2006;38:24–26 [DOI] [PubMed] [Google Scholar]
- 9. Sleegers K, Brouwers N, Gijselinck I, et al. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 2006;129:2977–2983 [DOI] [PubMed] [Google Scholar]
- 10. Rovelet-Lecrux A, Frebourg T, Tuominen H, Majamaa K, Campion D, Remes AM. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2007;78:1158–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brouwers N, Sleegers K, Engelborghs S, et al. Genetic risk and transcriptional variability of amyloid precursor protein in Alzheimer's disease. Brain 2006;129:2984–2991 [DOI] [PubMed] [Google Scholar]
- 12. Theuns J, Brouwers N, Engelborghs S, et al. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am J Hum Genet 2006;78:936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Athan ES, Lee JH, Arriaga A, Mayeux RP, Tycko B. Polymorphisms in the promoter of the human APP gene: functional evaluation and allele frequencies in Alzheimer disease. Arch Neurol 2002;59:1793–1799 [DOI] [PubMed] [Google Scholar]
- 14. Guyant-Marechal L, Rovelet-Lecrux A, Goumidi L, et al. Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology 2007;68:684–687 [DOI] [PubMed] [Google Scholar]
- 15. Hebert SS, Horre K, Nicolai L, et al. MicroRNA regulation of Alzheimer's amyloid precursor protein expression. Neurobiol Dis 2009;33:422–428 [DOI] [PubMed] [Google Scholar]
- 16. Bettens K, Brouwers N, Engelborghs S, et al. APP and BACE1 miRNA genetic variability has no major role in risk for Alzheimer disease. Hum Mutat 2009;30:1207–1213 [DOI] [PubMed] [Google Scholar]
- 17. Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J Neurosci 2008;28:1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer disease and other human CNS disorders. Curr Genomics 2009;10:154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kocerha J, Kauppinen S, Wahlestedt C. microRNAs in CNS disorders. Neuromolecular Med 2009;11:162–172 [DOI] [PubMed] [Google Scholar]
- 20. Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 2007;39:17–23 [DOI] [PubMed] [Google Scholar]
- 21. Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer's disease: the NIMH genetics initiative. Neurology 1997;48:139–147 [DOI] [PubMed] [Google Scholar]
- 22. Blacker D, Bertram L, Saunders AJ, et al. Results of a high-resolution genome screen of 437 Alzheimer's disease families. Hum Mol Genet 2003;12:23–32 [DOI] [PubMed] [Google Scholar]
- 23. Mohapatra G, Moore DH, Kim DH, et al. Analyses of brain tumor cell lines confirm a simple model of relationships among fluorescence in situ hybridization, DNA index, and comparative genomic hybridization. Genes Chromosomes Cancer 1997;20:311–319 [DOI] [PubMed] [Google Scholar]
- 24. Bertram L, Hiltunen M, Parkinson M, et al. Family-based association between Alzheimer's disease and variants in UBQLN1. N Engl J Med 2005;352:884–894 [DOI] [PubMed] [Google Scholar]
- 25. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39:906–913 [DOI] [PubMed] [Google Scholar]
- 27. Lange C, DeMeo D, Silverman EK, Weiss ST, Laird NM. PBAT: tools for family-based association studies. Am J Hum Genet 2004;74:367–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diskin SJ, Li M, Hou C, et al. Adjustment of genomic waves in signal intensities from whole-genome SNP genotyping platforms. Nucleic Acids Res 2008;36:e126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang K, Li M, Hadley D, et al. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res 2007;17:1665–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weaver S, Dube S, Mir A, et al. Taking qPCR to a higher level: analysis of CNV reveals the power of high throughput qPCR to enhance quantitative resolution. Methods 2010;50:271–276 [DOI] [PubMed] [Google Scholar]
- 32. Qin J, Jones RC, Ramakrishnan R. Studying copy number variations using a nanofluidic platform. Nucleic Acids Res 2008;36:e116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murrell JR, Hake AM, Quaid KA, Farlow MR, Ghetti B. Early-onset Alzheimer disease caused by a new mutation (V717L) in the amyloid precursor protein gene. Arch Neurol 2000;57:885–887 [DOI] [PubMed] [Google Scholar]
- 34. Nowotny P, Simcock X, Bertelsen S, et al. Association studies testing for risk for late-onset Alzheimer's disease with common variants in the β-amyloid precursor protein (APP). Am J Med Genet B Neuropsychiatr Genet 2007;144B:469–474 [DOI] [PubMed] [Google Scholar]
- 35. Bertram L. Alzheimer's genetics in the GWAS era: a continuing story of ‘replications and refutations. ' Curr Neurol Neurosci Rep 2011;11:246–253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.