Abstract
Objective:
To examine outcomes at age 4.5 years and compare to earlier ages in children with fetal antiepileptic drug (AED) exposure.
Methods:
The NEAD Study is an ongoing prospective observational multicenter study, which enrolled pregnant women with epilepsy on AED monotherapy (1999–2004) to determine if differential long-term neurodevelopmental effects exist across 4 commonly used AEDs (carbamazepine, lamotrigine, phenytoin, or valproate). The primary outcome is IQ at 6 years of age. Planned analyses were conducted using Bayley Scales of Infant Development (BSID at age 2) and Differential Ability Scale (IQ at ages 3 and 4.5).
Results:
Multivariate intent-to-treat (n = 310) and completer (n = 209) analyses of age 4.5 IQ revealed significant effects for AED group. IQ for children exposed to valproate was lower than each other AED. Adjusted means (95% confidence intervals) were carbamazepine 106 (102–109), lamotrigine 106 (102–109), phenytoin 105 (102–109), valproate 96 (91–100). IQ was negatively associated with valproate dose, but not other AEDs. Maternal IQ correlated with child IQ for children exposed to the other AEDs, but not valproate. Age 4.5 IQ correlated with age 2 BSID and age 3 IQ. Frequency of marked intellectual impairment diminished with age except for valproate (10% with IQ <70 at 4.5 years). Verbal abilities were impaired for all 4 AED groups compared to nonverbal skills.
Conclusions:
Adverse cognitive effects of fetal valproate exposure persist to 4.5 years and are related to performances at earlier ages. Verbal abilities may be impaired by commonly used AEDs. Additional research is needed.
Antiepileptic drugs (AEDs) are among the most common potentially teratogenic drugs taken by women of childbearing potential. Animal studies have demonstrated that fetal AED exposure at doses lower than those required for anatomic malformations can produce behavior deficits.1,2 Our group initiated a prospective study to establish if such effects can occur in humans. We have previously reported differential effects of fetal AED exposure on cognitive outcomes in children at age 3 years.3 Based largely on the data from this report, the Food and Drug Administration recently released a drug safety communication reporting an increased risk of impaired cognitive development in children whose mothers took valproate during pregnancy.4 Since deficits at age 3 years might change with maturation, we examined the effects of fetal AED exposure on cognitive functions at age 4.5 years and compared these findings to outcomes at ages 2 and 3 years.
METHODS
Design.
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study is an ongoing prospective observational investigation, which enrolled pregnant women with epilepsy who were on AED monotherapy (i.e., carbamazepine, lamotrigine, phenytoin, or valproate) from October 1999 through February 2004 across 25 epilepsy centers in the United States and United Kingdom. Sample sizes were estimated to detect a 0.5 SD in IQ outcome. These AEDs were the most frequently prescribed during pregnancy at our centers during enrollment. No other AEDs were employed in sufficient numbers to allow adequate sample sizes for analysis. Polytherapy was not included because of prior reports of poorer outcomes.5,6 A nonexposed control group was not included at the direction of the NIH review panel. This is a planned interim analysis.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at each center approved the study, and written informed consent was obtained prior to enrollment according to the Declaration of Helsinki. Study conduct and patient safety were monitored by an NIH appointed Data Safety Management Board. The study is registered at clinicaltrials.gov as NCT00021866.
Participants.
Pregnant women with epilepsy on the 4 monotherapies were enrolled. Since maternal IQ is the major predictor of child IQ in population studies,7 mothers with IQ <70 were excluded to avoid floor effects. Other exclusion criteria included positive syphilis or HIV serology, progressive cerebral disease, other major disease (e.g., diabetes), exposure to teratogenic agents other than AEDs, poor AED compliance, drug abuse in the prior year, or drug abuse sequelae.
Procedures.
Information was collected on potentially confounding variables, including maternal IQ, age, education, employment, race/ethnicity, seizure/epilepsy types and frequency, AED dosages, compliance, other medicines, socioeconomic status,8 UK/US site, preconception folate use, use of alcohol, tobacco, or other drugs during pregnancy, unwanted pregnancy, abnormalities and complications in present or prior pregnancies, enrollment and birth gestational age, birthweight, breastfeeding, and childhood medical diseases. Cognitive outcomes were evaluated by assessors (blinded to AED) using the Differential Ability Scales (DAS)9 at ages 3 and 4.5, and Bayley Scales of Infant Development (BSID)10 at age 2. Testing for age 4.5 outcomes ranged from 51 to 61 months of age. Standardized scores were calculated. Separate investigations with very similar designs in the United States and United Kingdom were merged after initiation. Maternal IQs were determined by different measures due to later merger: Test of Nonverbal Intelligence–third edition (TONI-3)11 in 267 mothers (67 UK), Wechsler Abbreviated Scale of Intelligence (WASI)12 in 20 (all UK), and National Adult Reading Test (NART)13 in 17 (all UK). Training and monitoring of neuropsychological evaluations were conducted to assure quality and consistency. Workshops were conducted for neuropsychological test batteries annually, and each assessor was required to identify errors and provide appropriate correction for videotaped testing sessions containing errors in administration and scoring. Assessors also had to submit videotape of their practice test session with record forms to the Neuropsychology Core for review, feedback, and approval. If assessors failed, they submitted additional video assessments for approval.
Therapeutic dosages (mg/day) vary across AEDs. In order to allow comparisons across AEDs, average AED dose during pregnancy was standardized, as in our prior report,3 relative to ranges observed within each AED group in the intent-to-treat population by the following calculation: 100 × (observed dose − minimum dose) ÷ range of doses (i.e., maximum − minimum).
Statistical analysis.
The primary analysis was intent-to-treat (n = 310 live births including 6 twin pairs). Secondary analysis was performed on children who completed testing at age 4.5 years (n = 209 including 6 twin pairs). Secondary analyses also included comparison to outcomes at ages 2 and 3 years, correlations of age 4.5 IQ to standardized doses and to maternal IQ for each AED, propensity score analyses, and comparison of verbal and nonverbal performances at age 4.5 years. Analyses were performed at the NEAD Data and Statistical Center using SAS and R.
For the primary analysis, linear regression models were used to examine group differences in IQ adjusting for maternal IQ, standardized AED dose, maternal and gestational age at delivery, years maternal education, race/ethnicity (self-reported), and alcohol use during pregnancy. Additional covariates examined were epilepsy/seizure types, seizure frequency during pregnancy, employment, socioeconomic status, US/UK site, tobacco use, birthweight, unwanted pregnancy, breastfeeding, prior pregnancy birth defects and complications, present pregnancy complications, AED compliance, and other medications used (most common in descending order were vitamins, non-narcotic analgesics, narcotics, antibiotics, iron, antiemetics, antacids, and local anesthetics). Our a priori hypothesis was that specific AED, dose, and maternal IQ were important covariates, so these were included as predictors in a linear model with child IQ as outcome. Other covariates were added individually to the model and were included if found to be significant (p < 0.05), or if they did not exhibit collinearity with existing predictors. When added to the model, none of the baseline variables substantively changed inferences regarding AED group differences.
For the intent-to-treat analysis, Markov Chain Monte Carlo methods were used to impute missing age 4.5 outcomes from available age 2 and 3 outcomes and from baseline variables related to outcome or missingness.14,15 Baseline variables in the imputation model included AED, dose, maternal IQ and age, gestational age at delivery, years of maternal education, alcohol use, race/ethnicity, and socioeconomic status (SES). Mothers of children with missing age 4.5 outcomes differed significantly from the rest on maternal IQ, years of maternal education, and SES (p < 0.05, t test for continuous variables, Fisher exact test for categorical variables). They had lower IQ, fewer years of education, and lower SES. These variables were included in the imputation model. Standard errors and confidence intervals of parameter estimates incorporated imputation uncertainty. Least squares mean IQs were estimated for each group adjusting for maternal IQ, maternal age, dose, gestational age, years maternal education, alcohol use, and race/ethnicity. Similar secondary analyses, without imputations for missing cognitive data, were conducted for the completer sample (i.e., children with cognitive testing at 4.5 years).
To investigate whether baseline differences in seizure type or other characteristics explain the association of valproate with poorer cognitive outcomes, post hoc subgroup analyses were conducted and forest plots were created. Subgroups were defined by 1) seizure type and 2) propensity scores.16 Further description of our propensity analysis approach is available in our prior report.3
To test the probability that the proportion of children with marked impairment of cognition (i.e., IQ < 70) is unchanged across time, Cochran Q statistic was used to test the null hypothesis using the sample of children with cognitive testing at all 3 ages (i.e., 2, 3, and 4.5 years). To test for differences across AEDs in % IQ <70 at age 4.5, Fisher exact test was used.
RESULTS
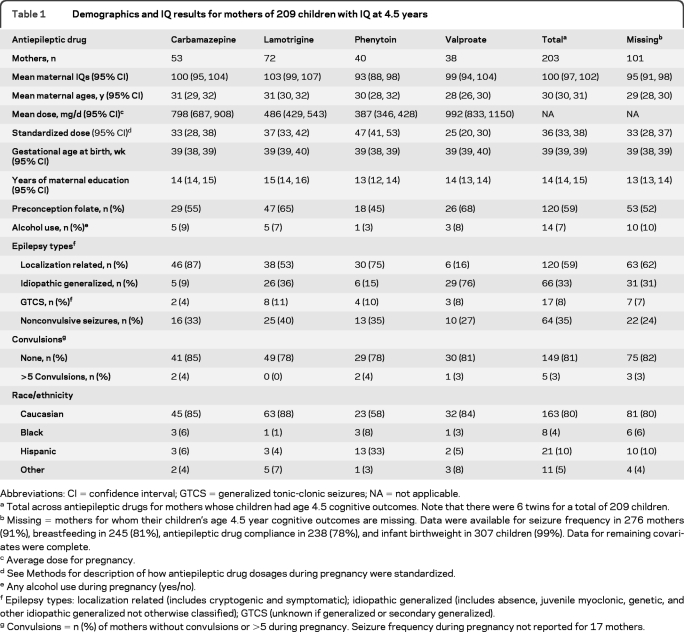
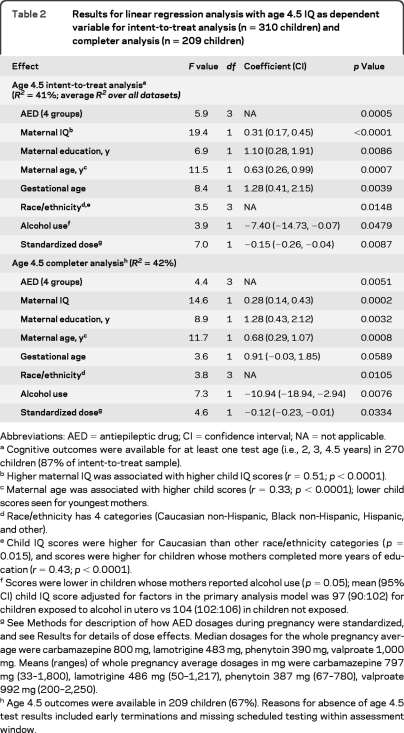
The primary intent-to-treat analysis included 304 mothers and 310 live births (6 sets of twins). Mean gestational age at enrollment was 18 weeks (range 4–37), which did not differ across drugs. The secondary completer analysis included 203 mothers and 209 children (6 sets of twins). Baseline maternal characteristics for the intent-to-treat sample were given in our prior publication,3 and for age 4.5 completers are depicted in table 1. Statistical results for the primary intent-to-treat and completer analyses with age 4.5 IQ as the dependent variable in the linear regression analyses are in table 2. Because different measures were used to estimate maternal IQ, a covariate for type of maternal IQ measure was added to the primary model; this covariate was nonsignificant (p = 0.39). In addition, reanalysis of the completer group analysis using only those with maternal TONI measure yielded results similar to the original analyses.
Table 1.
Demographics and IQ results for mothers of 209 children with IQ at 4.5 years
Abbreviations: CI = confidence interval; GTCS = generalized tonic-clonic seizures; NA = not applicable.
Total across antiepileptic drugs for mothers whose children had age 4.5 cognitive outcomes. Note that there were 6 twins for a total of 209 children.
Missing = mothers for whom their children's age 4.5 year cognitive outcomes are missing. Data were available for seizure frequency in 276 mothers (91%), breastfeeding in 245 (81%), antiepileptic drug compliance in 238 (78%), and infant birthweight in 307 children (99%). Data for remaining covariates were complete.
Average dose for pregnancy.
See Methods for description of how antiepileptic drug dosages during pregnancy were standardized.
Any alcohol use during pregnancy (yes/no).
Epilepsy types: localization related (includes cryptogenic and symptomatic); idiopathic generalized (includes absence, juvenile myoclonic, genetic, and other idiopathic generalized not otherwise classified); GTCS (unknown if generalized or secondary generalized).
Convulsions = n (%) of mothers without convulsions or >5 during pregnancy. Seizure frequency during pregnancy not reported for 17 mothers.
Table 2.
Results for linear regression analysis with age 4.5 IQ as dependent variable for intent-to-treat analysis (n = 310 children) and completer analysis (n = 209 children)
Abbreviations: AED = antiepileptic drug; CI = confidence interval; NA = not applicable.
Cognitive outcomes were available for at least one test age (i.e., 2, 3, 4.5 years) in 270 children (87% of intent-to-treat sample).
Higher maternal IQ was associated with higher child IQ scores (r = 0.51; p < 0.0001).
Maternal age was associated with higher child scores (r = 0.33; p < 0.0001); lower child scores seen for youngest mothers.
Race/ethnicity has 4 categories (Caucasian non-Hispanic, Black non-Hispanic, Hispanic, and other).
Child IQ scores were higher for Caucasian than other race/ethnicity categories (p = 0.015), and scores were higher for children whose mothers completed more years of education (r = 0.43; p < 0.0001).
Scores were lower in children whose mothers reported alcohol use (p = 0.05); mean (95% CI) child IQ score adjusted for factors in the primary analysis model was 97 (90:102) for children exposed to alcohol in utero vs 104 (102:106) in children not exposed.
See Methods for description of how AED dosages during pregnancy were standardized, and see Results for details of dose effects. Median dosages for the whole pregnancy average were carbamazepine 800 mg, lamotrigine 483 mg, phenytoin 390 mg, valproate 1,000 mg. Means (ranges) of whole pregnancy average dosages in mg were carbamazepine 797 mg (33–1,800), lamotrigine 486 mg (50–1,217), phenytoin 387 mg (67–780), valproate 992 mg (200–2,250).
Age 4.5 outcomes were available in 209 children (67%). Reasons for absence of age 4.5 test results included early terminations and missing scheduled testing within assessment window.
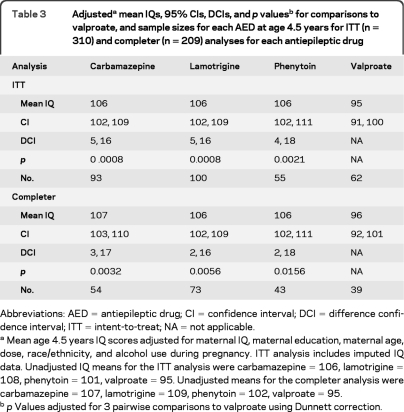
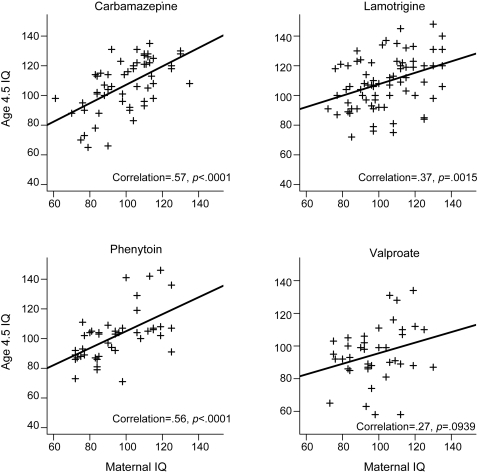
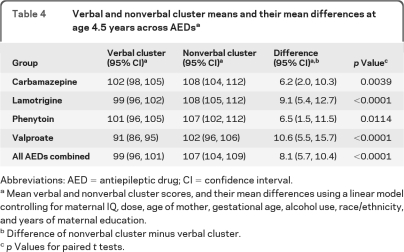
Adjusted mean age 4.5 IQ scores along with 95% confidence intervals (CIs) for each AED group are listed in table 3for the intent-to-treat and completer analyses. Children exposed in utero to valproate had significantly lower mean IQ than each of the other AEDs. Scatterplots and correlations (figure 1) depict the relationship of age 4.5 IQ with standardized dose. Dose-dependent effects were present for valproate but not for other AEDs. Similar results were found for separate analyses using first and third trimester dosages. Child and maternal IQ were significantly related for each AED except valproate (figure e-1 on the Neurology® Web site at www.neurology.org). Verbal abilities were significantly lower than nonverbal abilities across all drugs combined and for each AED (table 4).
Table 3.
Adjusteda mean IQs, 95% CIs, DCIs, and p valuesb for comparisons to valproate, and sample sizes for each AED at age 4.5 years for ITT (n = 310) and completer (n = 209) analyses for each antiepileptic drug
Abbreviations: AED = antiepileptic drug; CI = confidence interval; DCI = difference confidence interval; ITT = intent-to-treat; NA = not applicable.
Mean age 4.5 years IQ scores adjusted for maternal IQ, maternal education, maternal age, dose, race/ethnicity, and alcohol use during pregnancy. ITT analysis includes imputed IQ data. Unadjusted IQ means for the ITT analysis were carbamazepine = 106, lamotrigine = 108, phenytoin = 101, valproate = 95. Unadjusted means for the completer analysis were carbamazepine = 107, lamotrigine = 109, phenytoin = 102, valproate = 95.
p Values adjusted for 3 pairwise comparisons to valproate using Dunnett correction.
Figure 1. Scatterplots of age 4.5 IQ vs standardized dose for each antiepileptic drug during pregnancy.
.
Table 4.
Verbal and nonverbal cluster means and their mean differences at age 4.5 years across AEDsa
Abbreviations: AED = antiepileptic drug; CI = confidence interval.
Mean verbal and nonverbal cluster scores, and their mean differences using a linear model controlling for maternal IQ, dose, age of mother, gestational age, alcohol use, race/ethnicity, and years of maternal education.
Difference of nonverbal cluster minus verbal cluster.
p Values for paired t tests.
Age 4.5 IQ correlated with age 2 BSID (r = 0.66, p < 0.0001) and age 3 DAS IQ (r = 0.77, p < 0.0001). Adjusted mean cognitive scores (BSID and DAS IQ) and % marked intellectual impairment (<70 standard score) are in table e-1 for each AED across ages 2, 3, and 4.5 years for all children tested at each age and for those tested at all 3 ages. Overall, mean IQ scores improved (p < 0.0001) and % marked intellectual impairment decreased (p = 0.0037) as children became older. From age 3 to 4.5, IQ improved for carbamazepine (p = 0.008), lamotrigine (p < 0.0001), and phenytoin (p = 0.002), but not valproate (p = 0.57). At age 4.5, 10% of children exposed to valproate had marked intellectual impairment compared to 0%–4% for other AEDs (p = 0.0064); this AED difference was also present in the subset of children tested at all 3 time points (p = 0.0007).
Propensity score analysis demonstrates that the results are not due to differences in baseline variables related to either the child IQ outcome or chances of belonging to the valproate group. The forest plot displaying means by seizure type and AED group shows that AED group differences cannot be explained by imbalances in underlying seizure type (figure e-2). A subanalysis of patients with juvenile myoclonic epilepsy did not alter the results. The analysis examining sensitivity of results to missing data demonstrated that the results cannot be explained by incomplete data.
DISCUSSION
The present age 4.5 year results are consistent with our prior findings,3 and demonstrate reduced cognitive abilities in children exposed in utero to valproate monotherapy. Similar to our age 3 year findings,3 valproate dose was associated with lower child IQ at age 4.5, but the dose of the other AEDs was not. In addition, maternal IQ was associated with child IQ at age 4.5 for each AED except valproate, similar to our age 3 finding.3 Our findings are consistent with other studies reporting impaired cognition in children with fetal valproate exposure.17–22
The present results add confidence to our prior results, and also add new findings. Since age 4.5 outcomes are related significantly to age 2 and 3 outcomes, impairments may be detected early facilitating appropriate intervention programs. Cognitive improvements occurred as children aged. It is possible that this improvement could be due at least in part to practice effects. However, a different test was use at age 2 vs 3 and 4.5. Further, improvement did not appear to occur in valproate exposed children, in which 10% of the children had marked intellectual impairment range (i.e., <70 IQ) at age 4.5. If practice effects are occurring, they do not appear to be present in the valproate group.
Higher maternal IQ was associated with higher child IQ overall and for carbamazepine, lamotrigine, and phenytoin individually, but not for valproate. Greater maternal education and age were both associated with higher child IQ; lower child IQ was seen in very young mothers. Lower gestational age and maternal alcohol use were both associated with lower child IQ. Child IQ was higher in Caucasians. Similar results were found in our completer analysis.
The prospective design, blinded cognitive assessments using standardized measures, and detailed monitoring of multiple potential confounding factors are strengths of our study. Limitations include a relatively small non-population-based sample, loss of enrolled subjects to analysis, lack of randomization, lack of AED blood levels, and absence of an unexposed control group during pregnancy. Another weakness of our study is that different measures were used to estimate maternal IQ. However, the maternal IQ measures used in our study correlate well with a standard adult IQ measure (i.e., full-scale IQ of the Wechsler Adult Intelligence Scale).11–13 Further, regression analyses controlling for type of maternal IQ measure and analysis of the completer sample limited to mothers receiving the TONI both produce similar results. A potential confounding issue for observational studies is that differences in baseline characteristics might affect child IQ and alter results. However, the observed associations persist in analyses adjusted for baseline characteristics, including propensity scores and seizure type.
We previously reported that all 4 of these commonly used AEDs impair verbal abilities compared to nonverbal abilities at age 3.23 Here, we find that this verbal deficit persists for all 4 AEDs at age 4.5 years. The verbal and nonverbal measures are expressed as standard scores, so these scores should be equal based on normed data in healthy children. Performance IQ is more susceptible to practice effects than verbal IQ.24,25 Although practice effects could affect the cognitive outcomes, it is unlikely that such an effect is a major factor in our findings. The 30-day practice effects on the DAS for the verbal and nonverbal cluster scores are modest,9 and our test–retest interval was 1.5 years. Further, practice effect does not explain the verbal/nonverbal split at age 3 on the first exposure to DAS, nor the fact that the split does not widen across AEDs between age 3 and 4.5. In addition, several studies have found verbal impairments in children exposed to valproate,17,18,22 and one other study reported that language functions were impaired in children with antenatal AED exposure.26 Nevertheless, we caution that this finding requires replication in separate cohorts to determine if language abilities are particularly susceptible to fetal AED exposure. If this verbal impairment from fetal AED exposure is confirmed, it has important practical and theoretical implications.
Alcohol can induce widespread neuronal apoptosis in the immature brain,27 raising concern that AEDs might produce similar effects. Some AEDs induce neuronal apoptosis in the immature brain when given in monotherapy, and many AEDs can enhance apoptosis in polytherapy.28–33 The effect is dose dependent, occurs at therapeutically relevant blood levels, and requires only relatively brief exposure. Valproate appears to induce the apoptosis at dosages relatively lower in terms of effective therapeutic dose compared to other AEDs.28,29 However, many AEDs have not been tested in this model. In regards to the induced cognitive deficits, the neuronal apoptosis likely only contributes in part, and the more important factor may be impaired physiology in the surviving neurons, similar to alcohol.34 Although it is possible that widespread neuronal apoptosis or dysfunction might differentially affect verbal abilities, we have hypothesized that the greater verbal impairment might result from altered cerebral lateralization from fetal AED exposure.23 Additional research is needed to test this hypothesis and fully delineate the mechanisms of behavioral teratogenesis.
The treatment of epilepsy in women of childbearing potential requires balancing risks that seizures pose to the mother and child vs risks of teratogenesis (i.e., congenital malformations and cognitive deficits) in the child. A discussion of these risks should occur prior to pregnancy in all women of childbearing potential treated with AEDs. Based on the current literature, valproate poses the most consistent, frequent, and severe risks for both anatomic and behavioral teratogenesis.3,6 We contend that valproate is a poor first choice AED for most women of childbearing potential. Some women with generalized epilepsy can only be controlled by valproate.35 However, if other AEDs are ineffective in seizure control, then valproate can be used subsequently. When valproate is necessary, we recommend employing the lowest dose possible. Recent animal data suggest that peak drug levels may be an important risk factor for damage to the immature brain,36 but there are inadequate human data to determine if multiple daily doses or extended release formulations would reduce risk.
Our present knowledge remains inadequate on many issues to direct care of women with epilepsy who might become pregnant.6,37,38 If teratogenetic issues alone are considered, fetal risks are not fully delineated for most AEDs in regards to malformations or cognitive outcomes. Investigations of both anatomic and behavioral teratogenesis in humans are based on observational studies. Thus, a signal needs to be observed in multiple cohorts, as is the case for valproate. The recent EURAP results show dose-dependent malformation risks for not only valproate, but also carbamazepine, lamotrigine, and phenobarbital.39 Teratogens act in a dose-dependent manner, and delineation of dose effects for both anatomic and functional deficits in other AEDs may require larger sample sizes than valproate. Further, the relative risks of specific polytherapy combinations are unknown. Understanding these effects is critical to management of women with epilepsy who are of childbearing potential.
The risk for malformations is primarily due to exposure early in the first trimester, but the risk for cognitive deficits appears to be in the third trimester, similar to alcohol. While dose might be a reasonable surrogate for AED exposure early in pregnancy, it is not late in pregnancy due to considerable variability in AED clearance during pregnancy across AEDs and across individual patients.40 To date, no study of outcomes in children of women with epilepsy have controlled for this factor by assessing AED blood levels, which would be a much better measure of intrauterine AED exposure. Further, it is critical that our observation of language deficits in 4 commonly used AEDs be examined in other cohorts and in additional AEDs. Given the evolving nature of our evidence base, clinicians and patients should remain attentive to future information and its implications.
Supplementary Material
ACKNOWLEDGMENT
The investigators thank the children and families who gave their time to participate in the NEAD Study.
GLOSSARY
- AED
antiepileptic drug
- BSID
Bayley Scales of Infant Development
- CI
confidence interval
- DAS
Differential Ability Scales
- NART
National Adult Reading Test
- NEAD
Neurodevelopmental Effects of Antiepileptic Drugs
- SES
socioeconomic status
- TONI-3
Test of Nonverbal Intelligence–third edition
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Supplemental data at www.neurology.org
Contributor Information
David Labiner, Arizona Health Sciences Center, Tucson, AZ, site PI.
Jennifer Moon, Arizona Health Sciences Center, Tucson, AZ, co-I.
Scott Sherman, Arizona Health Sciences Center, Tucson, AZ, co-I.
Deborah T. Combs Cantrell, Baylor Medical Center, Irving Texas, site PI.
Cheryl Silver, University of Texas-Southwestern, Dallas TX, co-I.
Monisha Goyal, Case Western Reserve University, Cleveland, OH, site PI.
Mike R. Schoenberg, Case Western Reserve University, Cleveland, OH, Co-I.
Alison Pack, Columbia University, New York City, NY, site PI.
Christina Palmese, Columbia University, New York City, NY, Co-I.
Joyce Echo, Columbia University, New York City, NY, Co-I.
Kimford J. Meador, Emory University, Atlanta, GA, study PI.
David Loring, Emory University, Atlanta, GA, Co-Director Neuropsychology Core.
Page Pennell, Emory University, Atlanta, GA, Co-I.
Daniel Drane, Emory University, Atlanta, GA, Co-I.
Eugene Moore, Emory University, Atlanta, GA, Co-I.
Megan Denham, Emory University, Atlanta, GA, Co-I.
Charles Epstein, Emory University, Atlanta, GA, Co-I.
Jennifer Gess, Emory University, Atlanta, GA, Co-I.
Sandra Helmers, Emory University, Atlanta, GA, Co-I.
Thomas Henry, Emory University, Atlanta, GA, Co-I.
Gholam Motamedi, Georgetown University, Washington, DC, Co-I.
Erin Flax, Georgetown University, Washington, DC, Co-I.
Edward Bromfield, Harvard- Brigham & Women's, Boston, MA, site PI.
Katrina Boyer, Harvard- Brigham & Women's, Boston, MA, Co-I.
Barbara Dworetzky, Harvard- Brigham & Women's, Boston, MA, Co-I.
Andrew Cole, Harvard - Massachusetts General, Boston, MA, site PI.
Lucila Halperin, Harvard - Massachusetts General, Boston, MA, Co-I.
Sara Shavel-Jessop, Harvard - Massachusetts General, Boston, MA, Co-I.
Gregory Barkley, Henry Ford Hospital, Detroit, MI, site PI.
Barbara Moir, Henry Ford Hospital, Detroit, MI, Co-I.
Cynthia Harden, Medical College of Cornell University, New York City, NY, site PI.
Tara Tamny-Young, Medical College of Cornell University, New York City, NY, Co-I.
Gregory Lee, Medical College of Georgia, Augusta, GA, site PI.
Morris Cohen, Medical College of Georgia, Augusta, GA, Co-director Neuropsychology Core.
Patricia Penovich, Minnesota Epilepsy Group, St. Paul, MN, site PI.
Donna Minter, Minnesota Epilepsy Group, St. Paul, MN, Co-I.
Layne Moore, Ohio State University, Columbus, OH, site PI.
Kathryn Murdock, Ohio State University, Columbus, OH, Co-.
Joyce Liporace, Riddle Health Care, Media, PA, site PI.
Kathryn Wilcox, Riddle Health Care, Media, PA, Co-I.
Andres Kanner, Rush University Medical Center, Chicago, IL, site PI.
Michael N. Nelson, Rush University Medical Center, Chicago, IL, Co-I.
William Rosenfeld, The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO, site PI.
Michelle Meyer, The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO, Co-I.
Jill Clayton-Smith, St. Mary's Hospital, Manchester, UK, site PI.
George Mawer, St. Mary's Hospital, Manchester, UK, Co-I.
Usha Kini, St. Mary's Hospital, Manchester, UK, Co-I.
Roy Martin, University Alabama, Birmingham, AL, site PI.
Michael Privitera, University of Cincinnati, Cincinnati, OH, site PI.
Jennifer Bellman, University of Cincinnati, Cincinnati, OH, Co-I.
David Ficker, University of Cincinnati, Cincinnati, OH, Co-I.
Lyle Baade, University of Kansas School of Medicine - Wichita, Wichita, KA.
Kore Liow, University of Kansas School of Medicine - Wichita, Wichita, KA.
Gus Baker, University of Liverpool, Liverpool, UK, site PI.
Alison Booth, University of Liverpool, Liverpool, UK, Co-I.
Rebecca Bromley, University of Liverpool, Liverpool, UK, Co-I.
Miranda Casswell, University of Liverpool, Liverpool, UK, Co-I.
Claire Barrie, University of Liverpool, Liverpool, UK, Co-I.
Eugene Ramsay, University of Miami, Miami, FL, site PI.
Patricia Arena, University of Miami, Miami, FL, Co-I.
Laura Kalayjian, University of Southern California, Los Angeles, CA, site PI.
Christianne Heck, University of Southern California, Los Angeles, CA, Co-I.
Sonia Padilla, University of Southern California, Los Angeles, CA, Co-I.
John Miller, University of Washington, Seattle, WA, site PI.
Gail Rosenbaum, University of Washington, Seattle, WA, Co-I.
Alan Wilensky, University of Washington, Seattle, WA, Co-I.
Tawnya Constantino, University of Utah, Salt Lake City, UT, site PI.
Julien Smith, University of Utah, Salt Lake City, UT, Co-I.
Naghme Adab, Walton Centre for Neurology & Neurosurgery, Liverpool, UK, Co-I.
Gisela Veling-Warnke, Walton Centre for Neurology & Neurosurgery, Liverpool, UK, Co-I.
Maria Sam, Wake Forest University, Winston-Salem, NC, site PI.
Cormac O'Donovan, Wake Forest University, Winston-Salem, NC, Co-I.
Cecile Naylor, Wake Forest University, Winston-Salem, NC, Co-I.
Shelli Nobles, Wake Forest University, Winston-Salem, NC, Co-I.
Cesar Santos, Wake Forest University, Winston-Salem, NC, Co-I.
Gregory L. Holmes, Dartmouth Medical School, Hanover, NH, member Executive Committee.
Maurice Druzin, Stanford University, Stanford, CA, member Executive Committee.
Martha Morrell, Stanford University, Stanford, CA, member Executive Committee.
Lorene Nelson, Stanford University, Stanford, CA, member Executive Committee.
Richard Finnell, Texas A & M University Health Science Center, Houston, TX, member Executive Committee.
Mark Yerby, University of Oregon, Portland, OR, member Executive Committee.
Khosrow Adeli, University of Toronto, Toronto, Ontario, member Executive Committee.
Peter Wells, University of Toronto, Toronto, Ontario, member Executive Committee.
Nancy Browning, EMMES Corporation, Rockville, MD, Director Data & Statistical Center.
Temperance Blalock, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Todd Crawford, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Linda Hendrickson, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Bernadette Jolles, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Meghan Kelly Kunchai, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Hayley Loblein, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Yinka Ogunsola, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Steve Russell, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Jamie Winestone, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Mark Wolff, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Phyllis Zaia, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
Thad Zajdowicz, EMMES Corporation, Rockville, MD, Data & Statistical Center Co-I.
AUTHOR CONTRIBUTIONS
Dr. Meador drafted the manuscript and supervised the study. All other authors reviewed and revised the manuscript. Drs. Meador, Baker, Cohen, and Loring contributed to study design. Drs. Meador and Baker obtained funding. Dr. Browning conducted the statistical analyses and interpreted the data with Drs. Meador, Cohen, and Loring. All authors were involved in acquisition of data.
DISCLOSURE
Dr. Meador serves on the editorial boards of Neurology®, Behavior & Neurology, Epilepsy and Behavior, Epilepsy Currents, Epilepsy.com, and the Journal of Clinical Neurophysiology and on the Professional Advisory Board for the Epilepsy Foundation; has received travel support from sanofi-aventis; and received research support from GlaxoSmithKline, Eisai Inc., Marinus Pharmaceuticals, Inc., Myriad Genetics, Inc., NeuroPace, Inc., Pfizer Inc, SAM Technology Inc., Schwartz Pharma (UCB), the NIH/NINDS, and the Epilepsy Foundation. Dr. Baker has served on a scientific advisory board for sanofi-aventis; serves on the editorial board of Epilepsy and Behaviour; has received speaker honoraria from Eisai Inc.; has received educational support from UCB, sanofi-aventis, and Pfizer Inc; and receives research support from Epilepsy Research UK, Medical Research Council UK, and Epilepsy Action UK. Dr. Browning serves on a safety monitoring committee for Ligocyte Pharmaceuticals; receives research support from the NIH (NINDS, NIAID); and holds stock in Human Genome Sciences, Inc. Dr. Cohen serves on the editorial board of Developmental Neuropsychology; receives publishing royalties for Children's Memory Scale (Pearson, 1997); and receives research support from the NIH. Dr. Bromley has served on a scientific advisory board for sanofi-aventis; has received funding for travel from UCB; has served as an expert witness in a medico-legal case; and receives research support from UCB and sanofi-aventis. Dr. Clayton-Smith serves as Co-Editor for Clinical Dysmorphology and on the editorial board of European Journal of Human Genetics; has served as an expert witness in medico-legal cases; and receives research support from the NIH (NICDR), NHS Foundation Trust, and Action Medical. Dr. Kalayjian has received speaker honoraria from GlaxoSmithKline and Ortho-McNeil, and research support from Marinus Pharmaceuticals, Inc. and Medical College of Georgia. Dr. Kanner has served on scientific advisory boards or as a consultant for GlaxoSmithKline, Ortho McNeill, Pfizer Inc, Valeant Pharmaceuticals International, and UCB; has received speaker honoraria from GlaxoSmithKline, Ortho-McNeil, Pfizer Inc, and UCB; serves on the editorial boards of Epilepsy & Behavior, Epilepsia, and CNS Spectrums; receives publishing royalties for Psychiatric Issues in Epilepsy. Second Edition: A Practical Guide to Diagnosis and Treatment (Lippincott Williams & Wilkins, 2006) and Controversial Issues in Psychiatric Aspects of Epilepsy (Elsevier, 2008); has served on speakers' bureaus for GlaxoSmithKline, UCB, and Pfizer Inc; and has received research support from GlaxoSmithKline, Novartis, and Pfizer Inc. Dr. Liporace serves on the speakers' bureau for and has received speaker honoraria from UCB; receives publishing royalties for Crash Course Neurology (Elsevier, 2006); and serves on the Professional Board of Epilepsy Foundation of Eastern Pennsylvania. Dr. Pennell serves as a contributing editor to Epilepsy Currents and on the editorial board of Epilepsia; has received research support from the NIH, the Milken Family Foundation, the Epilepsy Foundation, UCB, and Marinus Pharmaceuticals; has received travel reimbursement from the NIH, the Milken Family Foundation, the Epilepsy Foundation, American Epilepsy Society, and the National EpiFellows Foundation; and serves as a volunteer member of the American Epilepsy Society Board of Directors and as chair of the Professional Advisory Board for the Epilepsy Foundation. Dr. Privitera has served on scientific advisory boards or as a consult for Ortho-McNeil-Janssen Pharmaceuticals, Inc., UCB, Johnson & Johnson, and the Epifellows Foundation; has received funding for travel and speaker honoraria from Ortho-McNeil-Janssen Pharmaceuticals, Inc., Pfizer Inc., GlaxoSmithKline, Janssen, and UCB; has served on speakers' bureaus for UCB, Pfizer Inc., GlaxoSmithKline, and Ortho-McNeil-Janssen Pharmaceuticals, Inc.; and has received research support from UCB, Ortho-McNeil-Janssen Pharmaceuticals, Inc., the NIH (K01-DA020485 [co-I], K23 NS052468 [co-Mentor], 1K23NS02170–01 [co-I], 2R01-NS38455 [site PI]), the American Epilepsy Society, and the Shor Foundation for Epilepsy Research. Dr. Loring serves on scientific advisory boards for the Epilepsy Foundation; serves on the Steering Committee for the NINDS Common Data Element Project, as Consulting Editor for the Journal of Clinical and Experimental Neuropsychology and for Epilepsy Research, and on the editorial boards of Epilepsia, Journal of Pediatric Epilepsy, and Neuropsychology Review; serves as a consultant for NeuroPace, Inc. and UCB; receives publishing royalties for Neuropsychological Assessment, 4th ed. (Oxford University Press, 2004) and INS Dictionary of Neuropsychology (Oxford University Press, 1999); estimates that 50% of his clinical effort involves neuropsychological testing including Wada testing; receives research support from the NIH/ NINDS, Epilepsy Foundation, and UCB; and receives travel reimbursement from the NIH.
REFERENCES
- 1. Fisher JE, Vorhees CV. Developmental toxicity of antiepileptic drugs: relationship to postnatal dysfunction. Pharmacol Res 1992;26:207–221 [DOI] [PubMed] [Google Scholar]
- 2. Gaily E, Meador KJ. Neurodevelopmental effects. In: Engel J, Pedley TA. eds. Epilepsy: A Comprehensive Textbook, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007: 1225–1233 [Google Scholar]
- 3. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA Drug Safety Communication. Children born to mothers who took Valproate products while pregnant may have impaired cognitive development (6/30/2011). [Accessed March 1, 2012.]. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm261543.htm.
- 5. Kluger BM, Meador KJ. Teratogenicity of antiepileptic medications. Semin Neurol 2008;28:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harden CL, Meador KJ, Pennell PB, et al. Practice Parameter update: management issues for women with epilepsy: focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sattler JM. Assessment of Children, 3rd ed. San Diego: Jerome M. Sattler; 1992 [Google Scholar]
- 8. Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975 [Google Scholar]
- 9. Elliott CD. Differential Abilities Scales, 2nd ed. San Antonio, TX: The Psychological Corporation; 1990 [Google Scholar]
- 10. Bayley N. Bayley Scales of Infant Development, 2nd ed. San Antonio, TX: The Psychological Corporation; 1993 [Google Scholar]
- 11. Brown L, Sherbenou RJ, Johnsen SK. Test of Non-verbal Intelligence (TONI-3), 3rd ed. Austin, TX: Pro-Ed; 1996 [Google Scholar]
- 12. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX,: Psychological Corporation; 1999 [Google Scholar]
- 13. Nelson H, Willison J. National Adult Reading Test (NART). Oxford, UK: NFER-Nelson Publishing; 1991 [Google Scholar]
- 14. Li KH. Imputation using Markov chains. J Stat Comput Simul 1988;30:57–79 [Google Scholar]
- 15. Little RJA, Rubin DB. Statistical Analysis with Missing Data, 2nd ed. New York: John Wiley & Sons, Inc.; 2002 [Google Scholar]
- 16. Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55 [Google Scholar]
- 17. Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 2004;75:1575–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology 2004;62:28–32 [DOI] [PubMed] [Google Scholar]
- 19. Thomas SV, Ajaykumar B, Sindhu K, Nair MK, George A, Sarma PS. Motor and mental development of infants exposed to antiepileptic drugs in utero. Epilepsy Behav 2008;13:229–236 [DOI] [PubMed] [Google Scholar]
- 20. Bromley RL, Mawer G, Love J, et al. Liverpool and Manchester Neurodevelopment Group [LMNDG]. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia 2010;51:2058–2065 [DOI] [PubMed] [Google Scholar]
- 21. Shallcross R, Bromley RL, Irwin B, Bonnett LJ, Morrow J, Baker GA. Liverpool Manchester Neurodevelopment Group, UK Epilepsy and Pregnancy Register. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 2011;76:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadebaum C, Anderson V, Vajda F, Reutens D, Barton S, Wood A. The Australian brain and cognition and antiepileptic drugs study: IQ in school-aged children exposed to sodium valproate and polytherapy. J Int Neuropsychol Soc 2011;17:133–142 [DOI] [PubMed] [Google Scholar]
- 23. Meador KJ, Baker GA, Browning N, et al. NEAD Study Group Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain 2011;134:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sawrie SM, Chelune GJ, Naugle RI, Luders HO. Empirical methods for assessing meaningful neuropsychological change following epilepsy surgery. JINS 1996;2:556–564 [DOI] [PubMed] [Google Scholar]
- 25. Hermann BP, Seidenberg M, Schoenfeld J, Peterson J, Leveroni C, Wyler AR. Empirical techniques for determining the reliability, magnitude, and pattern of neuropsychological change after epilepsy surgery. Epilepsia 1996;37:942–950 [DOI] [PubMed] [Google Scholar]
- 26. Thomas SV, Sukumaran S, Lukose N, George A, Sarma PS. Intellectual and language functions in children of mothers with epilepsy. Epilepsia 2007;48:2234–2240 [DOI] [PubMed] [Google Scholar]
- 27. Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 2000;287:1056–1060 [DOI] [PubMed] [Google Scholar]
- 28. Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA 2002;99:15089–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann NY Acad Sci 2003;993:103–114 [DOI] [PubMed] [Google Scholar]
- 30. Glier C, Dzietko M, Bittigau P, Jarosz B, Korobowicz E, Ikonomidou C. Therapeutic doses of topiramate are not toxic to the developing rat brain. Exp Neurol 2004;187:403–409 [DOI] [PubMed] [Google Scholar]
- 31. Manthey D, Asimiadou S, Stefovska V, et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol 2005;193:497–503 [DOI] [PubMed] [Google Scholar]
- 32. Katz I, Kim J, Gale K, Kondratyev A. Effects of lamotrigine alone and in combination with MK-801, phenobarbital, or phenytoin on cell death in the neonatal rat brain. J Pharmacol Exp Ther 2007;322:494–500 [DOI] [PubMed] [Google Scholar]
- 33. Kim JS, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia 2007;48 (suppl 5): 19–26 [DOI] [PubMed] [Google Scholar]
- 34. Medina AE, Krahe TE, Coppola DM, Ramoa AS. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci 2003;23:10002–10012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 2007;369:1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondratyev A, Gale K, Tsukerman S.Phenobarbital-induced apoptosis in the neonatal rat brain occurs in a limited compartment of neurons: American Epilepsy Society 2010. abstract 2.156. [Accessed March 1, 2012]. Available at: http://www.aesnet.org/go/publications/aes-abstracts/abstract-search/mode/display/st/gale/sy/2010/sb/All/id/12750.
- 37. Harden CL, Hopp J, Ting TY, et al. Practice Parameter update: management issues for women with epilepsy: focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:126–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harden CL, Pennell PB, Koppel BS, et al. Practice Parameter update: Management issues for women with epilepsy: focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tomson T, Battino D, Bonizzoni E, et al. for the EURAP study group. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609–617 [DOI] [PubMed] [Google Scholar]
- 40. Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol 2008;83:227–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.