Abstract
Purpose of review
This review assesses the recent progress in xenograft rejection by innate immune responses, with a focus on innate cellular xenoreactivity.
Recent findings
Current literature was reviewed for new insights into the role of innate cellular immunity in xenograft rejection. Increasing evidence confirms that vigorous innate immune cell activation is accounted for by a combination of xenoantigen recognition by activating receptors, and incompatibility in inhibitory receptor-ligand interactions. Although both innate humoral and cellular xenoimmune responses are predominantly elicited by preformed and induced xenoreactive antibodies in nonhuman primates following porcine xenotransplantation, innate immune cells can also be activated by xenografts in the absence of antibodies. The latter antibody-independent response will likely persist in recipients even when adaptive xenoimmune responses are suppressed. In addition to xenograft rejection by recipient innate immune cells, phagocytic cells within liver xenografts are also deleterious to recipients by causing thrombocytopenia.
Summary
Strategies of overcoming innate immune responses are required for successful clinical xenotransplantation. In addition to developing better immunosuppressive and tolerance induction protocols, endeavors towards further genetic modifications of porcine source animals are ultimately important for successful clinical xenotransplantation.
Keywords: Innate immunity, macrophages, NK cells, xenotransplantation
Introduction
Transplants across discordant species barriers are subject to vigorous immunologic rejection, which poses a major hurdle to successful xenotransplantation. Due to extensive molecular incompatibilities between the donor and host, innate immune responses play a much greater role in the rejection of xenografts than in allograft rejection [1]. The innate immune response is also involved in the rejection of α1,3-galactosyltransferase gene-knockout (GalT-KO) porcine organ xenografts [2]. The innate immune system is composed mainly of phagocytic cells (monocytes/macrophages and neutrophils), natural killer (NK) cells, cells producing inflammatory mediators (basophils, eosinophils, and mast cells), and complement proteins. Innate immune cell activation is triggered by the recognition of pathogen-associated molecular patterns (PAMPs), and is downregulated by the recognition of ‘self’ molecules. In xenotransplantation, species differences in glycosylation patterns and in receptor-ligand intersections could result in recognition of the graft by host innate immune cells, and in failure to downregulate the activation of such cells. There are examples of both phenomena in the xenotransplantation literature. This review summarizes the most recent insights into the role of innate immune cells in xenotransplantation.
NK cell xenoreactivity
On balance, many xenogeneic NK-cell–target-cell interactions appear to be activating interactions rather than inhibitory ones, resulting in higher levels of reactivity. Previous studies have shown that human NK cells can be activated via recognition of porcine glycosylphosphatidylinositol-anchored proteins and carbohydrate epitopes [3;4], and by insufficient self-MHC-mediated inhibitory signals due to the extensive disparity in MHC molecules [5;6]. Thus, it is likely that a combination of approaches, to block activating receptor signals and enhance inhibitory receptor signaling, is required for suppressing NK xenoreactivity. There is emerging evidence indicating that, although expression of human HLA class I molecules on porcine cells could mediate protection against human NK cytotoxicity, this approach has not been able to completely protect porcine cells from lysis by polyclonal human NK cells [7;8]. A recent study tested a broad array of NK receptors including NKp46, 2B4, CD49d, CD48, CD2, and NKG2D, and found that only CD2 and NKG2D were involved in both cytotoxicity and cytokine production against porcine targets [9]. Simultaneous blocking of CD2 and NKG2D significantly suppressed xenogeneic NK cell responses. Furthermore, addition of an extracellular signal-regulated kinase (ERK) inhibitor further reduced NK cell xenoresponses, implicating an important role for ERK in NK xenoreactivity [9]. Considering the fact that NK cells are comprised of heterogeneous cell populations that express different panels of activating and inhibitory receptors, optimal protection would likely require simultaneously targeting multiple receptors.
Human anti-pig NK cell xenoreactivity has thus far only been evaluated by in vitro assays. A recent study provided detailed analysis of baboon NK cells [10]. NK cells in baboons are IL-2 responsive and exhibit a CD3−NKp46+CD8dimCD16+/− or CD3−CD8dimCD16bright phenotype. These results will help to more precisely identify NK cells in baboons, and to better use baboons as a preclinical model for studying the role of NK cells in porcine xenograft rejection.
CD47 incompatibility and macrophage xenoresponses
Macrophages mediate robust rejection of donor hematopoietic cells in highly disparate xenogeneic settings [11;12], and such powerful xenoreactivity results from the combined effect of xenogeneic receptors in activating macrophages [13;14], and ineffective inhibitory receptor signaling (e.g., CD47-SIRPα signaling; see discussion below) [15;16]. CD47 is a pentaspan membrane glycoprotein expressed ubiquitously in all tissues [17]. Previous studies have shown that CD47 serves as a ‘marker of self’ for macrophages, and that its interaction with the inhibitory receptor, SIRPα (signal regulatory protein α), on macrophages prevents engulfment of autologous hematopoietic cells [18–20]. The lack of interaction between donor CD47 and recipient SIRPα was found to induce rapid rejection of xenogeneic hematopoietic cells [15;16], which poses a strong barrier to tolerance induction via bone marrow chimerism that has been successfully applied to small and large allogeneic models [1].
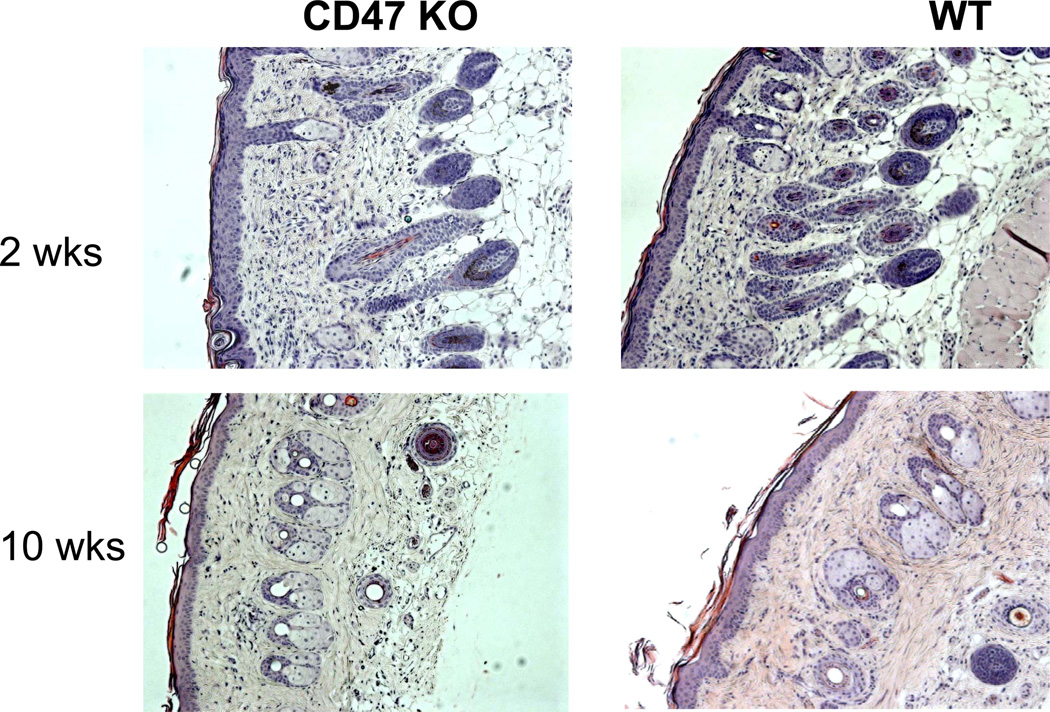
However, recent studies indicate that the CD47-SIRPα pathway may play a different role in controlling macrophage responses to non-hematopoietic tissues or cells. When fetal thymus from CD47-deficient mice was transplanted into syngeneic CD47-competent mice, CD47-deficient thymic epithelial cells survived and supported thymopoiesis and T cell development, while CD47-deficient thymocytes within the graft were rejected [21]. Lack of CD47 expression also did not result in rejection of skin (Figure 1) or heart (Wang Y and Yang YG, unpublished data) grafts in syngeneic mice. These results suggest that CD47 as a ‘marker of self’ for macrophages may not apply to all types of cells, and non-hematopoietic cells may not need CD47 to prevent phagocytosis by macrophages. Alternatively, long-term survival of CD47-deficient grafts in these studies could be due to a less important role of CD47 in controlling macrophage activation in organ grafts. In the latter case, CD47 expression may still play an important role in controlling macrophage activation after non-hematopoietic cellular xenotransplantation.
Figure 1. CD47-deficient skin grafts survived long-term with no sign of rejection at histology in syngeneic CD47-competent mice.
Wild-type (WT) B6 mice were grafted with CD47 KO and WT (control) B6 skin. Shown are representative histological sections (H&E) of skin grafts at weeks 2 and 10 post-transplantation.
Hepatocyte xenotransplantation is considered a potential therapy for liver diseases. Hepatocyte transplantation obviates the need to remove the native liver, and to a certain degree, the latter might offset incompatibilities in liver-produced proteins between pigs and humans. We have recently assessed the role of CD47 expression in a mouse model of hepatocyte transplantation. Intrasplenic transplantation of CD47-deficient, but not CD47-expressing, hepatocytes led to rapid activation and recruitment of monocytes/macrophages, which was associated with poor graft survival [22]. These results provide the first evidence that lack of CD47 expression on non-hematopoietic cells may also induce macrophage activation, implicating the potential contribution of CD47 incompatibility in macrophage-mediated rejection of xenogeneic hepatocytes. Since innate immune activation plays a crucial role in priming of adaptive immune responses [23;24], activation of innate immune cells following CD47-deficient cell transplants may also augment the subsequent T cell response to donor antigens. In support of this possibility, injection of CD47-deficient cells was found to stimulate recipient dendritic cell (DC) activation and promote anti-donor T cell alloresponses [25].
Although previous attempts at clinical islet xenotransplantation were unsuccessful [26], encouraging results in preclinical diabetic models [27;28] and in humans [29] suggest that pancreatic islet xenotransplantation from pigs has potential to be the first successful clinical application of xenotransplantation (reviewed in [30]). A recent study showed that CD47 interspecies incompatibility contributes to xenogeneic insulinoma rejection by macrophages, in which transgenic expression of mouse CD47 improved rat insulinoma cell survival in T and B cell-deficient mice [31]. This study suggests that the inability of porcine CD47 to functionally interact with human SIRPα may present an additional barrier to porcine islet xenotransplantation in humans.
Taken together, these studies indicate that the lack of cross-species inhibitory interaction in the CD47-SIRPα pathway largely accounts for the vigorous rejection of cellular xenografts by triggering macrophage and DC activation. Since chronic depletion of recipient macrophages is not clinically practical, researchers are currently seeking to develop transgenic pigs expressing human CD47. A recent report showed that codominant expression of human CD47 gene in the α1,3GalT locus does not appear to have deleterious effects on fetal development, suggesting the feasibility of developing α1,3GalT KO pigs with transgenic expression of human CD47 [32].
Macrophages in the rejection of xenogeneic red blood cells
Blood transfusion from animals has been considered a potential solution to severe shortage of red blood cells (RBCs) for clinical transfusion [33;34]. Although some advantages for using bovine over porcine RBCs were previously reported [33], the latter species will likely offer a greater chance for clinical use considering the continuous efforts in making genetically manipulated or humanized pigs [35–37]. Besides sharing many similarities with human RBCs, porcine RBCs are considered less immunogenic than other cells due to the lack of MHC antigens, and free of risk for transmission of porcine endogenous retroviruses due to the lack of nuclei [34]. However, it should be emphasized that RBCs are much more vulnerable than other types of grafts to destruction by immune attack. Porcine RBCs are subject to immediate rejection after transfusion into non-human primates and only limited progress has been made in understanding the cause of rejection. Most early studies have been focused on antibody-mediated destruction/rejection of xenogeneic RBCs, which involve complement-mediated cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC). A recent study showed that RBCs from α1,3GalT KO pigs are significantly less susceptible than those from wild-type pigs to antibody-dependent complement-mediated cytotoxicity and ADCC by human macrophages [38], indicating that α1,3Gal is an important antigen causing destruction of porcine RBCs. However, depletion of α1,3Gal is not enough to overcome rejection, and α1,3GalT KO porcine RBCs were still rapidly and vigorously rejected after infusion into baboons [39]. The ability of human macrophages to phagocytose porcine RBCs in the absence of anti-pig antibodies was controversial in previous studies [38;40]. A recent study indicated that efficient phagocytosis of porcine RBCs by human macrophages was only detected in the presence of anti-pig antibodies [38], while human macrophages were found in an earlier report to spontaneously phagocytose porcine RBCs via an antibody-/complement-independent mechanism [40]. However, further studies are needed to determine the contribution of antibody-dependent versus -independent mechanisms to porcine RBC rejection in xenogeneic recipients. Although it has been impossible to directly assess the human innate immune response to porcine RBCs in vivo, some insights were provided by studies in other species combinations.
Recent studies using immunodeficient mice demonstrated rapid rejection of xenogeneic human RBCs by recipient macrophages in the absence of xenoreactive antibodies [41]. Human RBCs survived in macrophage-depleted, but were rapidly rejected in control, T and B cell-deficient NOD/SCID mice. Although CD47-SIRPα incompatibility has been shown to induce vigorous phagocytosis of RBCs [18], this pathway is unlikely to contribute to the rejection of human RBCs observed in NOD/SCID mice, as human CD47 has been shown to cross-react with NOD mouse SIRPα [42]. This possibility was further supported by the observation that NOD/SCID mice rejected human RBCs more rapidly than CD47-deficient mouse RBCs [41]. These studies indicate that xenogeneic RBCs may also activate macrophages via mechanisms other than failed CD47-SIRPα interactions. Thus, macrophages pose a strong barrier to the use of xenogeneic RBCs for blood transfusions in humans.
Thrombocytopenia after liver xenotransplantation: a role of donor phagocytic cells
Liver is a major metabolic organ, and produces the majority of blood proteins, including enzymes, complement and coagulate proteins. While short-term porcine liver perfusion studies have documented the ability of a pig liver to restore coagulation and clear ammonium from human plasma [43;44], the question remains as to whether porcine liver can fully restore human liver function in the long term. Nonetheless, it has been suggested that porcine liver xenotransplantation or extra-corporeal porcine liver perfusion might most appropriately be evaluated as a bridge to allotransplantation in patients suffering from acute, fulminant hepatic failure, for whom an allogeneic donor is not available [45]. However, extra-corporeal porcine liver perfusion has been shown to cause thrombocytopenia in patients with hepatic failure [43]. A recent study demonstrated that, although hyperacute rejection, which was observed after wild-type porcine liver transplantation, was prevented, rapid and profound thrombocytopenia developed in baboons receiving orthotopic liver xenotransplantation from α1,3GalT KO/human CD46-transgenic pigs [46]. The failure of recipient splenectomy performed before liver graft reperfusion to attenuate thrombocytopenia raised the possibility that the liver xenograft was directly responsible for platelet loss [46]. Liver endothelial cells have been shown to not only directly phagocytose cells [47], but to also potentiate the phagocytic activity of macrophages [48;49]. Ex vivo porcine liver perfusion studies showed that liver sinusoidal endothelial cells (LSECs) and Kupffer cells are responsible for the loss of human platelets [50]. Freshly isolated LSEC-enriched cells showed strong phagocytic activity against human platelets, and blocking pig Fc receptors failed to inhibit phagocytosis [50]. The lack of contribution of antibody opsonization to phagocytosis of human platelets in this in vitro assay was presumably due to the absence or low levels of anti-human platelet xenoantibodies in the system, as antibody opsonization has been shown to strongly enhance phagocytosis in various settings. Nonetheless, these results indicate that LSECs and Kupffer cells may recognize xenogeneic platelets in the absence of opsonization. Previous studies have shown that lack of CD47-SIRPα signaling can lead to phagocytosis of hematopoietic cells and platelets [51]. In a recent report, porcine LSECs and Kupffer cells were found to express SIRPα that does not interact with human CD47, and transgenic expression of human SIRPα on porcine LSECs was capable of suppressing their phagocytic activity against human platelets [52]. These results indicate that transgenic expression of human SIRPα on porcine phagocytic cells may attenuate thrombocytopenia after liver xenotransplantation.
Fc receptor-independent phagocytosis of xenogeneic platelets was also seen in T and B cell-deficient mice on the NOD background (Hu Z and Yang YG, Unpublished data). Since human CD47 is capable of cross interacting with NOD mouse SIRPα [42], phagocytosis of human platelets by mouse macrophages in these mice indicated that phagocytosis of xenogeneic platelets can be induced in the absence of antibody opsonization by mechanisms other than failed CD47-SIRPα interaction. Recent studies showed that the asialoglycoprotein receptor (ASGR) [53] and CD18 [54] on porcine LSECs may lead to binding and phagocytosis of human platelets.
Conclusions
Due to the extensive molecular incompatibilities between the donor and host, innate cellular immune responses play a much greater role in the rejection of xenografts than in allograft rejection. Activated innate immune cells destroy xenografts not only by direct cytotoxicity, but also by augmenting subsequent T cell xenoresponses. Xenograft rejection by cells of the innate immune system involves multiple effector mechanisms, which cannot be prevented by a single approach. Our increased understanding of the mechanisms underlying xenoantigen recognition by innate immune cells has helped identify molecules to target for genetic manipulation in donor pigs to suppress innate xenoimmune responses. For example, transgenic expressions of HLA molecules and human CD47 were used to suppress xenograft rejection by NK cells and macrophages, respectively. It is expected that porcine source animals with multiple genetic manipulations will be needed for clinical xenotransplantation.
Key points.
Further in vivo studies in nonhuman primates is required for better understanding the role of NK cells in rejection of porcine cellular and organ xenografts.
Lack of cross-species interaction in the CD47-SIRPα inhibitory pathway triggers rejection of both hematopoietic and non-hematopoietic cellular xenografts by macrophages, but its role in organ xenograft rejection remains to be defined.
Intragraft Kupffer cells and sinusoidal endothelial cells play an important role in the development of rapid and profound thrombocytopenia in nonhuman primates after porcine liver transplantation or extra-corporeal porcine liver perfusion; overcoming this problem is crucial for clinical liver xenotransplantation.
Acknowledgements
The authors thank Dr. Waichi Wong for critical reading of the manuscript. We apologize to those investigators whose work could not be cited as a result of space limitations. The work from the authors’ laboratory discussed in this review was supported by grants from NIH (R01 AI064569, RC1 HL100117, PO1 CA111519, and PO1 AI045897), JDRF (1-2005-72), and AHA (SDG 0930361N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
Reference
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 2.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lilienfeld BG, Garcia-Borges C, Crew MD, Seebach JD. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J Immunol. 2006;177:2146–2152. doi: 10.4049/jimmunol.177.4.2146. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen D, Mouhtouris E, Milland J, et al. Recognition of a carbohydrate xenoepitope by human NKRP1A (CD161) Xenotransplantation. 2006;13:440–446. doi: 10.1111/j.1399-3089.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 5.Seebach JD, Comrack C, Germana S, et al. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunology. 1997;159:3655–3661. [PubMed] [Google Scholar]
- 6.Sharland A, Patel A, Lee JH, et al. Genetically modified HLA class I molecules able to inhibit human NK cells without provoking alloreactive CD8+ CTLs. J Immunol. 2002;168:3266–3274. doi: 10.4049/jimmunol.168.7.3266. [DOI] [PubMed] [Google Scholar]
- 7.Weiss EH, Lilienfeld BG, Mller S, et al. HLA-E/Human [beta]2-Microglobulin Transgenic Pigs: Protection Against Xenogeneic Human Anti-Pig Natural Killer Cell Cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 8.Forte P, Baumann BC, Schneider MKJ, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16:19–26. doi: 10.1111/j.1399-3089.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 9. Kim TJ, Kim N, Kim EO, et al. Suppression of human anti-porcine natural killer cell xenogeneic responses by combinations of monoclonal antibodies specific to CD2 and NKG2D and extracellular signal-regulated kinase kinase inhibitor. Immunology. 2010;130:545–555. doi: 10.1111/j.1365-2567.2010.03253.x. • This study shows that simultaneous blocking of CD2 and NKG2D is more effective than blocking of a single receptor in suppressing NK cell xenoresponses, indicating that the two receptors play distinct roles in porcine xenograft recognition by human NK cells.
- 10. Kennett SB, Porter CM, Horvath-Arcidiacono JA, Bloom ET. Characterization of baboon NK cells and their xenogeneic activity. Xenotransplantation. 2010;17:288–299. doi: 10.1111/j.1399-3089.2010.00591.x. • This study provides phenotypic and functional analysis of baboon NK cells.
- 11.Abe M, Cheng J, Qi J, et al. Elimination of porcine hemopoietic cells by macrophages in mice. J Immunol. 2002;168:621–628. doi: 10.4049/jimmunol.168.2.621. [DOI] [PubMed] [Google Scholar]
- 12.Basker M, Alwayn IP, Buhler L, et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation. 2001;72:1278–1285. doi: 10.1097/00007890-200110150-00017. [DOI] [PubMed] [Google Scholar]
- 13.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine kupffer cells. Transplantation. 2005;80:344–352. doi: 10.1097/01.tp.0000162974.94890.9f. [DOI] [PubMed] [Google Scholar]
- 14.Jin R, Greenwald A, Peterson MD, Waddell TK. Human monocytes recognize porcine endothelium via the interaction of galectin 3 and {alpha}-GAL. J Immunol. 2006;177:1289–1295. doi: 10.4049/jimmunol.177.2.1289. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, VerHalen J, Madariaga ML, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ide K, Wang H, Liu J, et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 18.Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 19.Blazar BR, Lindberg FP, Ingulli E, et al. CD47 (Integrin-associated Protein) Engagement of Dendritic Cell and Macrophage Counterreceptors Is Required to Prevent the Clearance of Donor Lymphohematopoietic Cells. J Exp Med. 2001;194:541–550. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Madariaga ML, Wang S, et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci U S A. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Wang H, Wang S, et al. Survival and function of CD47-deficient thymic grafts in mice. Xenotransplantation. 2010;17:160–165. doi: 10.1111/j.1399-3089.2010.00578.x. •• After transplantation of CD47 KO mouse fetal thymic tissue into CD47-competent mice, CD47-deficient thymic epithelial cells survived and supported thymopoiesis, suggesting that non-hematopoietic grafts may be less susceptible to rejection by macrophages, or that CD47-SIRPα signaling is not required for inhibiting macrophage activation in tissue grafts.
- 22. Navarro-Alvarez N, Yang YG. Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft rejection: a potential barrier to hepatocyte xenotransplantation. Am J Transplant. 2011;11(S2):107–108. doi: 10.3727/096368913X663604. • This study shows that CD47-SIRPα interaction plays a critical role in suppressing innate immune cell activation after hepatocyte transplantation.
- 23.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 24.Kratky W, Reis e Sousa C, Oxenius A, Sporri R. Direct activation of antigen-presenting cells is required for CD8+ T-cell priming and tumor vaccination. Proc Natl Acad Sci U S A. 2011;108:17414–17419. doi: 10.1073/pnas.1108945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Wu X, Wang Y, et al. CD47 is required for suppression of allograft rejection by donor-specific transfusion. J Immunol. 2010;184:3401–3407. doi: 10.4049/jimmunol.0901550. •• This study demonstrates that CD47 expression on donor cells is required for preventing DC activation following donor-specific transfusion, indicating that CD47-SIRPα incompatibility may also enhance adaptive xenoimmune responses by promoting DC activation.
- 26.Groth CG, Korsgren O, Tibell A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 27.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 28.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 29.Elliott RB, Escobar L, Tan PLJ, et al. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Hering B. Islet and cellular xenotransplants. Curr Opin Organ Transplant. 2012;17 In Press. [Google Scholar]
- 31. Teraoka Y, Ide K, Tahara H, et al. Genetic induction of mouse CD47 on rat insulinoma cells prevents macrophage-mediated xenograft rejection through CD47-SIRPα inhibitory signaling in mice. Am J Transplant. 2011;11(S2):107. • Mouse CD47 expression was found to inhibit rat insulinoma cell rejection by macrophages in mice, suggesting that CD47 interspecies incompatibility is likely to trigger islet xenograft rejection by macrophages.
- 32. Tena A, Turcotte N, Leto Barone AA, et al. Miniature swine expressing human CD47 to enhance bone marrow engraftment in non-human primates. Xenotransplantation. 2011;18:271. • This study shows the feasibility of developing human CD47-transgenic pigs.
- 33.Johnstone JE, MacLaren LA, Doucet J, McAlister VC. In vitro studies regarding the feasibility of bovine erythrocyte xenotransfusion. Xenotransplantation. 2004;11:11–17. doi: 10.1111/j.1399-3089.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 34.Cooper DKC. Porcine red blood cells as a source of blood transfusion in humans. Xenotransplantation. 2003;10:384–386. doi: 10.1034/j.1399-3089.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 35.Schuurman HJ, Pierson RN., III Progress towards clinical xenotransplantation. Front Biosci. 2008;13:204–220. doi: 10.2741/2671. [DOI] [PubMed] [Google Scholar]
- 36.Sachs DH, Galli C. Genetic manipulation in pigs. Curr Opin Organ Transplant. 2009:14. doi: 10.1097/mot.0b013e3283292549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gock H, Nottle M, Lew AM, et al. Genetic modification of pigs for solid organ xenotransplantation. Transplantation Reviews. 2011;25:9–20. doi: 10.1016/j.trre.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Long C, Hara H, Pawlikowski Z, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49:2418–2429. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 39.Rouhani FJ, Dor FJ, Cooper DK. Investigation of red blood cells from alpha1,3-galactosyltransferase-knockout pigs for human blood transfusion. Transfusion. 2004;44:1004–1012. doi: 10.1111/j.1537-2995.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 40.Ide K, Ohdan H, Kobayashi T, et al. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 41. Hu Z, van Rooijen N, Yang YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011 doi: 10.1182/blood-2010-11-321414. (In press). • This study shows that macrophages may mediate antibody-independent phagocytosis of xenogeneic RBCs via mechanisms other than failed CD47-SIRPα interactions.
- 42.Takenaka K, Prasolava TK, Wang JCY, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 43.Chari RS, Collins BH, Magee JC, et al. Treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med. 1994;331:234–237. doi: 10.1056/NEJM199407283310404. [DOI] [PubMed] [Google Scholar]
- 44.Makowka L, Cramer DV, Hoffman A, et al. The use of a pig liver xenograft for temporary support of a patient with fulminant hepatic failure. Transplantation. 1995;59:1654–1659. doi: 10.1097/00007890-199506270-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ekser B, Gridelli B, Veroux M, Cooper DKC. Clinical pig liver xenotransplantation: how far do we have to go? Xenotransplantation. 2011;18:158–167. doi: 10.1111/j.1399-3089.2011.00642.x. [DOI] [PubMed] [Google Scholar]
- 46. Ekser B, Long C, Echeverri GJ, et al. Impact of Thrombocytopenia on Survival of Baboons with Genetically Modified Pig Liver Transplants: Clinical Relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. •• Profound thrombocytopenia developing within hours after porcine liver transplantation is the major factor causing early mortality in baboons after orthotopic liver transplantation from genetically manipulated pigs.
- 47.Dini L, Lentini A, Diez GD, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108(Pt 3):967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 48.Owaki T, Meneshian A, Maemura K, et al. Endothelial cells potentiate phagocytic killing by macrophages via platelet-activating factor release. Am J Physiol Heart Circ Physiol. 2000;278:H269–H276. doi: 10.1152/ajpheart.2000.278.1.H269. [DOI] [PubMed] [Google Scholar]
- 49.Potoka DA, Takao S, Owaki T, et al. Endothelial cells potentiate oxidant-mediated Kupffer cell phagocytic killing. Free Radic Biol Med. 1998;24:1217–1227. doi: 10.1016/s0891-5849(97)00453-x. [DOI] [PubMed] [Google Scholar]
- 50. Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010;17:350–361. doi: 10.1111/j.1399-3089.2010.00605.x. •• Human platelets were found to be rapidly removed by porcine LSECs via phagocytosis in the absence of endothelial cell or platelet activation in an ex vivo porcine liver perfusion model.
- 51.Olsson M, Bruhns P, Frazier WA, et al. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paris LL, Reyes LM, Tector AJ. SIRPa interspecies incompatibilities lead to xenogeneic phagocytosis of platelets by liver cells. Xenotransplantation. 2011;18:293. • Transgenic expression of human SIRPα on porcine LSECs was found protective against phagocytosis of human platelets.
- 53. Paris LL, Chihara RK, Reyes LM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18:245–251. doi: 10.1111/j.1399-3089.2011.00639.x. • This study shows that LSECs express ASGR1 that mediates phagocytosis of human platelets by porcine LSECs.
- 54.Chihara RK, Paris LL, Reyes LM, et al. Primary Porcine Kupffer Cell Phagocytosis of Human Platelets Involves the CD18 Receptor. Transplantation. 2011;92:739–744. doi: 10.1097/TP.0b013e31822bc986. [DOI] [PubMed] [Google Scholar]