Abstract
The B-type lamins are widely assumed to be essential for mammalian cells. In part, this assumption is based on a highly cited study that found that RNAi-mediated knockdown of lamin B1 or lamin B2 in HeLa cells arrested cell growth and led to apoptosis. Studies indicating that B-type lamins play roles in DNA replication, the formation of the mitotic spindle, chromatin organization and regulation of gene expression have fueled the notion that B-type lamins must be essential. But surprisingly, this idea had never been tested with genetic approaches. Earlier this year, a research group from UCLA reported the development of genetically modified mice that lack expression of both <i>Lmnb1</i> and <i>Lmnb2</i> in skin keratinocytes (a cell type that proliferates rapidly and participates in complex developmental programs). They reasoned that if lamins B1 and B2 were truly essential, then keratinocyte-specific lamin B1/lamin B2 knockout mice would exhibit severe pathology. Contrary to expectations, the skin and hair of lamin B1/lamin B2-deficient mice were quite normal, indicating that the B-type lamins are dispensable in some cell types. The same UCLA research group has gone on to show that lamin B1 and lamin B2 are critical for neuronal migration in the developing brain and for neuronal survival. The absence of either lamin B1 or lamin B2, or the absence of both B-type lamins, results in severe neurodevelopmental abnormalities.
Keywords: lamin B1, lamin B2, nuclear envelope, nuclear lamina
The B-type lamins are widely assumed to be essential proteins
The main protein constituents of the nuclear lamina in mammals are lamins A, C, B1, and B2.1,2 Lamins A and C are alternatively spliced isoforms of LMNA,3 while lamins B1 and B2 are products of independent genes (LMNB1 and LMNB2, respectively).4-6 The B-type lamins are expressed in most cell types, starting early in development, while the A-type lamins are expressed mainly in differentiated cells, and generally later in development.7-9
Over the past few years, the general perception has been that the B-type lamins play essential roles in mammalian cells, while A-type lamins are important for more specialized functions in differentiated cells. For example, in a recent review on nuclear lamins, Broers et al.10 wrote, “expression of B-type lamins is essential for nuclear integrity, cell survival, and normal development,” adding that “B-type lamins are the fundamental building blocks of the nuclear lamina, while the A-type lamins have more specialized functions.” Numerous studies underlie the notion that the B-type lamins are particularly important for cells. One highly cited study reported that RNAi-mediated knockdown of either LMNB1 or LMNB2 in HeLa cells blocked cell growth and induced apoptosis, whereas a knockdown of LMNA did not.11 The authors concluded that lamins B1 and B2 are essential proteins in mammalian cells. Many other studies have provided indirect support for this idea. For example, by studying lamin B localization in CHO cells, Belmont et al.12 concluded that the B-type lamins are likely important for organizing heterochromatin at the nuclear rim. Other investigators have suggested that the B-type lamins have unique and vital roles in chromatin organization, DNA replication and gene transcription.13-15 One high-profile study indicated that the B-type lamins are crucial for the organization and function of the mitotic spindle,16 while another reported that a knockdown of lamin B1 in HeLa cells compromised the formation of nucleoli.17 Together, these disparate cell biology studies suggested that mammalian cells would be unlikely to thrive in the absence of B-type lamins.
Recent insights from Lmnb1 and Lmnb2 knockout mice
Studies of conventional knockout mice have complemented the aforementioned cell biology studies. A Lmna knockout did not interfere with development but led to premature death from cardiomyopathy/muscular dystrophy by ~6 weeks of age,9 implying that lamins A/C are not required for cell growth or development but are essential for the function of certain differentiated cell types. In contrast, Lmnb1- and Lmnb2-deficient mice do not survive beyond birth. Lmnb1-deficient mice were initially generated from a gene-trap ES cell clone that led to the production of a lamin B1–βgeo fusion protein.18 Lmnb1-deficient fibroblasts grew in culture, but they exhibited misshapen nuclei, premature senescence, and aneuploidy.18 Lmnb1-deficient embryos were small and died soon after birth with immature lungs and abnormalities in the cranium and other bones. And, as discussed further in the last few paragraphs of this article, Coffinier and coworkers have shown that lamin B1 deficiency leads to severe neurodevelopmental abnormalities.
Coffinier et al.19,20 also created Lmnb2-deficient mice and found that those mice also died at birth. Lmnb2-deficient embryos were virtually normal in size, and the only noteworthy pathological findings were severe neurodevelopmental defects involving the cerebral cortex and cerebellum. In contrast to lamin B1-deficient fibroblasts, Lmnb2-deficient fibroblasts grew normally in culture and were free of misshapen nuclei and chromosomal abnormalities.20
The fact that both the Lmnb1-deficient mice and Lmnb2-deficient mice survived through embryonic development—and that fibroblasts from these mice grew in culture—implied that neither lamin B1 nor lamin B2 is absolutely required for the viability of mammalian cells. But this conclusion was subject to caveats. First, in the case of Lmnb1, there was a formal possibility that the lamin B1–βgeo fusion protein retained partial function, permitting “lamin B1–deficient” cells to survive. Subsequent studies by Coffinier and coworkers have excluded that caveat as a serious consideration. Second, it seemed possible that the two B-type lamins might play redundant functions, and that the capacity of Lmnb1-deficient embryos and Lmnb2-deficient embryos to survive until birth reflected their capacity to produce one of the two B-type lamins. Functional redundancy of the two B-type lamins is not a farfetched possibility; lamins B1 and B2 are both intermediate filament proteins of the nuclear lamina, and their amino acid sequences are much more similar to each other than to the A-type lamins.4 Also, both of the B-type lamins share a carboxyl-terminal farnesyl lipid anchor (unlike mature lamin A or lamin C).
The field of human genetics has yielded few insights into the functional relevance of the B-type lamins. Unlike LMNA, where hundreds of missense, nonsense, and frameshift mutations have been identified and characterized,21 no one has yet uncovered any clinically significant loss-of-function mutations in LMNB1 or LMNB2. The only bona fide association of B-type lamins to human disease is LMNB1 gene duplications in patients with adult-onset autosomal-dominant leukodystrophy.22 The absence of loss-of-function mutations in LMNB1 and LMNB2 (at least so far) suggests several possibilities. One—which is consistent with the knockout mice results—is that the loss of lamin B1 or lamin B2 in humans is incompatible with postnatal life. A second possibility is that the two B-type lamins are functionally redundant, and therefore the loss of one is fully compensated by the other. Still another possibility is that neither B-type lamin is crucial as long as LMNA is expressed at a respectable level.
Keratinocyte-specific Lmnb1 and Lmnb2 knockout mice
Lingering uncertainties about the in vivo relevance of the B-type lamins prompted Yang et al.23 to investigate the effects of inactivating both Lmnb1 and Lmnb2 in specific tissues. They generated conditional knockout alleles for both genes (Lmnb1fl and Lmnb2fl) and then took advantage of a well-characterized keratin 14–Cre (K14-Cre) transgene24,25 to create mice lacking both Lmnb1 and Lmnb2 in skin keratinocytes. They chose to examine skin keratinocytes for two reasons. First, the formation of a cornified epithelium and the appendages of the skin (e.g., hair, nails) involve complex developmental programs, and they imagined that these programs would be compromised by the loss of the B-type lamins. Second, skin keratinocytes proliferate rapidly, and they suspected that a deficiency of the B-type lamins would interfere with cell growth, particularly in light of the literature suggesting that B-type lamins are important for DNA replication and gene transcription.13-15
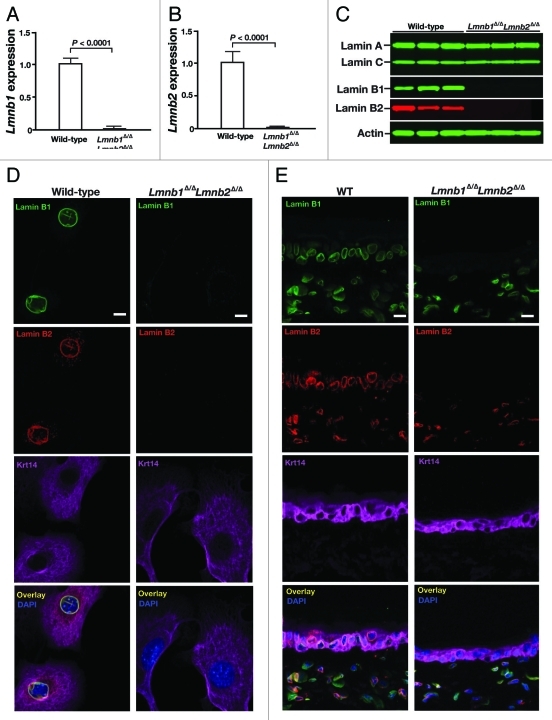
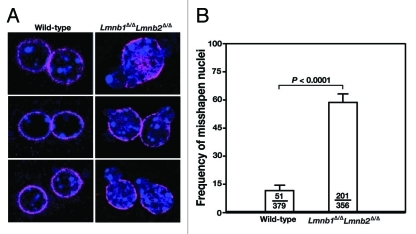
The newly created Lmnb1 and Lmnb2 conditional knockout alleles worked as planned, and the K14-Cre transgene led to high levels of recombination in keratinocytes.23 But surprisingly, the keratinocyte-specific double knockout mice (Lmnb1fl/flLmnb2fl/flK14Cre mice, designated Lmnb1Δ/ΔLmnb2Δ/Δ) survived development and appeared grossly normal at birth.23 The skin keratinocytes of Lmnb1Δ/ΔLmnb2Δ/Δ mice express negligible levels of Lmnb1 and Lmnb2 transcripts, as judged by qRT-PCR (Fig. 1A–B), and lamin B1 and lamin B2 proteins were undetectable by protein gel blots (Fig. 1C). The absence of both B-type lamins was confirmed by immunocytochemistry in freshly isolated Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes (Fig. 1D) and by immunohistochemistry in skin biopsies from Lmnb1Δ/ΔLmnb2Δ/Δ mice (Fig. 1E). Occasional misshapen nuclei, mainly cells with nuclear blebs, were observed in cultured Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes (Fig. 2), but nuclear shape abnormalities were never observed in skin biopsies, as judged by immunofluorescence microscopy.23
Figure 1.
Inactivation of Lmnb1 and Lmnb2 in skin keratinocytes. (A, B) qRT-PCR studies of Lmnb1 (A) and Lmnb2 (B) expression in keratinocytes from wild-type and Lmnb1fl/flLmnb2fl/flK14Cre (Lmnb1Δ/ΔLmnb2Δ/Δ) mice (n = 4/genotype). Data were normalized to β2-microglobulin and gene expression was compared with that in the Lmnb1+/+ mice (which was set at 1.0). (C) protein gel blot analysis of lamin expression in keratinocytes isolated from wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ mice (n = 3/group) with antibodies against lamin A/C, lamin B1, and lamin B2. Actin was used as a loading control. (D, E) Immunofluorescence microscopy of primary keratinocytes (D) and ear skin (E) from wild-type (WT) and Lmnb1Δ/ΔLmnb2Δ/Δ mice (4 mice/group were examined) with antibodies against lamin B1 (green), lamin B2 (red), and keratin 14 (magenta). DNA was visualized with DAPI (blue). Scale bar, 20 μm. Reproduced with permission from Yang et al.23
Figure 2.
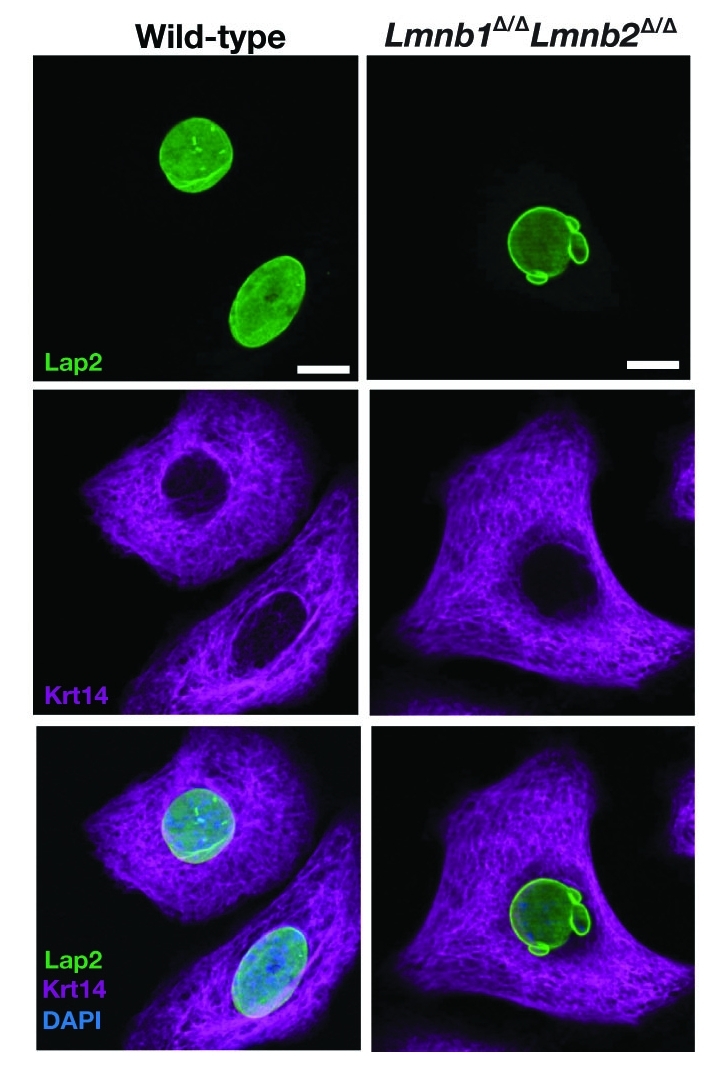
Immunohistochemical staining of keratinocytes from wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ mice with antibodies against Lap2 (green) and keratin 14 (Krt14; magenta), revealing an increased frequency of nuclear blebbing in Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes. DNA was visualized with DAPI (blue). Reproduced with permission from Yang et al.23
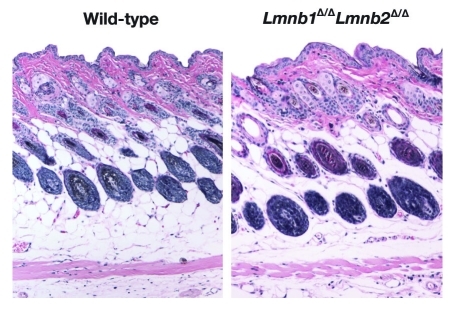
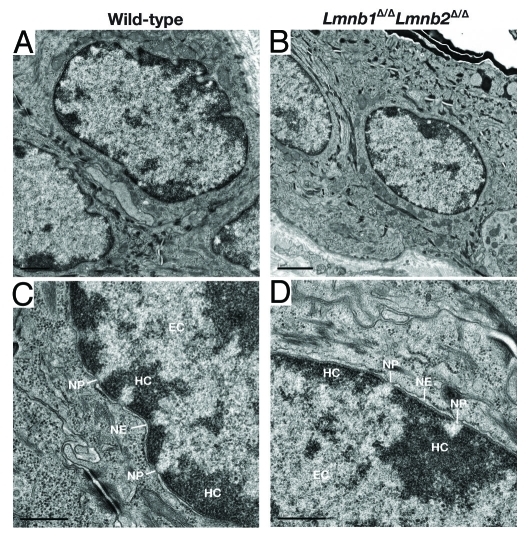
The absence of lamin B1 and lamin B2 in skin keratinocytes had no apparent deleterious consequences. Lmnb1Δ/ΔLmnb2Δ/Δ mice survived for > 2 y, and the skin, hair, and nails were indistinguishable from those of wild-type mice.23 The histology of the skin and hair was entirely normal, as judged by hematoxylin and eosin–stained histological sections (Fig. 3). The proliferation of keratinocytes in the skin of Lmnb1Δ/ΔLmnb2Δ/Δ mice was normal, as judged by BrdU incorporation, and the growth of cultured Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes was also normal.23 Unlike lamin B1–deficient fibroblasts described by Vergnes et al.,18 Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes did not exhibit aneuploidy. Interestingly, wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes were indistinguishable by electron microscopy (Fig. 4), and in particular, the distribution of heterochromatin at the periphery of the cell nucleus was similar. In parallel, Yang et al.23 isolated embryonic fibroblasts lacking both lamin B1 and lamin B2 (by treating Lmnb1fl/flLmnb2fl/fl fibroblasts with Cre-adenovirus), immortalized the fibroblasts by serial passaging, and uncovered no defects in growth or chromosome number. The complete absence of Lmnb1 and Lmnb2 expression did not result in upregulatation of Lmna expression. Those observations strongly support the dispensability of B-type lamins in skin keratinocytes and fibroblasts and challenged the notion that B-type lamins have unique and essential roles in DNA replication, cell proliferation, or gene expression.13-15
Figure 3.
Hematoxylin and eosin staining on skin sections from 40-d-old wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ mice. Ten × magnification. Reproduced with permission from Yang et al.23
Figure 4.
Electron micrographs of keratinocyte nuclei in the skin from wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ mice, showing heterochromatin (HC), euchromatin (EC), nuclear pores (NP), and nuclear envelope (NE). Magnification in A–B, × 22700. Scale bar, 1.0 μm. Magnification in C–D, × 74200. Scale bar, 0.5 μm. Reproduced with permission from Yang et al.23
Knocking out both Lmnb1 and Lmnb2 in hepatocytes
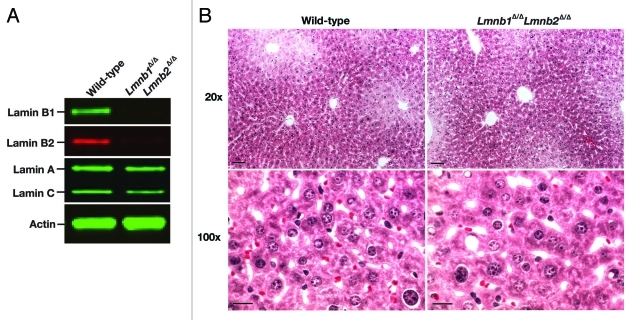
We considered the possibility that the dispensability of B-type lamins in keratinocytes might be a peculiarity of that cell type. Therefore, we took advantage of an albumin-Cre transgenic line26 and created mice lacking both lamin B1 and lamin B2 in liver hepatocytes (Lmnb1fl/flLmnb2fl/flAlbCre, or Hep-Lmnb1Δ/ΔLmnb2Δ/Δ). Once again, Cre-mediated recombination was efficient; levels of Lmnb1 and Lmnb2 transcripts were negligible in Hep-Lmnb1Δ/ΔLmnb2Δ/Δ hepatocytes, and lamin B1 and lamin B2 proteins were virtually undetectable (Fig. 5A). The Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice exhibited normal vitality, and liver function tests were entirely normal (Table 1). Liver histology was normal in Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice, as judged by hematoxylin and eosin—stained sections (Fig. 5B). Misshapen nuclei were not observed in frozen sections of liver from Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice, although we did observe an increased frequency of nuclear blebs in cultured Hep-Lmnb1Δ/ΔLmnb2Δ/Δ hepatocytes (Fig. 6).
Figure 5.
Inactivation of Lmnb1 and Lmnb2 in hepatocytes. A, protein gel blot analysis of lamin expression in hepatocytes isolated from wild-type (WT) and Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice (n = 3/group) with antibodies against lamin A/C, lamin B1, and lamin B2. Actin was used as a loading control. B, Hematoxylin and eosin–stained liver sections from 6-mo-old wild-type and Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice. Upper panel: 20 × magnification. Scale bar: 30 μm. Lower panel: 100 × magnification. Scale bar: 10 μm.
Table 1. Evaluation of liver function in wild-type and Hep-Lmnb1Δ/ΔLmnb2Δ/Δ mice.
| Serum Chemistry | Wild-type | Hep-Lmnb1Δ/ΔLmnb2Δ/Δ | Normal range | |
|---|---|---|---|---|
| ALT (U/L) |
137 ± 96 |
80 ± 57 |
7–227 |
|
| ALP |
83 ± 6 |
92 ± 12 |
13–291 |
|
| AST (U/L) |
169 ± 120 |
134 ± 67 |
37–329 |
|
| CHOL (mg/dl) |
80 ± 9 |
73 ± 19 |
34–129 |
|
| GLU (mg/dl) |
222 ± 36 |
242 ± 52 |
46–279 |
|
| ALB (g/dl) |
2.35 ± 0.21 |
2.3 ± 0.28 |
2.5–5.4 |
|
| DBILI (mg/dl) |
0.20 ± 0.04 |
0.24 ± 0.09 |
0–0.3 |
|
| TBILI (mg/dl) |
0.67 ± 0.15 |
0.3 ± 0.07 |
0.1–1.1 |
|
| ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CHOL, cholesterol; GLU, glucose; ALB, albumin; DBILI, direct bilirubin; TBILI, total bilirubin. | ||||
Figure 6.
Increased frequency of nuclear blebbing in Hep-Lmnb1Δ/ΔLmnb2Δ/Δ hepatocytes. (A) Immunohistochemical staining of hepatocytes from wild-type and Lmnb1Δ/ΔLmnb2Δ/Δ mice with antibodies against lamin A (magenta); DNA was visualized with DAPI (blue). (B) Frequency of misshapen nuclei in wild-type and Hep-Lmnb1Δ/ΔLmnb2Δ/Δ hepatocytes (n = 3/genotype tested). Bars indicate the frequency of misshapen nuclei; the number of cells with nuclear blebs and the total number of cells examined are provided within each bar. Hep-Lmnb1Δ/ΔLmnb2Δ/Δ hepatocytes had more nuclear blebs (p < 0.0001, chi-square test).
The fact that nuclear blebs were more frequent in isolated Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes and hepatocytes implies that the B-type lamins play a role in the structural integrity of the nuclear lamina. We were not surprised, however, by the failure to detect misshapen nuclei in the tissues of the mutant mice. Earlier, our group found severe nuclear shape abnormalities in fibroblasts from mice that synthesize exclusively mature lamin A (“mature lamin A–only” or LmnaLAO/LAO mice); however, we could not identify any abnormalities in the cell nuclei in tissues of LmnaLAO/LAO mice.27 We suspect that growing cells on plastic surfaces, where the nuclei tend to be pancake-shaped, makes it easier to detect subtle changes in the integrity of the nuclear envelope.
Interpretation of experiments with tissue-specific Lmnb1/Lmnb2 knockout mice
Keratinocytes and hepatocytes were free of significant pathology in the absence of the B-type lamins, suggesting that these proteins are dispensable in those cell types. Given the host of publications pointing to many key functions of the B-type lamins in the cell nucleus, how should these knockout studies be interpreted? Can one reconcile the earlier cell culture studies with the absence of phenotypes in tissue-specific knockout mice? Some might be tempted to conclude that the B-type lamins have no roles in DNA replication, gene transcription, organization of the mitotic spindle, etc., and that the earlier reports were simply incorrect, but we would advise against jumping to this sort of conclusion. First, the studies by Yang et al.23 merely assessed cell viability and gross phenotypes, and more subtle studies of nuclear functions are yet to be performed. One could also argue that the significance of the mouse experiments were limited because no efforts were made to “stress the system” (e.g., no wound healing or liver regeneration studies were performed). This type of criticism is reasonable, but on the other hand, it is noteworthy that both the keratin 14–Cre and albumin–Cre transgenes are expressed early in development (by E14.5–15.5).26,28 Thus, deficiencies of the B-type lamins appear to have little effect on the vitality of the liver or skin during periods of robust differentiation and cell growth. Also, the studies by Yang et al.23 examined only two differentiated cell types, and it is entirely possible that unique functions of the B-type lamins would be more obvious in other tissues. Third, and most importantly, the B-type lamins have been conserved through vertebrate evolution, and we seriously doubt that this would have occurred in the absence of unique and important functions. At this point, more research is needed to identify and define those functions.
It is important to emphasize that keratinocytes and hepatocytes—the cell types where the B-type lamins appear dispensable—represent differentiated cells that express not only the B-type lamins but also lamins A and C. It seems possible that the A-type lamins possess redundant functional capacities and are capable of carrying out all of the crucial “lamin functions” in Lmnb1/Lmnb2-deficient keratinocytes and hepatocytes. Interestingly, the consequences of lamin B1 and lamin B2 deficiencies are quite striking in at least one tissue where lamin A/C expression levels are minimal low. Coffinier and coworkers have shown that lamin B1 and lamin B2 deficiencies lead to severe defects in the developing brain,20,29 a tissue where lamin A/C expression is very low and is only activated late in development.7
In considering results with conditional knockout alleles, one should always worry about “leaky alleles” and the possibility that recombined alleles produce proteins with partial function.30 In the case of the Lmnb1 and Lmnb2 conditional knockout alleles, our combination of qRT-PCR, protein gel blot, and immunohistochemistry studies provided little evidence of persistent protein expression. For both alleles, the recombination event excises exon 2 and yields a frameshift; neither allele resulted in the production of β-galactosidase fusion proteins. In the case of Lmnb1, exon 1 encodes 121 amino acids; in the case of Lmnb2, exon 1 encodes 66 amino acids. Any residual “exon 1 truncated proteins” produced by the recombined alleles would lack important protein domains, including the vast majority of the rod domain, the carboxyl-terminal globular domain, the nuclear localization signal, and the carboxyl-terminal CaaX motif. We doubt that significant amounts of exon 1 truncated proteins are produced. When we measured Lmnb1 and Lmnb2 transcripts in Lmnb1Δ/ΔLmnb2Δ/Δ keratinocytes by qRT-PCR with exon 1 primers, we found only trace levels, likely reflecting rapid turnover of the mutant transcripts.
We anticipate that some cell biologists may be skeptical of the mouse knockout mouse experiments, and they may even contend that the functions of the B-type lamins in DNA replication, the mitotic spindle formation, gene transcription, etc. are so crucial that nature has evolved back-up systems to deal with their absence. Over the next few years, the nuclear lamin field will undoubtedly need to sort out those types of arguments with additional experiments. In any case, genetically modified mice—and cell lines derived from them—will remain important tools for defining both unique and redundant roles of the different nuclear lamins in the cell nucleus.
What is the in vivo functional relevance of B-type lamins?
The B-type lamins may be dispensable in the skin and liver, but they are clearly important in the brain.19,20 In a 2010 paper in PNAS, Coffinier et al.20 made a seminal observation regarding about the function of B-type lamins in mammals. They demonstrated, in a comprehensive fashion and with a variety of experimental techniques, that a deficiency of Lmnb2 in mice causes defective neuronal migration in the developing cerebral cortex, resulting in defective layering of cortical neurons. Layering of neurons depends on the timely migration of neurons from the ventricular zone, where they are born, to the proper layer in the cortical plate. Interestingly, migration of neurons to the cortical plate is utterly dependent on moving the cell nucleus forward in the direction of cell migration. The movement of the cell nucleus, which depends on a network of microtubules and cytoplasmic motors, likely exerts significant deformational forces on the cell nucleus. We suspect that the nucleus is unable to withstand those forces in the absence of lamin B2, which would explain defective migration of the neurons. We have suggested that the B-type lamins might be particularly well suited for ensuring the integrity of the nuclear envelope since they are anchored to the inner nuclear membrane by a farnesyl lipid.21 We would not be surprised if lamin B1 were also essential for the development of the mouse brain, given the earlier observation of abnormal cranial vaults in Lmnb1 knockout mice.18 This topic needs to be investigated further.
Given that lamin B2 plays an important role in brain development,20 and given that the cranial vault in lamin B1 knockout mice is abnormal in size and shape,20 Coffinier and coworkers29 were suspicious that lamin B1 might also play an important role in brain development. Their suspicions were confirmed! In a 2011 Mol. Biol. Cell paper,29 Coffinier and coworkers established, with multiple experimental approaches, that lamin B1 is critical for neuronal survival, neuronal migration, and proper layering of neurons in the brain. Interestingly, a deficiency of lamin B1 in neurons resulted in striking abnormalities in the shape of cortical neurons. Most cortical neurons in lamin B1–deficient mice contained a solitary nuclear bleb; also, many cortical neurons exhibited an asymmetric distribution of lamin B2. In contrast, lamin B2 deficiency led to increased numbers of neurons with elongated nuclei. In addition to studies with conventional lamin B1– and lamin B2–knockout mice, Coffinier and coworkers29 created conditional knockout alleles for both Lmnb1 and Lmnb2 and went on to breed forebrain-specific knockout mice. Both forebrain-specific Lmnb1 knockout embryos and forebrain-specific Lmnb2 knockout embryos had small forebrains with disordered layering of neurons and nuclear shape abnormalities, similar to the abnormalities in the conventional knockout embryos. In adult forebrain-specific knockout mice, brain pathology was even more striking than in the embryos. A far more severe phenotype, complete atrophy of the cortex, was observed in forebrain-specific Lmnb1/Lmnb2 double knockout mice. No viable neurons were found in the forebrains of adult Lmnb1/Lmnb2 double knockout mice, indicating that neuronal survival requires the expression of one of the two B-type lamins. The studies by Coffinier et al.29 established, for the first time, that deficiencies in lamin B1 and lamin B2 lead to severe neurodevelopmental abnormalities, expanding the realm of disorders associated with lamin defects. Sooner or later, we suspect that LMNB1 and LMNB2 mutations will be identified in infants with severe neurodevelopmental abnormalities. Also, given that neuronal migration abnormalities have been implicated in milder neurological diseases, including epilepsy, it would not be particularly surprising if geneticists and neurologists ultimately uncovered LMNB1 and/or LMNB2 mutations in outpatients in neurology clinics.
Interestingly, Lmna expression is very low in the developing brain29—at a time period when Lmnb1 and Lmnb2 expression in the brain are so essential.20,29 The fact that Lmna expression is low in the developing brain raises many questions, including: Why is Lmna expression low in the developing brain when it is robust in other tissues? Would the synthesis of lamin A/C in neurons interfere with neuronal migration? Would the synthesis of lamin A/C in developing neurons effectively substitute for the roles of lamin B2 and rescue Lmnb2-deficient mice from their neurodevelopment abnormalities? Are the farnesyl lipid anchors in lamins B1 and B2 crucial for the development of the brain? These are intriguing questions, and we strongly suspect that efforts to address these questions will yield important insights into the unique and redundant functions of the nuclear lamins in mammalian development and cell biology.
Acknowledgments
This work was supported by the National Institutes of Health Grants [HL76839, CA099506–07, HL086683, HL089781]; the Ellison Medical Foundation Senior Scholar Program; and a March of Dimes Grant [6-FY2007–1012]; a Beginning Grant-in-aid from the American Heart Association, Western States Affiliate [0865262F]; an American Heart Association Scientist Development Award [0835489N] and a fellowship from the Vascular Biology Program at UCLA [2 T32 HL069766:06]. We would like to thank Dr. Pinchas Cohen (University of California, Los Angeles) for providing the albumin-Cre transgenic mouse strain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/18085
References
- 1.Stewart CL, Roux KJ, Burke B. Blurring the boundary: the nuclear envelope extends its reach. Science. 2007;318:1408–12. doi: 10.1126/science.1142034. [DOI] [PubMed] [Google Scholar]
- 2.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol. 2010;2:a000547. doi: 10.1101/cshperspect.a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–6. [PubMed] [Google Scholar]
- 4.Höger TH, Zatloukal K, Waizenegger I, Krohne G. Characterization of a second highly conserved B-type lamin present in cells previously thought to contain only a single B-type lamin. Chromosoma. 1990;99:379–90. doi: 10.1007/BF01726689. [DOI] [PubMed] [Google Scholar]
- 5.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–53. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechat T, Adam SA, Goldman RD. Nuclear lamins and chromatin: when structure meets function. Adv Enzyme Regul. 2009;49:157–66. doi: 10.1016/j.advenzreg.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Röber RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–78. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 8.Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–92. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 11.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 12.Belmont AS, Zhai Y, Thilenius A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J Cell Biol. 1993;123:1671–85. doi: 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–21. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moir RD, Montag-Lowy M, Goldman RD. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol. 1994;125:1201–12. doi: 10.1083/jcb.125.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, et al. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J Cell Sci. 2008;121:1014–24. doi: 10.1242/jcs.020982. [DOI] [PubMed] [Google Scholar]
- 16.Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, et al. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–93. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 17.Martin C, Chen S, Maya-Mendoza A, Lovric J, Sims PF, Jackson DA. Lamin B1 maintains the functional plasticity of nucleoli. J Cell Sci. 2009;122:1551–62. doi: 10.1242/jcs.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–33. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffinier C, Fong LG, Young SG. LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins. Nucleus. 2010;1:407–11. doi: 10.4161/nucl.1.5.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, et al. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci USA. 2010;107:5076–81. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–36. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–23. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 23.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y. Jong PJd, Fong LG, Young SG. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of the skin and hair. Hum Mol Genet. 2011;20:3537–44. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 25.Lee R, Chang SY, Trinh H, Tu Y, White AC, Davies BS, et al. Genetic studies on the functional relevance of the protein prenyltransferases in skin keratinocytes. Hum Mol Genet. 2010;19:1603–17. doi: 10.1093/hmg/ddq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisend CM, Kundert JA, Suvorova ES, Prigge JR, Schmidt EE. Cre activity in fetal albCre mouse hepatocytes: Utility for developmental studies. Genesis. 2009;47:789–92. doi: 10.1002/dvg.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffinier C, Jung HJ, Li Z, Nobumori C, Yun UJ, Farber EA, et al. Direct synthesis of lamin A, bypassing prelamin A processing, causes misshapen Nuclei in fibroblasts but No detectable pathology in mice. J Biol Chem. 2010;285:20818–26. doi: 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cang Y, Zhang J, Nicholas SA, Kim AL, Zhou P, Goff SP. DDB1 is essential for genomic stability in developing epidermis. Proc Natl Acad Sci USA. 2007;104:2733–7. doi: 10.1073/pnas.0611311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, Fong LG, Young SG. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011;22:4683–93. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SH, Bergo MO, Farber E, Qiao X, Fong LG, Young SG. Caution! Analyze transcripts from conditional knockout alleles. Transgenic Res. 2009;18:483–9. doi: 10.1007/s11248-008-9237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]