Abstract
Purpose
To investigate the genetic associations of polypoidal choroidal vasculopathy (PCV), the genetic difference between PCV and age-related macular degeneration (AMD), and the genotype-phenotype correlation of PCV.
Methods
A systematic review and meta-analysis were performed. Published articles about genetic associations of PCV identified from a literature search were reviewed. The following data from individual studies were extracted and analyzed: 1) comparison of genetic polymorphisms between PCV and controls; 2) comparison of genetic polymorphisms between PCV and AMD; and 3) comparison of phenotypes between different genotype groups.
Results
A total of 33 articles fulfilled the inclusion criteria. With meta-analyses, variants in four genes were found to be significantly associated with PCV: LOC387715 rs10490924 (n=9, allelic odds ratio [OR]=2.27, p<0.00001), HTRA1 rs11200638 (n=4, OR=2.72, p<0.00001), CFH rs1061170 (n=4, OR=1.72, p<0.00001), CFH rs800292 (n=5, OR=2.10, p<0.00001), and C2 rs547154 (n=3, OR=0.56, p=0.01). LOC387715 rs10490924 was the only variant showing a significant difference between PCV and wet AMD (n=5, OR=0.66, p<0.00001). The risk genotypes of rs10490924 were associated with larger lesion size, greater chance of vitreous hemorrhage, and worse therapeutic response in PCV.
Conclusions
LOC387715 rs10490924 was associated with PCV and its clinical manifestations, and showed a discrepant distribution between PCV and AMD. Variants in HTRA1, CFH, and C2 were also associated with PCV.
Introduction
Polypoidal choroidal vasculopathy (PCV) is a sight-threatening disease in older adults. Clinically, it shares several common manifestations with wet age-related macular degeneration (AMD) such as subretinal exudation and hemorrhage involving the macular region. PCV was first identified as distinct from wet AMD in 1990 by Yannuzzi et al. [1], who reported a series of patients with peculiar polypoidal, subretinal vascular lesions associated with serous and hemorrhagic detachment of the retinal pigment epithelium, and named the clinical subtype PCV. Later, researchers reported that under indocyanine green angiography (ICGA), PCV demonstrated the distinct characteristic of a branching network of vessels and dilation at the ends of the vascular network in the inner choroid [2], thus making ICGA a standard investigation for diagnosing PCV.
The etiology of PCV remains largely unknown. It is likely a multifactorial disease sharing some mechanisms with AMD. To date, it is well recognized that genetic factors play an important role in the pathogenesis of AMD. In 2005, a coding single nucleotide polymorphism (SNP) rs1061170 (Y402H) at the Complement factor H (CFH) gene was found to be strongly associated with AMD in a genome-wide association study (GWAS) [3-7] in Caucasians. Subsequently, the SNP rs10490924 at LOC387715 (also known as ARMS2) and rs11200638 at HTRA1 were found to be associated with AMD in Caucasian and Asian populations [8-11]. Later, other genes or loci were also discovered as risk or protective factors for AMD, such as Complement factor B (CFB) [12], Complement factor C2 [12], Complement factor C3 [13], SERPING1 [14], and so on. The phenotypic similarities between AMD and PCV lead to the hypothesis that genes involved in AMD may also play a role in PCV. Therefore, investigating AMD genes involved in PCV may easily reveal candidate genes for PCV [15,16], providing insights into the pathogenesis of PCV; in addition, differentiating the genetic profiles between PCV and AMD may provide clues to whether PCV is a subtype of wet AMD or a distinct disease [17], shedding some light on the pathogenesis of the respective phenotypes. Furthermore, genotype-phenotype correlation analysis, especially genetic predictors of therapy, may provide guidelines for better management of patients with AMD or PCV [18]. Thus far, however, findings on the genetic profiles of PCV compared to AMD remain controversial among different reports [17,19,20].
Here, in an attempt to give the overall effects and solve the controversies, we report a systematic review and meta-analysis summarizing all published results of genetic associations in PCV. This study 1) investigated which genetic variants are significantly associated with PCV and their effect sizes, 2) examined whether there are differences between the genetic risks of PCV and AMD, and 3) summarized the results of genotype-phenotype correlations in PCV.
Methods
Literature search
A systematic literature search using the databases PubMed, Embase, Web of Science, and the Chinese Biomedical Database was conducted on October 30, 2011, to identify all published studies on the association of genetic polymorphisms with PCV and/or its phenotypes. The search strategy (polypoidal choroidal vasculopathy OR PCV) AND (gene OR genetic OR polymorphism OR variant OR DNA or SNP) in All Fields was used. We did not use the option of language limitation on PubMed, Embase, or Web of Science.
Review process
Two reviewers (H.Y.C. and K.L.) independently reviewed the retrieved records. The following inclusion criteria were applied during the review process: 1) association study of genetic variants with PCV and/or its phenotypes; 2) raw data of allele or genotype frequencies or counts available; 3) the type of article an original research study, not a review, case report, or editorial comment. For studies published by the same group on the same gene and markers, only the most recent article or the article with the largest sample size was included for analysis. Independent review and resolution by a third reviewer (L.J.C.) was sought if the two reviewers disagreed.
Data extraction
The following data were extracted: author, year of publication, ethnicity of study subjects, whether the Hardy–Weinberg equilibrium (HWE) was examined in controls, the numbers and demographic characteristics of the patients and controls, and the allele and genotype counts or frequencies of each SNP in the patients and controls. The allele counts were calculated from the genotype counts when needed. We also calculated the allele or genotype counts from the frequencies, rounding to the closest integer, for studies [21,22] in which the genotype counts were not given. For the analysis of the genotype-phenotype correlation, the following phenotype information were extracted at each genotype group: the gender and bilaterality counts, the case number, the mean and standard deviation of age of onset, the best-corrected visual acuity (BCVA), greatest linear diameter (GLD) of lesion on fluorescence fundus angiography (FFA) and ICGA, and BCVA at 12 months after photodynamic therapy or combined therapy. For the study [23] in which the standard deviation was not given, we obtained it by communicating with the corresponding author. The data extraction and data input processes were performed by two reviewers (H.Y.C. and K.L.) independently. Further independent review and resolution by a third reviewer (L.J.C.) was sought if the two reviewers disagreed.
Data analysis
To investigate the associations of gene variants with PCV, the allele and genotype frequencies of the SNPs were compared between PCV and normal controls. Six genetic models were used in the association analysis: allele, homozygote, heterozygote, dominant, recessive, and additive. To investigate whether PCV and wet AMD have different genetic risks, the allele frequencies of the SNPs were compared between the patients with PCV and wet AMD in the studies that included both disorders. To investigate the genotype-phenotype correlation, the phenotype characters were compared between patients carrying one or two risk alleles and patients without the risk allele. The results of individual studies were pooled using the software Review Manager (RevMan, version 5.1.4, The Cochrane Collaboration, Copenhagen, Denmark). In all of the meta-analyses, the odds ratios (ORs) or mean differences (MDs) and 95% confidence interval (CIs) were estimated with the fixed or random model according to the heterogeneity test. When the heterogeneity test α was <0.1, a random model was applied; otherwise, a fixed model was applied. Egger’s test was used to evaluate possible publication bias in the meta-analysis with the number of included studies >2.
Results
A total of 175 articles were identified from literature search, including 43 from PubMed, 63 from Embase, 65 from Web of Science, and four from the Chinese Biomedical Literature Database. Among them, 79 were excluded because of duplication, 38 were excluded because of unrelated topics, and 16 were excluded because of the publication type, such as a review. The full text of the remaining 42 records was retrieved and reviewed. Nine articles were excluded after the full text was reviewed (Figure 1). Finally, 33 articles were included for the meta-analysis. All the studies were case-control studies, and none was family based. The characteristics of these articles are listed in Table 1.
Figure 1.
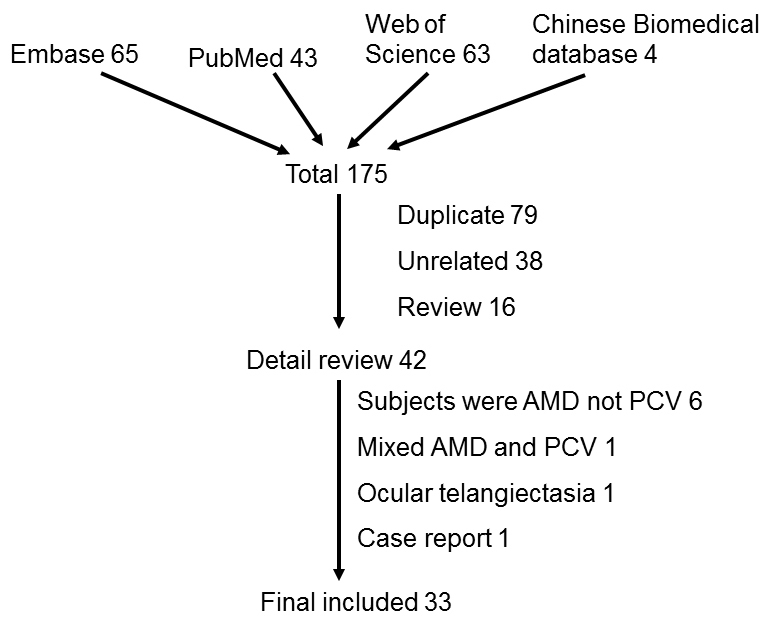
Flow diagram of literature screening. Flow diagram depicted the screening process of retrieved articles, including the number and reason of exclusion.
Table 1. Characteristics of the included studies.
| Author | Year | Ethnicity | HWE | PCV | AMD | Control | Gene(s)/locus investigated | Ref. |
|---|---|---|---|---|---|---|---|---|
| Gotoh |
2004 |
Japanese |
yes |
58 |
85 |
82 |
APOE |
[15] |
| Gotoh |
2008 |
Japanese |
no |
204 |
116 |
- |
HTRA1, CFH |
[35] |
| Gotoh |
2009 |
Japanese |
yes |
55 |
56 |
77 |
LOC387715 |
[24] |
| Gotoh |
2010 |
Japanese |
yes |
181 |
84 |
276 |
LOC387715, HTRA1 |
[25] |
| Yamashiro |
2011 |
Japanese |
no |
518 |
408 |
336 |
Elastin |
[20] |
| Yamashiro |
2011 |
Japanese |
no |
154 |
216 |
142 |
Elastin |
[20] |
| Nakanishi |
2010 |
Japanese |
yes |
375 |
- |
847 |
LOC387715, CFH |
[26] |
| Hayashi |
2010 |
Japanese |
yes |
518 |
408 |
1351 |
LOC387715, CFH |
[27] |
| Nakata |
2011 |
Japanese |
yes |
167 |
- |
- |
PEDF, SERPINF1 |
[49] |
| Nakata |
2011 |
Japanese |
yes |
510 |
401 |
336 |
SERPING1 |
[37] |
| Tsujikawa |
2011 |
Japanese |
no |
88 |
- |
- |
LOC387715 |
[45] |
| Kondo |
2007 |
Japanese |
yes |
76 |
73 |
94 |
LOC387715, HTRA1 |
[16] |
| Kondo |
2008 |
Japanese |
yes |
103 |
78 |
104 |
Elastin |
[17] |
| Kondo |
2009 |
Japanese |
yes |
130 |
- |
173 |
CFH |
[21] |
| Kondo |
2009 |
Japanese |
yes |
136 |
- |
183 |
CFB, C2, RDBP, SKIV2L |
[21] |
| Kondo |
2009 |
Japanese |
yes |
140 |
116 |
189 |
SOD2 |
[40] |
| Bessho |
2009 |
Japanese |
yes |
140 |
116 |
189 |
PEDF |
[38] |
| Bessho |
2011 |
Japanese |
no |
119 |
68 |
- |
LOC387715 |
[34] |
| Sakurada |
2008 |
Japanese |
yes |
109 |
- |
85 |
LOC387715 |
[30] |
| Sakurada |
2009 |
Japanese |
yes |
92 |
- |
- |
LOC387715 |
[44] |
| Sakurada |
2010 |
Japanese |
yes |
71 |
- |
- |
LOC387715 |
[18] |
| Sakurada |
2011 |
Japanese |
yes |
226 |
- |
- |
LOC387715, CFH |
[23] |
| Goto |
2009 |
Japanese |
yes |
100 |
100 |
190 |
LOC387715, CFH, C3 |
[29] |
| Fuse |
2011 |
Japanese |
yes |
60 |
50 |
138 |
LOC387715, LOXL1 |
[28] |
| Tanaka |
2011 |
Japanese |
yes |
287 |
- |
277 |
LOC387715, CFH |
[31] |
| Park |
2011 |
Korea |
yes |
103 |
- |
112 |
LOC387715, HTRA1 |
[33] |
| Park |
2011 |
Korea |
yes |
51 |
- |
LOC387715, HTRA1 |
[43] |
|
| Li |
2010 |
Chinese |
yes |
118 |
- |
115 |
SERPING1 |
[36] |
| Zhang |
2011 |
Chinese |
yes |
177 |
131 |
182 |
9p21 |
[42] |
| Wu |
2011 |
Chinese |
yes |
177 |
131 |
182 |
PEDF |
[39] |
| Sng |
2011 |
Chinese |
yes |
120 |
126 |
274 |
Toll-like receptor 3 |
[41] |
| Lee |
2008 |
Chinese |
yes |
72 |
- |
93 |
LOC387715, CFH, CFB, C2 |
[32] |
| Lima |
2011 |
Caucasian |
no |
56 |
368 |
368 |
Elastin |
[19] |
| Lima | 2010 | Caucasian | no | 55 | 368 | 368 | LOC387715, CFH, CFB, C2 | [22] |
HWE: Hardy–Weinberg equilibrium; PCV: polypoidal choroidal vasculopathy; AMD: Age-related macular degeneration. Ref: Reference
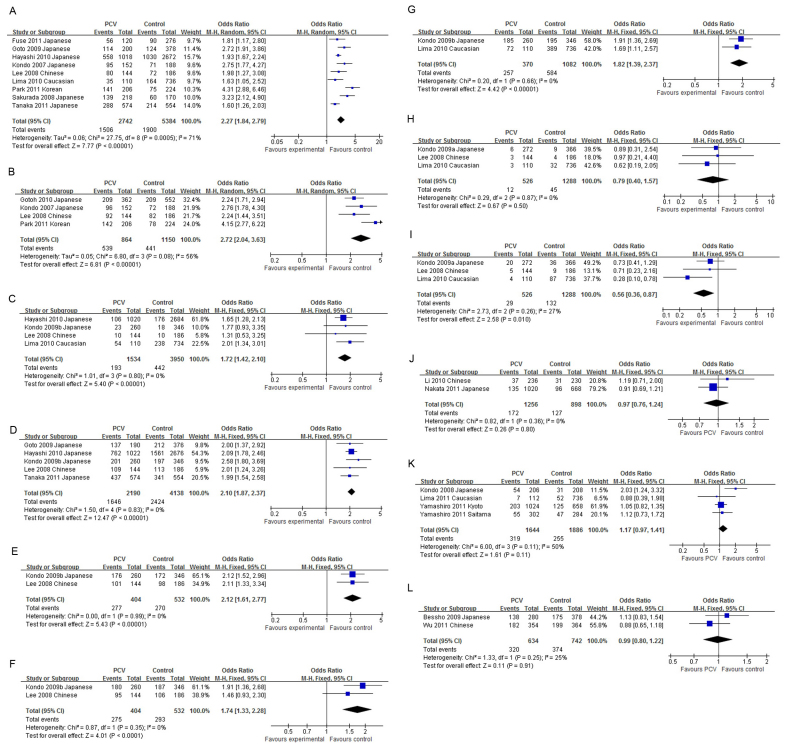
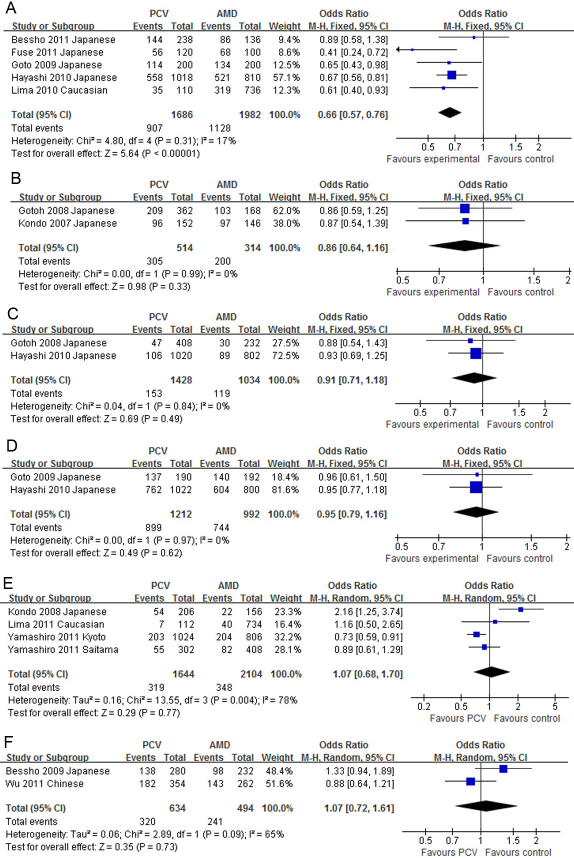
All reported genetic associations in PCV are summarized in Table 2. The SNP rs10490924 at LOC387715 was the most investigated SNP in PCV. Four articles [24-27] were from the same study group; only the most recent was used [27]. Nine studies were included in the meta-analysis [16,22,27-33]. The minor allele, T, was more frequent in PCV than in the controls in all articles. The allelic OR in an individual study ranged from 1.63 to 4.31, with a pooled OR of 2.27 (95% CI: 1.84–2.79, p<0.00001, Figure 2A). No significant publication bias was detected (Egger’s test p=0.202). The pooled ORs were 4.90, 1.74, 2.44, 3.26, and 1.65 for the homozygote, heterozygote, dominant, recessive, and additive models, respectively, with all p<0.0001 (Appendix 1). The allele frequencies of rs10490924 in PCV and AMD were reported in five studies [22,27-29,34]. The T allele frequency was lower in PCV compared to AMD in all reports, with a pooled OR of 0.66 (95% CI: 0.57–0.76, p<0.00001, Figure 3A). No significant publication bias was detected (Egger’s test p=0.627).
Table 2. Summary of allelic odds ratios of gene variants in polypoidal choroidal vasculopathy.
| Gene/locus | SNP | Allele | PCV versus control OR (95% CI) | Case | Control | N | Ref | PCV versus AMD OR (95% CI) | N | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| LOC387715 |
rs10490924 |
T |
2.27 (1.84–2.79) |
2742 |
5383 |
9 |
Figure 2A |
0.66 (0.57–0.76) |
5 |
Figure 3A |
| HTRA1 |
rs11200638 |
A |
2.72 (2.04–3.63) |
864 |
1150 |
4 |
Figure 2B |
0.86 (0.64–1.16) |
2 |
Figure 3B |
| CFH |
rs1061170 |
C |
1.72 (1.42–2.10) |
1534 |
3950 |
4 |
Figure 2C |
0.91 (0.71–1.18) |
2 |
Figure 3C |
| CFH |
rs800292 |
G |
2.10 (1.87–2.37) |
2190 |
4138 |
5 |
Figure 2D |
0.95 (0.79–1.16) |
2 |
Figure 3D |
| CFH |
rs3753394 |
T |
2.12 (1.61–2.77) |
404 |
532 |
2 |
Figure 2E |
NA |
|
|
| CFH |
rs1329428 |
T |
1.74 (1.33–2.28) |
404 |
532 |
2 |
Figure 2F |
NA |
|
|
| CFH |
rs1410996 |
C |
1.82 (1.39–2.37) |
370 |
1082 |
2 |
Figure 2G |
0.71 (0.47–1.06) |
1 |
[22] |
| CFB |
rs415667 |
A |
0.79 (0.40–1.57) |
526 |
1288 |
3 |
Figure 2H |
1.35 (0.38–4.73) |
1 |
[22] |
| C2 |
rs547154 |
T |
0.56 (0.36–0.87) |
526 |
1288 |
3 |
Figure 2I |
0.73 (0.26–2.10) |
1 |
[22] |
| SERPING1 |
rs2511989 |
A |
0.97 (0.76–1.24) |
1256 |
898 |
2 |
Figure 2J |
0.91 (0.70–1.20) |
1 |
[37] |
| Elastin |
rs2301995 |
G |
1.17 (0.97–1.41) |
1644 |
1886 |
4 |
Figure 2K |
1.07 (0.68–1.70) |
4 |
Figure 3E |
| PEDF |
rs1136287 |
T |
0.99 (0.80–1.22) |
634 |
742 |
2 |
Figure 2L |
1.07 (0.72–1.61) |
2 |
Figure 3F |
| SOD2 |
rs4880 |
C |
0.81 (0.52–1.26) |
140 |
189 |
1 |
[40] |
1.11 (0.66–1.88) |
1 |
[40] |
| TLR3 |
rs3775291 |
T |
1.27 (0.93–1.73) |
120 |
274 |
1 |
[41] |
1.16 (0.81–1.66) |
1 |
[41] |
| 9p21 |
rs10757278 |
A |
1.44 (1.08–1.94) |
177 |
182 |
1 |
[42] |
1.12 (0.81–1.54) |
1 |
[42] |
| RDBP |
rs3880457 |
C |
0.31 (0.13–0.71) |
136 |
183 |
1 |
[21] |
NA |
|
|
| SKIV2L |
rs2075702 |
C |
0.31 (0.13–0.71) |
136 |
183 |
1 |
[21] |
NA |
|
|
| C3 | rs2241394 | C | 3.47 (1.48–8.38) | 100 | 190 | 1 | [29] | NA |
SNP: single nucleotide polymorphism; PCV: polypoidal choroidal vasculopathy; AMD: age-related macular degeneration; OR: odds ratio; CI: confidence interval; NA: not available. N: number of study cohorts. Ref: reference.
Figure 2.
The forest plots of meta-analysis compared the allelic frequencies between polypoidal choroidal vasculopathy and control. Squares indicate the study-specific odds ratio (OR). The size of the box is proportional to the weight of the study. Horizontal lines indicate 95% confidence interval (CI). A diamond indicates the summary OR with its corresponding 95% CI. A: LOC387715 rs10490924; B: HTRA1 rs11200638; C: Complement factor H (CFH) rs1061170; D: CFH rs800292; E: CFH rs3753394; F: CFH rs1329428; G: CFH rs1410996; H: CFB rs415667; I: C2 rs547154; J: SERPING1 rs2511989; K: Elastin rs2301995; L: PEDF rs1136287.
Figure 3.
The forest plots of meta-analysis compared the allelic frequencies between polypoidal choroidal vasculopathy and age-related macular degeneration. Squares indicate the study-specific odds ratio (OR). The size of the box is proportional to the weight of the study. Horizontal lines indicate 95% confidence interval (CI). A diamond indicates the summary OR with its corresponding 95% CI. A: LOC387715 rs10490924; B: HTRA1 rs11200638; C: Complement factor H (CFH) rs1061170; D: CFH rs800292; E: Elastin rs2301995; F: PEDF rs1136287.
The SNP rs11200638 located at the promoter of HTRA1 was investigated in five studies [16,25,32,33,35]. Two articles [25,35] were from the same study group, and the earlier one [35] was excluded. The A allele was more prevalent in PCV than in the controls in all studies, with individual allelic ORs ranging from 2.24 to 4.15. The pooled allelic OR was 2.72 (95% CI: 2.04–3.63, p<0.00001, Figure 2B). No significant publication bias was detected (Egger’s test p=0.539). The pooled ORs were 6.43, 1.75, 2.95, 4.50, and 2.08 in the homozygote, heterozygote, dominant, recessive, and additive models, respectively (all p<0.001, Appendix 2). The distributions of rs11200638 in PCV and AMD were reported in two studies [16,25]. Although the frequencies of the A allele in PCV were lower than in AMD in both studies, neither the individual ORs nor the pooled OR (0.86, 95% CI: 0.64–1.16, p=0.33, Figure 3B) was statistically significant.
The association of CFH rs1061170 (Y402H) with PCV was investigated in five studies [21,22,27,32,35], among which one [35] was excluded because the authors were from the same group as those in a later article [27]. The C allele was more frequent in PCV than in the controls. The OR in an individual study was statistically significant in two studies [22,27] but not in the other two [21,32]. The pooled allelic OR was 1.72 (95% CI: 1.42–2.10, p<0.000001, Figure 2C). No significant publication bias was detected (Egger’s test p=0.912). The pooled ORs were statistically significant in the heterozygote (1.72), dominant (1.71), and additive (1.57) models, but not for the homozygote (1.48) or recessive (1.37) model (Appendix 3). There was no significant difference in the allele frequencies of rs1061170 between PCV and AMD, with a pooled OR of 0.91 (95% CI: 0.71–1.18, p=0.49, Figure 3C). The association of CFH rs800292 (I62V) with PCV was investigated in five studies [21,27,29,31,32]. The G allele was more frequent in PCV than in the controls, with a pooled allelic OR of 2.10 (95% CI: 1.87–2.37, p<0.00001, Figure 2D). No significant publication bias was detected (Egger’s test, p=0.780). The pooled ORs were 4.06, 1.92, 2.81, 2.42, and 1.67 in the homozygote, heterozygote, dominant, recessive, and additive models, respectively, with all p<0.00001 (Appendix 4). The allelic distributions of rs800292 in PCV and AMD were reported in two studies [27,29], but neither the individual studies nor the pooled analysis (OR=0.95, 95% CI: 0.79–1.16, p=0.62, Figure 3D) found a significant difference between PCV and AMD. The CFH rs3753394 was reported in two studies [21,32] and the pooled OR was 2.12 for the T allele (95% CI: 1.61–2.77, p<0.00001, Figure 2E). The CFH rs1329428 was reported in two studies [21,32] and the pooled OR was 1.74 for the C allele (95% CI: 1.33–2.28, p<0.0001, Figure 2F). The CFH rs1410996 was reported in two studies [21,22], and the pooled OR was 1.82 for the C allele (95% CI: 1.39–2.37, p<0.0001, Figure 2G).
At the CFB-C2 locus, the association of CFB rs415667 with PCV was investigated in three studies [21,22,32]. Neither the individual OR nor the pooled OR (0.79, 95% CI: 0.40–1.57, p=0.50, Figure 2H) was statistically significant. No significant publication bias was detected (Egger’s test p=0.907). Only one study compared the allele frequency of rs415667 between PCV and wet AMD, and the OR was 1.35 (p=0.50) [22]. The association of C2 rs547154 with PCV was investigated in three studies [21,22,32]. The individual ORs for the T allele ranged from 0.28 to 0.73, and only one was statistically significant [22]. The pooled OR was 0.56 (95% CI 0.36–0.87, p=0.01, Figure 2I). No significant publication bias was detected (Egger’s test p=0.601). Only one study compared the allele frequency of rs547154 between PCV and wet AMD, and the OR was 0.74 (p=0.82) [22].
Regarding the other genes, the SNP rs2511989 at the SERPING1 gene was reported in two studies [36,37], but none of the original studies or the pooled analysis (OR=0.97, 95% CI: 0.76–1.24, p=0.80, Figure 2J) showed a statistically significant association with PCV. A significant association between rs2301995 in elastin and PCV was reported in one study cohort [17], but not in the other three cohorts [19,20]. The pooled OR was 1.17 (95% CI: 0.97–1.41, p=0.11, Figure 2K). No significant publication bias was detected (Egger’s test p=0. 0.721). A significant difference in the distributions of rs2301995 between PCV and AMD was shown in one cohort [17] but not in the other three [19,20], and the pooled OR was 1.07 (95% CI: 0.68–1.70, p=0.77, Figure 3E, Egger’s test p=0.233). Association of PEDF rs1136287 with PCV was reported in two studies [38,39], but no individual studies or the pooled analysis showed a significant association (Figure 2L). The allelic distributions of rs1136287 in PCV and AMD were not significantly different (Figure 3F). The associations of SNPs at SOD2 [40], PEDF [38], TLR3 [41], 9p21 [42], RDBP [21], SKIV2L [21], and C3 [29] with PCV were reported in one article (Table 2). No meta-analysis was performed on these genes/loci.
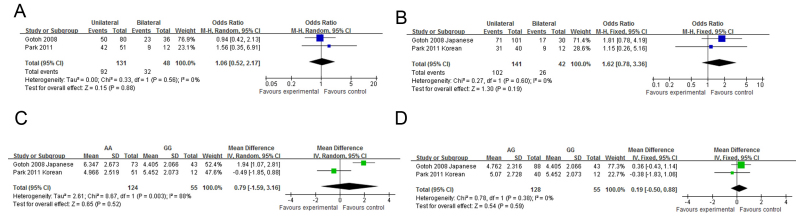
Genotype-phenotype correlations of LOC387715 rs10490924 were investigated in nine articles [18,23,30,31,33,34,43-45]. Four articles [18,23,30,44] were written by Sakurada’s group and another two [33,43] by Park’s group. Only the latest or largest data were included in the meta-analysis. Correlation of gender with rs10490924 was investigated in three studies [23,34,43]. Neither the original studies nor the meta-analysis showed a statistically significant difference in gender between different genotype groups (Figure 4A,B). One study [23] reported the mean age of onset in the TT genotype group was younger than in the GG genotype group. However, two other studies [34,43] and the pooled analysis showed a lack of significant difference in age of onset between different genotype groups (Figure 4C,D). A study reported that patients with the TT genotype of rs10490924 were more likely to have bilateral involvement compared to patients with the GG genotype [23]. However, a lack of significant correlation was reported in another study [33], and revealed by our meta-analysis (Figure 4E). Two studies reported that patients with the risk genotypes TT and TG had worse BCVA compared to those with the GG genotype [23,33]. However, in another study the result was reversed, although the difference was not statistically significant [34]. The meta-analysis did not support a significant difference in BCVA between the genotype groups (Figure 4G,H). Studies have reported that the FFA GLD in patients with the TT genotype was larger compared to those with the GG genotype in three studies [18,33,34]. Pooled analyses showed the mean difference was 1.21 mm (TT versus GG, 95% CI: 0.64–1.77, p<0.0001, Figure 4I), while the mean difference in FFA GLD between the TG and GG genotype groups was not statistically significant (Figure 4J). There was also a statistically significant difference in the ICGA GLD between the TT and GG genotype groups (pooled MD=0.57 mm, 95% CI: 0.17–0.96 mm, p=0.005, Figure 4K), as well as between the TG and GG genotype groups (pooled MD=0.46 mm, 95% CI: 0.05–0.87, p=0.03, Figure 4L). A study also reported that the T allele or TT genotype was associated with larger PCV (lesion size greater than 1 disc diameter) [45]. However, no mean or standard deviation for each genotype was reported in the article; therefore, it was not included in the meta-analysis. Association of rs10490924 with the risk of vitreous hemorrhage was reported in two studies [30,33]. The pooled OR was 6.52 (95% CI: 0.83–51.03, p=0.07, Figure 4M) and 1.00 (95% CI: 0.10–10.04, p=1.00, Figure 4N) for homozygote and heterozygote, respectively. Since the heterozygote OR was 1, we analyzed it using the recessive and allelic models, and the pooled ORs were 12.15 (95% CI: 2.72–54.21, p=0.001, Figure 4O) and 10.41 (95% CI: 2.47–43.88, p=0.001 Figure 4P), respectively. The BCVA 12 months after PDT or combined therapy was better in the GG genotype group than in the TT genotype group; the mean difference was 0.39 LogMAR (95% CI 0.10–0.68, p=0.008, Figure 4Q). The mean difference between the TG and GG genotype groups was 0.20 LogMAR lines but was not statistically significant (p=0.20, Figure 4R). The associations of bilaterality and lesion size of PCV with HTRA1 rs11200638 were also investigated [33,35]. However, neither the original studies nor the pooled analysis showed a significant correlation (Figure 5).
Figure 4.
The forest plots of meta-analysis compared the phenotypes of polypoidal choroidal vasculopathy between different genotypes of LOC387715 rs10490924. Blue squares indicate the study-specific odds ratio (OR). Green squares indicate the study-specific mean difference (MD). The size of the box is proportional to the weight of the study. Horizontal lines indicate 95% confidence interval (CI). A diamond indicates the summary OR (blue) or MD (green) with its corresponding 95% CI. A, C, E, G, I, K, M and Q: comparison between TT and GG; B, D, F, H, J, L, N and R: comparison between TG and GG. O: comparison between TT and TG + GG; P: comparison between T and G allele. A and B: Gender distribution; C and D: Age of onset; E and F: Bilaterality; G and H: Best-corrected visual acuity; I and J: Greatest linear diameter on fundus fluorescence angiography; K and L: Greatest linear diameter on indocyanine green angiography; M-P: Vitreous hemorrhage; Q and R: Best-corrected visual acuity at 12 months after photodynamic therapy or combined therapy.
Figure 5.
The forest plots of meta-analysis compared the phenotypes of polypoidal choroidal vasculopathy between different genotypes of HTRA1 rs11200638. Blue squares indicate the study-specific odds ratio (OR). Green squares indicate the study-specific mean difference (MD). The size of the box is proportional to the weight of the study. Horizontal lines indicate 95% confidence interval (CI). A diamond indicates the summary OR (blue) or MD (green) with its corresponding 95% CI. A and C: Comparison between TT and GG; B and D: Comparison between TG and GG. A and B: Bilaterality; C and D: Greatest linear diameter on fundus fluorescence angiography.
Discussion
In this systematic review and meta-analysis, we identified 33 genetic studies of PCV. After the individual results were pooled, the LOC387715 rs10490924, HTRA1 rs11200638, CFH rs1061170, rs800292, rs3753394, rs1329428 and rs1410996, and C2 rs547154 were found to be significantly associated with PCV. In addition, by comparing the variants between PCV and AMD, only the rs10490924 showed a statistical difference. Furthermore, by analyzing the genotype-phenotype correlation, we found that the risk genotypes of rs10490924 were associated with larger lesion size, greater chance of vitreous hemorrhage, and worse response to therapy in PCV.
To date, a group of genetic risk factors for AMD has been identified. This helps us understand not only the etiology and pathogenesis but also the diagnosis and management of this ophthalmic condition. In view of the phenotypic similarities between AMD and PCV, the genes for AMD are good candidates for genetic studies of PCV. Until now, almost all reported AMD-associated genes have been investigated in PCV, including LOC387715, HTRA1, CFH, C2, CFB, C3, SERPING1, PEDF, Elastin, TLR3, RDBP, and SKIV2L.
With this systematic review and meta-analysis, the association of LOC387715 rs10490924 was confirmed, with the overall allelic OR 2.27. The homozygote and heterozygote ORs were 4.90 and 1.74, respectively, suggesting an additive genetic effect. A study reported that LOC387715 encoded a mitochondrial membrane protein and was expressed in the retina [46]. The association of LOC387715 with PCV suggests that mitochondrial disorders may play an important role in PCV pathogenesis. Another variant at the 10q26 region, HTRA1 rs11200638, was also confirmed to be associated with PCV. A study reported that HTRA1 transgenic mice had retinal pigment epithelium induced choroidal branching vascular networks, polypoidal lesions, severe degeneration of the elastic laminae, and tunica media of choroidal vessels [47], suggesting that overexpression of HTRA1 may predispose individuals to PCV. To date, whether the LOC387715 or the HTRA1 at 10q26 is the gene responsible for AMD and PCV remains in question because of the strong linkage disequilibrium between them. However, this issue cannot be solved in our current meta-analysis, and awaits further functional characterizations. The associations of CFH rs1061170, rs800292, rs3753394, rs1329428, and rs1410996, and C2 rs547154 were also confirmed, suggesting that the complement system and inflammatory pathways may also play an important role in the pathogenesis of PCV. The variants RDBP rs3880457, SKIV2L rs2075702, and C3 rs2241394 were also reported in one article to be associated with PCV, suggesting a role for the immunological system in PCV. In contrast, polymorphisms at SERPING1, elastin, SOD2, PEDF, and TLR3 were not associated with PCV.
There are some common and distinct clinical characteristics between PCV and AMD. Both PCV and wet AMD usually involve older adults. However, patients with PCV tend to be younger. The prevalence of AMD is higher in Caucasians than in Asian and black populations, while the prevalence of PCV is higher in Asians and Africans than in Caucasians. Eyes with PCV usually lack drusen—a characteristic sign of early AMD. However, some cases have demonstrated clinical manifestations of PCV and dry or wet AMD. Although PDT and anti-vascular endothelial growth factor (VEGF) therapies are therapeutic opinions for PCV and AMD, the responses to treatments between these two diseases are different. PCV seemingly has a better response to PDT but poorer response to anti-VEGF agents such as bevacizumab [48]. In view of such controversies, whether PCV is a subtype of AMD or a specific entity of disorder remains unsolved, and one solution is to compare the genetic etiology of PCV and AMD.
Through a systematic review, we identified 22 articles reporting the genetic associations of 11 genes with PCV and AMD, including LOC387715, HTRA1, CFH, CFB, C2, SERPING1, elastin, SOD2, PEDF, TLR3, and 9p21. Among them, only LOC387715 rs10490924 was statistically different between PCV and AMD, with an allelic OR of 0.66 (95% CI: 0.57–0.76, p<0.00001). This difference suggests that although LOC387715 is associated with PCV and AMD, its effect could be less strong in PCV than in AMD. In view of the distinct difference in the prevalence of PCV between Caucasian and Asian populations with a comparable frequency of the risk allele, there could be yet-to-be-identified genetic or environmental factors guiding the development of each phenotype. However, the variants in other genes were not statistically different between PCV and AMD, including HTRA1 rs11200638, CFH rs1061170, CFH rs800292, and C2 rs547154. The failure in differentiation may be due to the small overall ample size from a limited number of studies, especially for the eight variants studied in only one article, and needs further investigation.
Genotype-phenotype correlation may shed light on the pathogenesis and clinical management of disease. In this meta-analysis, we found that LOC387715 rs10490924 was statistically associated with lesion size and vitreous hemorrhage in PCV, with the risk genotype TT associated with a larger lesion and a greater risk of vitreous hemorrhage, that is, more severe phenotypes. This may support the role of LOC387715 in the pathogenesis of PCV. The association of rs10490924 with gender, age of onset, bilaterality, and BCVA is controversial. The association of HTRA1 rs11200638 and bilaterality or BCVA is also controversial. The rs10490924 genotype was also correlated with the therapeutic response in PCV. The risk genotypes, TT or TG, are associated with poorer therapeutic response, and the mean difference was 0.39 and 0.20 LogMAR lines, respectively. These results provide pharmacogenetics evidence for estimating the visual prognosis after therapies for PCV.
One advantage of this systematic review and meta-analysis is an overview of all published genetic studies in PCV, demonstrating the overall effects and, at least partially, resolving the controversies. There are also some limitations in this study. First, the number of original studies was limited for some genes, and the conclusions may not be sufficiently strong. Second, the quality of the meta-analysis depends on the quality of the original studies. In some studies, the HWE was not examined in the control group; thus, quality control was lacking. Third, there was significant heterogeneity among studies of some polymorphisms. The source of heterogeneity may include the small sample size in some studies and the different clinical characteristics of patients in different studies. A random effect model was used for the meta-analysis when statistically significant heterogeneity was met. Since most reports were by Eastern Asians, especially Japanese, and there was only one report by one group about a Caucasian population, no subgroup analysis was performed. Fourth, there may be an imbalance between the case and control groups. For example, the gender or age may be different between groups in some articles. The imbalance can be corrected with multivariate analysis in the original studies, but cannot be handled in the meta-analysis. This may also be a cause of heterogeneity. Fifth, we did not search Japanese databases. Although some Japanese medical journals are indexed in PubMed and Embase, we still may have missed some articles in Japanese or other languages. Finally, only one GWAS of PCV [29] has been published, and we could not perform a meta-analysis of GWASs to identify new loci/polymorphisms associated with PCV.
In conclusion, in this systematic review and meta-analysis of 33 articles reporting genetic associations in PCV, polymorphisms at LOC387715, HTRA1, CFH, and C2 were found to be significantly associated with PCV. LOC387715 rs10490924 was the only variant showing a significant difference between PCV and AMD. This variant was also correlated with lesion size, vitreous hemorrhage, and therapeutic response in PCV. Further investigations are necessary to confirm the roles of those genes reported in a limited number of original studies.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (30901646 and 81170853), Guangdong Science and Technology Project (2011B031300013), Guangdong Medical Research Foundation (B2010230), and Science and Technology Project of Shantou City, China (2009–70).
Appendix 1. Meta-analysis of the association of LOC387715 rs10490924 with polypoidal choroidal vasculopathy in different genetic models.
Squares indicate study-specific OR; the size of the box is proportional to the weight of the study; horizontal lines indicate 95% confidence interval (CI); diamond indicates summary OR with its corresponding 95% CI. A: homozygote; B: heterozygote; C: dominant model; D: recessive model; E: additive model. To access the data, click or select the words “Appendix 1.” This will initiate the download of a compressed (pdf) archive that contains the file.
Appendix 2. Meta-analysis of the association of HTRA1 rs11200638 with polypoidal choroidal vasculopathy in different genetic models.
Squares indicate study-specific OR; the size of the box is proportional to the weight of the study; horizontal lines indicate 95% confidence interval (CI); diamond indicates summary OR with its corresponding 95% CI. A: homozygote; B: heterozygote; C: dominant model; D: recessive model; E: additive model. To access the data, click or select the words “Appendix 2.” This will initiate the download of a compressed (pdf) archive that contains the file.
Appendix 3. Meta-analysis of the association of Complement factor H rs1061170 with polypoidal choroidal vasculopathy in different genetic models.
Squares indicate study-specific OR; the size of the box is proportional to the weight of the study; horizontal lines indicate 95% confidence interval (CI); diamond indicates summary OR with its corresponding 95% CI. A: homozygote; B: heterozygote; C: dominant model; D: recessive model; E: additive model. To access the data, click or select the words “Appendix 3.” This will initiate the download of a compressed (pdf) archive that contains the file.
Appendix 4. Meta-analysis of the association of Complement factor H rs800292 with polypoidal choroidal vasculopathy in different genetic models.
Squares indicate study-specific OR; the size of the box is proportional to the weight of the study; horizontal lines indicate 95% confidence interval (CI); diamond indicates summary OR with its corresponding 95% CI. A: homozygote; B: heterozygote; C: dominant model; D: recessive model; E: additive model. To access the data, click or select the words “Appendix 4.” This will initiate the download of a compressed (pdf) archive that contains the file.
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 2.Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–10. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 5.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 6.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–53. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–92. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–3. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 10.Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39:1200–1. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 14.Ennis S, Jomary C, Mullins R, Cree A, Chen X, Macleod A, Jones S, Collins A, Stone E, Lotery A. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008;372:1828–34. doi: 10.1016/S0140-6736(08)61348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotoh N, Kuroiwa S, Kikuchi T, Arai J, Arai S, Yoshida N, Yoshimura N. Apolipoprotein E polymorphisms in Japanese patients with polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Am J Ophthalmol. 2004;138:567–73. doi: 10.1016/j.ajo.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007;144:608–12. doi: 10.1016/j.ajo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. Elastin gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:1101–5. doi: 10.1167/iovs.07-1145. [DOI] [PubMed] [Google Scholar]
- 18.Sakurada Y, Kubota T, Imasawa M, Mabuchi F, Tanabe N, Iijima H. Association of LOC387715 A69S genotype with visual prognosis after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2010;30:1616–21. doi: 10.1097/IAE.0b013e3181e587e3. [DOI] [PubMed] [Google Scholar]
- 19.Lima LH, Merriam JE, Freund KB, Barbazetto IA, Spaide RF, Yannuzzi LA, Allikmets R. Elastin rs2301995 polymorphism is not associated with polypoidal choroidal vasculopathy in caucasians. Ophthalmic Genet. 2011;32:80–2. doi: 10.3109/13816810.2010.544362. [DOI] [PubMed] [Google Scholar]
- 20.Yamashiro K, Mori K, Nakata I, Tsuchihashi T, Horie-Inoue K, Nakanishi H, Tsujikawa A, Saito M, Iida T, Yamada R, Matsuda F, Inoue S, Awata T, Yoneya S, Yoshimura N. Association of elastin gene polymorphism to age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2011;52:8780–4. doi: 10.1167/iovs.11-8205. [DOI] [PubMed] [Google Scholar]
- 21.Kondo N, Honda S, Kuno S, Negi A. Coding variant I62V in the complement factor H gene is strongly associated with polypoidal choroidal vasculopathy. Ophthalmology. 2009;116:304–10. doi: 10.1016/j.ophtha.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Lima LH, Schubert C, Ferrara DC, Merriam JE, Imamura Y, Freund KB, Spaide RF, Yannuzzi LA, Allikmets R. Three major loci involved in age-related macular degeneration are also associated with polypoidal choroidal vasculopathy. Ophthalmology. 2010;117:1567–70. doi: 10.1016/j.ophtha.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurada Y, Kubota T, Imasawa M, Mabuchi F, Tateno Y, Tanabe N, Iijima H. Role of complement factor H I62V and age-related maculopathy susceptibility 2 A69S variants in the clinical expression of polypoidal choroidal vasculopathy. Ophthalmology. 2011;118:1402–7. doi: 10.1016/j.ophtha.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Gotoh N, Nakanishi H, Hayashi H, Yamada R, Otani A, Tsujikawa A, Yamashiro K, Tamura H, Saito M, Saito K, Iida T, Matsuda F, Yoshimura N. ARMS2 (LOC387715) variants in Japanese patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;147:1037–41. doi: 10.1016/j.ajo.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Gotoh N, Yamashiro K, Nakanishi H, Saito M, Iida T, Yoshimura N. Haplotype analysis of the ARMS2/HTRA1 region in Japanese patients with typical neovascular age-related macular degeneration or polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2010;54:609–14. doi: 10.1007/s10384-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi H, Yamashiro K, Yamada R, Gotoh N, Hayashi H, Nakata I, Saito M, Iida T, Oishi A, Kurimoto Y, Matsuo K, Tajima K, Matsuda F, Yoshimura N. Joint effect of cigarette smoking and CFH and LOC387715/HTRA1 polymorphisms on polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2010;51:6183–7. doi: 10.1167/iovs.09-4948. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi H, Yamashiro K, Gotoh N, Nakanishi H, Nakata I, Tsujikawa A, Otani A, Saito M, Iida T, Matsuo K, Tajima K, Yamada R, Yoshimura N. CFH and ARMS2 Variations in Age-Related Macular Degeneration, Polypoidal Choroidal Vasculopathy, and Retinal Angiomatous Proliferation. Invest Ophthalmol Vis Sci. 2010;51:5914–9. doi: 10.1167/iovs.10-5554. [DOI] [PubMed] [Google Scholar]
- 28.Fuse N, Mengkegale M, Miyazawa A, Abe T, Nakazawa T, Wakusawa R, Nishida K. Polymorphisms in ARMS2 (LOC387715) and LOXL1 genes in the Japanese with age-related macular degeneration. Am J Ophthalmol. 2011;151:550–6. doi: 10.1016/j.ajo.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 29.Goto A, Akahori M, Okamoto H, Minami M, Terauchi N, Haruhata Y, Obazawa M, Noda T, Honda M, Mizota A, Tanaka M, Hayashi T, Tanito M, Ogata N, Iwata T. Genetic analysis of typical wet-type age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese population. J Ocul Biol Dis Infor. 2009;2:164–75. doi: 10.1007/s12177-009-9047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakurada Y, Kubota T, Mabuchi F, Imasawa M, Tanabe N, Iijima H. Association of LOC387715 A69S with vitreous hemorrhage in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2008;145:1058–62. doi: 10.1016/j.ajo.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Nakayama T, Mori R, Sato N, Kawamura A, Mizutani Y, Yuzawa M. Associations of Complement Factor H (CFH) and Age-Related Maculopathy Susceptibility 2 (ARMS2) Genotypes with Subtypes of Polypoidal Choroidal Vasculopathy. Invest Ophthalmol Vis Sci. 2011;52:7441–4. doi: 10.1167/iovs.11-7546. [DOI] [PubMed] [Google Scholar]
- 32.Lee KY, Vithana EN, Mathur R, Yong VH, Yeo IY, Thalamuthu A, Lee MW, Koh AH, Lim MC, How AC, Wong DW, Aung T. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:2613–9. doi: 10.1167/iovs.07-0860. [DOI] [PubMed] [Google Scholar]
- 33.Park DH, Kim IT. Association of ARMS2/HTRA1 variants with polypoidal choroidal vasculopathy phenotype in a Korean population. Jpn J Ophthalmol. 2012;56:60–7. doi: 10.1007/s10384-011-0089-0. [DOI] [PubMed] [Google Scholar]
- 34.Bessho H, Honda S, Kondo N, Negi A. The association of age-related maculopathy susceptibility 2 polymorphisms with phenotype in typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Mol Vis. 2011;17:977–82. [PMC free article] [PubMed] [Google Scholar]
- 35.Gotoh N, Yamada R, Nakanishi H, Saito M, Iida T, Matsuda F, Yoshimura N. Correlation between CFH Y402H and HTRA1 rs11200638 genotype to typical exudative age-related macular degeneration and polypoidal choroidal vasculopathy phenotype in the Japanese population. Clin Experiment Ophthalmol. 2008;36:437–42. [PubMed] [Google Scholar]
- 36.Li M, Wen F, Zuo C, Zhang X, Chen H, Huang S, Luo G. SERPING1 polymorphisms in polypoidal choroidal vasculopathy. Mol Vis. 2010;16:231–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Nakata I, Yamashiro K, Yamada R, Gotoh N, Nakanishi H, Hayashi H, Tsujikawa A, Otani A, Saito M, Iida T, Oishi A, Matsuo K, Tajima K, Matsuda F, Yoshimura N. Association between the SERPING1 gene and age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese. PLoS ONE. 2011;6:e19108. doi: 10.1371/journal.pone.0019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bessho H, Kondo N, Honda S, Kuno S, Negi A. Coding variant Met72Thr in the PEDF gene and risk of neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Mol Vis. 2009;15:1107–14. [PMC free article] [PubMed] [Google Scholar]
- 39.Wu K, Wen F, Zuo C, Li M, Zhang X, Chen H, Zeng R. Lack of association with PEDF Met72Thr variant in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in a Han Chinese population. Curr Eye Res. 2012;37:68–72. doi: 10.3109/02713683.2011.618289. [DOI] [PubMed] [Google Scholar]
- 40.Kondo N, Bessho H, Honda S, Negi A. SOD2 gene polymorphisms in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Mol Vis. 2009;15:1819–26. [PMC free article] [PubMed] [Google Scholar]
- 41.Sng CC, Cackett PD, Yeo IY, Thalamuthu A, Venkatraman A, Venkataraman D, Koh AH, Tai ES, Wong TY, Aung T, Vithana EN. Toll-like receptor 3 polymorphism rs3775291 is not associated with choroidal neovascularization or polypoidal choroidal vasculopathy in Chinese subjects. Ophthalmic Res. 2011;45:191–6. doi: 10.1159/000321387. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Wen F, Zuo C, Li M, Chen H, Wu K. Association of genetic variation on chromosome 9p21 with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:8063–7. doi: 10.1167/iovs.11-7820. [DOI] [PubMed] [Google Scholar]
- 43.Park DH, Kim IT. Loc387715/Htra1 variants and the response to combined photodynamic therapy with intravitreal bevacizumab for polypoidal choroidal vasculopathy. Retina. 2012;32:299–307. doi: 10.1097/IAE.0b013e318225290f. [DOI] [PubMed] [Google Scholar]
- 44.Sakurada Y, Kubota T, Imasawa M, Tsumura T, Mabuchi F, Tanabe N, Iijima H. Angiographic lesion size associated with LOC387715 A69S genotype in subfoveal polypoidal choroidal vasculopathy. Retina. 2009;29:1522–6. doi: 10.1097/IAE.0b013e3181af0d72. [DOI] [PubMed] [Google Scholar]
- 45.Tsujikawa A, Ojima Y, Yamashiro K, Nakata I, Ooto S, Tamura H, Nakanishi H, Hayashi H, Otani A, Yoshimura N. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151:961–72. doi: 10.1016/j.ajo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci USA. 2007;104:16227–32. doi: 10.1073/pnas.0703933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones A, Kumar S, Zhang N, Tong Z, Yang JH, Watt C, Anderson J, Amrita, Fillerup H, McCloskey M, Luo L, Yang Z, Ambati B, Marc R, Oka C, Zhang K, Fu Y. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc Natl Acad Sci USA. 2011;108:14578–83. doi: 10.1073/pnas.1102853108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, Wong TY, Aung T. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29. doi: 10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Nakata I, Yamashiro K, Yamada R, Gotoh N, Nakanishi H, Hayashi H, Tsujikawa A, Otani A, Ooto S, Tamura H, Saito M, Saito K, Iida T, Oishi A, Kurimoto Y, Matsuda F, Yoshimura N. Genetic variants in pigment epithelium-derived factor influence response of polypoidal choroidal vasculopathy to photodynamic therapy. Ophthalmology. 2011;118:1408–15. doi: 10.1016/j.ophtha.2010.12.011. [DOI] [PubMed] [Google Scholar]