SUMMARY
The histone H3K27 methyltransferase EZH2 plays an important role in oncogenesis, by mechanisms that are incompletely understood. Here we show that the JmjC domain histone H3 demethylase NDY1 synergizes with EZH2 to silence the EZH2 inhibitor miR-101. NDY1 and EZH2 repress miR-101 by binding its promoter in concert, via a process triggered by upregulation of NDY1. Whereas EZH2 binding depends on NDY1, the latter binds independently of EZH2. However, both are required to repress transcription. NDY1 and EZH2 acting in concert, upregulate EZH2 and stabilize the repression of miR-101 and its outcome. NDY1 is induced by FGF-2 via CREB phosphorylation and activation, downstream of DYRK1A, and mediates the FGF-2 and EZH2 effects on cell proliferation, migration and angiogenesis. The FGF-2-NDY1/EZH2-miR-101-EZH2 axis described here, was found to be active in bladder cancer. These data delineate a novel oncogenic pathway that functionally links FGF-2 with EZH2 via NDY1 and miR-101.
INTRODUCTION
Developing tumors progress through multiple stages. Thus, a carcinoma may start in a given organ as a “carcinoma in situ” and it may progress into an invasive carcinoma and then into a metastatic tumor. The progressive acquisition of malignant properties depends on sequential genetic and epigenetic changes, which occur either in a cell autonomous fashion or in response to environmental cues from the surrounding stroma. Epigenetic changes depend in part on the activity of polycomb repressor complexes (Cao et al., 2002). The co-recruitment of two of these complexes (PRC1 and PRC2) to Polycomb responsive genes promotes trimethylation of histone H3 at K27 and ubiquitination of histone H2A at K119 and represses gene expression (Gil et al., 2005; Simon and Kingston, 2009).
Enhancer of zeste homolog-2 (EZH2) is a histone H3K27 methyltransferase that is associated with the Polycomb repressor complex-2 (PRC2). Overexpression of EZH2 in tumor cells, which is common in both hematopoietic malignancies and solid tumors, promotes cell proliferation and cell migration and invasiveness, while overespression of EZH2 in the tumor stroma stimulates angiogenesis (Gil et al., 2005; Lu et al., 2010). Finally, pharmacologic disruption of the EZH2-containing PRC2 complex selectively induces apoptosis in cancer cells (Tan et al., 2007). The upregulation of EZH2 in different tumors may be transcriptional or postranscriptional. The latter may be due to the downregulation of miR-101, which targets EZH2 (Varambally et al., 2008). Although the downregulation of miR-101 may be due to deletion of the miR-101 locus, in many tumors it is due to unknown mechanisms (Friedman et al., 2009; Varambally et al., 2008). The data presented in this report show that the repression of miR-101 can be the result of chromatin modifications induced by the overexpression of the recently described histone demethylase NDY1.
NDY1/KDM2B is a histone H3K36me2, H3K36me1 and H3K4me3 demethylase that is activated by provirus integration in Moloney murine leukemia virus (MoMuLV)-induced rodent T cell lymphomas (Pfau et al., 2008). The upregulation of NDY1 in these tumors is therefore cell-autonomous. Here we show that the upregulation of NDY1 in tumor cell lines and primary tumors may also be driven by growth factor signals, prominent among them being signals initiated by Fibroblast Growth Factor-2 (FGF-2). FGF-2 or basic fibroblast growth factor (bFGF) is one of the 23 members of the FGF growth factor family (Wiedlocha and Sorensen, 2004). FGF-2 is produced by a variety of cell types and is presented to the FGF receptors (primarily FGFR-1 and -2), by binding to various heparan sulfate proteoglycans (HSPGs) whose expression and composition regulate its activity (Sanderson et al., 2005). In human cancer, FGF-2 is produced by the tumor cells or by the surrounding stroma and promotes tumor cell proliferation, migration and invasiveness, as well as angiogenesis and the cycling of cancer stem cells (Cao et al., 2008; Dvorak et al., 2006). In addition, it promotes the cycling of normal stem cells during development (Dvorak et al., 2006; Liu et al., 2007). Its expression is under the control of a variety of transcription factors that are known to contribute to oncogenesis (Fahmy and Khachigian, 2007; Hara et al., 2007; Wu et al., 2006).
The dramatic effects of FGF-2 in cancer and stem cells result from FGF-2-induced shifts in gene expression. In this paper we present evidence for a novel pathway that may promote such shifts in FGF-2-stimulated cells. Specifically we show that FGF-2 upregulates NDY1 via CREB phosphorylation and activation, downstream of the DYRK1A kinase. The upregulation of NDY1 triggers the recruitment of basally expressed EZH2, which binds and represses the miR-101 locus in concert with NDY1. The repression of miR-101 leads to the upregulation of EZH2, which in addition to its direct effects on gene expression, stabilizes the NDY1-initiated repression of miR-101 and regulates miR-101 target genes. The FGF-2-NDY1/EZH2-miR-101-EZH2 axis was documented in primary fibroblasts in culture and in FGF-2-producing human tumor cell lines. The same axis was also active in a set of human bladder carcinomas. These data identify a novel signaling pathway that links FGF-2 to EZH2 and other miR-101 target genes in human cancer and they show that the link is epigenetically regulated via NDY1.
RESULTS
The overexpression of NDY1 in mouse embryo fibroblasts upregulates EZH2 by downregulating miR-101
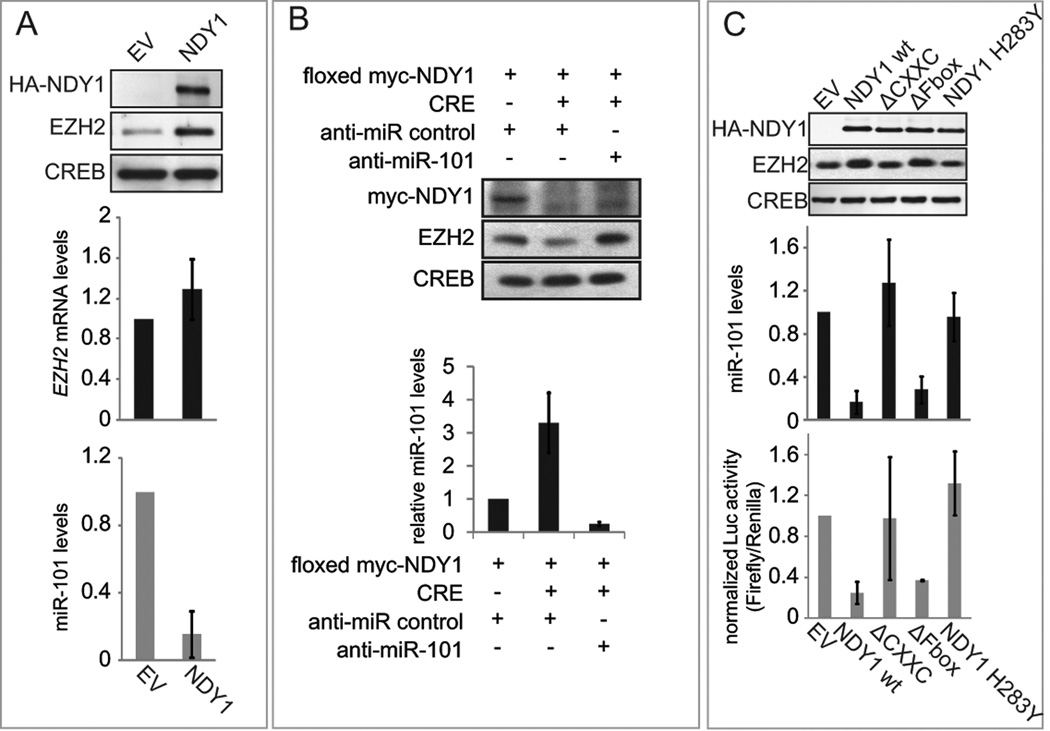
Transduction of wild type MEFs with MigR1-based retroviral constructs of NDY1 bypasses replicative senescence and promotes immortalization (Pfau et al., 2008) by repressing the lnk4a/Arf locus, in concert with EZH2, which is upregulated in NDY1-expressing cells (Tzatsos et al., 2009). Here we confirmed that NDY1 upregulates both EZH2 and the global levels of histone H3K27me3. Also, we showed that it represses the expression of EZH2 targets genes (Fig S1A). However the upregulation of the EZH2 mRNA was only modest, suggesting that NDY1 upregulates EZH2 via post transcriptional mechanisms (Fig 1A, upper and middle panels, Fig S1A).
Figure 1. NDY1 downregulates miR-101 and upregulates EZH2.
(A) (Left panel) Nuclear extracts of MEFs transduced with HA-NDY1 or pBabe (EV) were probed with the indicated antibodies. (Middle panel) EZH2 mRNA levels were measured by real time RT-PCR. (Right panel) miR-101 levels were measured by real time RT-PCR. The data are presented as mean ± SD.
(B) MEFs transduced with the loxp.myc-NDY1.loxp MigR1 construct were super-infected with MigR1-Cre or the empty vector. Subsequently, they were transfected with anti-miR-101 or anti-miR-control. (Top panel) Nuclear lysates harvested from these cells were probed with the indicated antibodies. (Bottom panel) miR-101 levels were measured in the same loxP.myc-NDY1.loxP-transduced MEFs by real time RT-PCR. The data are presented as mean ± SD.
(C) (Upper panel) Nuclear lysates of MEFs transduced with wild type or mutant NDY1 were probed with the indicated antibodies. (Middle panel) MiR-101 levels were measured in the cells shown in the upper panel, by real time RT-PCR. (Lower panel) The same cells were transfected with a miR-101 promoter-luciferase reporter construct (Fig S1E) and the relative luciferase activities in the transfected cells were measured as in Fig S1B. The data are presented as mean ± SD.
Recent studies had shown that EZH2 is regulated posttranscriptionally by miR-101 (Friedman et al., 2009; Varambally et al., 2008). This suggested that NDY1 may regulate EZH2 via miR-101. The results in Figure 1A (bottom panel) provided support to this hypothesis by showing that ectopic expression of NDY1 indeed represses miR-101. Deletion of myc-NDY1 by Cre, in MEFs transduced with a floxed myc-NDY1 construct, led to the rapid upregulation of miR-101 and the parallel downregulation of EZH2 (Fig S1B, left and middle panels). Transduction of the cells in which myc-NDY1 was deleted with anti-miR-101, restored EZH2 expression (Fig 1B) and confirmed that the upregulation of EZH2 by NDY1 is due to the repression of miR-101. In agreement with the preceding findings, the activity of a miR-101-luciferase reporter (Fig S1E) was enhanced upon excision of the floxed myc-NDY1 transgene (Fig S1B, right).
To test the hypothesis that miR-101 is responsible for the regulation of EZH2 by NDY1, we first examined whether miR-101 regulates the mouse EZH2 gene (Fig S1C). Transfection of miR-101 confirmed that it inhibits the expression of EZH2 both in wild type MEFs, and in MEFs transduced with HA-NDY1 (Fig S1D). The functional role of the NDY1-miR-101 module, was confirmed with additional experiments, which showed that HA-NDY1 regulates the expression of Ataxin-1 (Fig S1F), another validated target of miR-101 (Lee et al., 2008).
To determine whether the NDY1-induced repression of miR-101 depends on the demethylase activity of NDY1, we probed nuclear lysates of early passage MEFs transduced with the indicated wild type or mutant NDY1 constructs (Fig 1C, upper) (Pfau et al., 2008), with anti-HA (NDY1), anti-EZH2 or anti-CREB antibodies. The results revealed that only the wild type NDY1 and the ΔFbox mutant upregulate EZH2 and repress miR-101 (Fig 1C, upper and middle). Transfection of the miR-101 promoter/luciferase reporter construct (Fig S1E) in the same cells confirmed that only the wild type NDY1 and NDY1-ΔFbox repress the activity of the promoter (Fig 1C, bottom). These data collectively suggest that NDY1 represses miR-101 by binding the miR-101 locus and directing the regional modification of histones.
NDY2/KDM2A, an NDY1 homolog, shares both enzymatic activity and substrate specificity with NDY1 and immortalizes MEFs (Pfau et al., 2008). Here we addressed the question whether NDY2/KDM2A also represses miR-101. To our surprise, we found that it does not (Fig S1G), despite its sequence and enzymatic similarities with NDY1.
The repression of miR-101 by NDY1 depends on the concerted binding of NDY1 and EZH2 to the miR-101 locus
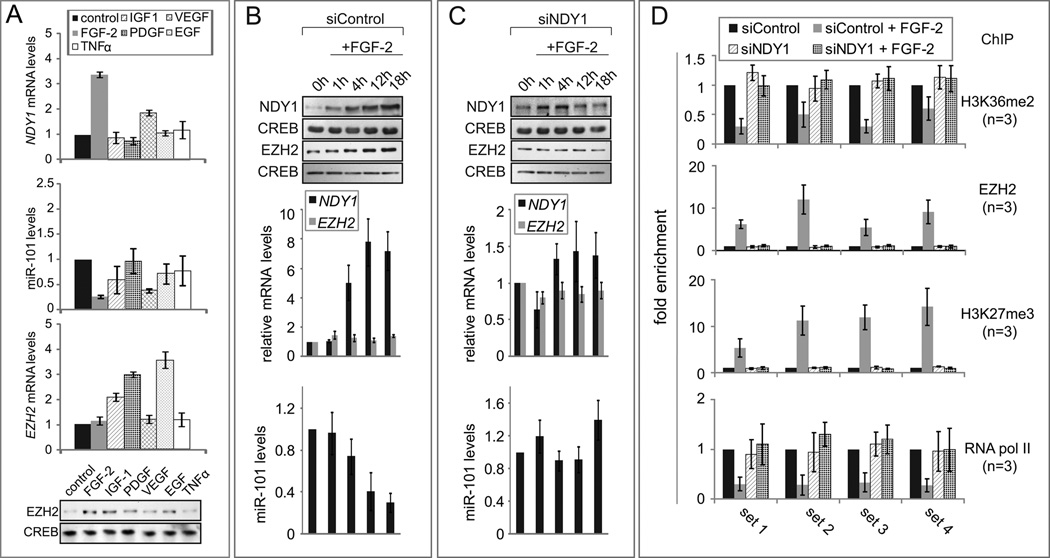
Chromatin immunoprecipitation (ChIP) addressing the binding of NDY1 and EZH2 to the miR-101 locus and the neighboring DNA region revealed that they bind, together with BMI1, at overlapping sites (Fig 2A, HA-NDY1, EZH2 and BMI1) and that their binding is associated with changes in histone H3 mehylation at K36 and K27 (Fig 2A, H3K36me2 and H3K27me3). This leads to transcriptional repression of miR-101 as evidenced by the displacement of RNA polymerase II (Fig 2A, RNA pol II). The NDY1 binding and its consequences were specific for miR-101, as they were not observed in the prdx2 locus (negative control) (Fig S2A). These findings suggest that NDY1 binds DNA in concert with EZH2 and represses transcription by coupling histone H3K36me2 demethylation to histone H3K27 trimethylation.
Figure 2. Overexpressed NDY1 recruits basally expressed EZH2, which binds in concert with NDY1 to the miR-101 locus.
(A) NDY1 silences miR-101 by binding the locus in concert with EZH2 and BMI1. (Upper panel) miR-101 (box) and flanking sequences. Set 1 through Set 4 are the primer sets used for ChIP. The numbers above the line show the position of the primers relative to the transcriptional start site. (Lower panels) MEFs were transduced with EV or pBabe-HA-NDY1 and they were used for ChIP with the indicated antibodies. The bars show the fold change in binding (mean ± SD) in the NDY1/KBM2B relative to the vector-transduced cells.
(B) In the absence of EZH2, NDY1 binds to the promoter of miR-101 but it does not silence its expression. MEFs were transduced with a MigR1 construct of NDY1 or with the EV and they were superinfected with lentiviral shEZH2 or with shControl. (Left panel) MiR-101 levels measured by real time RT-PCR. Data shown as mean ± SD. (Right panel) The same cells were used for ChIP with the indicated antibodies. The bars show the fold change in NDY1 binding or H3K36me2 abundance (mean ± SD) relative to the EV-shControl-transduced cells.
(C) EZH2 overexpression does not silence the expression of miR-101. MEFs were transduced with a pBabe construct of EZH2 or with the EV. (Left and middle panel) NDY1 mRNA and miR-101 levels were measured by real time RT-PCR. Data are expressed as mean ± SD. (Right panel) The same cells were used for ChIP with the indicated antibodies. The bars show the binding (mean ± SD) in EZH2 relative to vector-transduced cells.
(D) The demethylase activity of NDY1 is essential for the recruitment of EZH2 to the miR-101 locus and the silencing of miR-101. MEFs were transduced with pBabe/HA-NDY1wt, pBabe/HA-NDY1 H283Y or with the empty vector. Transduced cells were used for ChIP with the indicated antibodies. The bars show the fold change in HA-NDY1/EZH2 binding and H3K36me2 abundance (mean ± SD) in NDY1wt or NDY1H283Y relative to the vector-transduced cells.
To determine whether EZH2, a known transcriptional repressor (Schuettengruber et al., 2007), is required for the repression of miR-101 by NDY1, we transduced MEFs with a MigR1 construct of myc-NDY1, or with empty MigR1 (EV), and we super-infected them with a pLKO.1-shEZH2 lentiviral construct, or with pLKO.1-shControl. The efficiency of the knockdown was confirmed by western blotting (Fig S2B). Real time RT-PCR addressing the expression of miR-101 (Fig 2B, Left panel), and ChIP analyses addressing the binding of myc-NDY1 and the abundance of H3K36me2 in the miR-101 locus (Fig 2B, Right panel), revealed that EZH2 is not required for NDY1 binding and the regional demethylation of histone H3K36me2, but it is required for the silencing of miR-101.
To determine whether EZH2 is also sufficient for the repression of miR-101, we overexpressed EZH2 in MEFs and we examined whether it binds and represses the miR-101 locus, in the absence of NDY1 upregulation. The overexpression of EZH2 was confirmed by western blotting (Fig S1C). Real time RT-PCR revealed that EZH2 does not alter the expression of either NDY1, or miR-101 (Fig 2C, Left and Middle panels). Moreover, ChIP analyses revealed that in the absence of NDY1 upregulation, the overexpression of EZH2 does not increase the binding of EZH2 to the miR-101 locus. We conclude that although EZH2 is required for the repression of miR-101, its binding to the miR-101 locus and its silencing activity depend on the overexpression of NDY1.
Given that overexpression of the demethylase-deficient NDY1 mutant in MEFs failed to repress miR-101 (Fig 1C), we hypothesized that the binding of EZH2 to the miR-101 promoter in cells overexpressing NDY1 may depend on H3K36me2 demethylation. ChIP assays on MEFs transduced with wild type NDY1, its JmjC domain mutant NDY1 H283Y, or the empty vector revealed that both the wild type and the mutant NDY1 bind to the locus, but only the wild type downregulates the abundance of histone H3K36me2 (Fig 2D). EZH2 binding to the miR-101 locus was also increased, but only in cells with low abundance of H3K36me2 (Fig 2D). This suggests that H3K36me2 may hinder the binding of EZH2 to the miR-101 promoter, and that NDY1 binds the promoter in concert with EZH2 by downregulating the abundance of H3K36me2.
The NDY1-miR-101-EZH2 axis is regulated by FGF-2 and VEGF
To determine whether NDY1 is a physiological regulator of miR-101 and EZH2 we knocked down NDY1 in cultured MEFs. This led to the slight downregulation of EZH2 but it did not affect the expression of miR-101 (Fig S3A), suggesting that miR-101 and EZH2 are not under the control of NDY1 in MEFs cultured under standard conditions.
MEFs passaged in culture downregulate NDY1 (Tzatsos et al., 2009) and EZH2 (Bracken et al., 2007; Tzatsos et al., 2009) and undergo senescence. A repeat of this experiment showed that whereas both NDY1 and EZH2 are downregulated during senescence, miR-101 is not upregulated (Fig S3B). We conclude that miR-101 is not responsible for the downregulation of EZH2 during senescence.
The preceding data suggested that the physiological activity of the NDY1-miR-101-EZH2 pathway is reserved for signals other than the ones that regulate EZH2 expression in cultured MEFs growing normally or undergoing senescence. To identify these signals, we stimulated serum-starved MEFs with FGF-2, IGF-1, PDGF, EGF, VEGF or TNFα. Of these factors only FGF-2, and to a lesser degree VEGF, induced EZH2 at the protein level, 24 hours later, by downregulating miR-101. IGF-1, PDGF and EGF induced EZH2 at the RNA level via pathways that are independent of NDY1 and miR-101 (Fig 3A). We conclude that FGF-2 and VEGF are physiological regulators of the NDY1-miR-101-EZH2 axis. The levels of FGF-2-induced NDY1 were comparable to the levels of exogenous protein in cells transduced with an NDY1 retroviral construct (Supplemental fig. S3E).
Figure 3. FGF-2 induces NDY1, which upregulates EZH2 by repressing miR-101.
(A) Serum starved MEFs were stimulated with growth factors and they were harvested 12 hours later. (Top three panels) NDY1, EZH2 and miR-101 were measured in these lysates by real time RT-PCR. Data expressed as mean ± SD. (Lower panel) Nuclear lysates were probed with anti-EZH2 or anti-CREB.
(B) (Upper panel) Nuclear extracts of siControl-transfected MEFs were harvested after stimulation with FGF-2 and they were probed with the indicated antibodies. (Middle panel) NDY1 and EZH2 mRNAs were measured with real time RT-PCR. (Lower panel) miR-101 levels were measured with real time RT-PCR. Data in the middle and lower panels are expressed as mean ± SD.
(C) The same experiments as in the B were done using MEFs transduced with siNDY1.
(D) FGF-2-induced NDY1 upregulation promotes the recruitment of EZH2, which binds the miR-101 locus, in concert with NDY1. MEFs were transfected with siNDY1 or siControl. Serum starved cells were stimulated with FGF-2 and they were used for ChIP. Bars show the fold change in the binding of EZH2 and RNA Pol II, or in the abundance of selected histone modifications in FGF-2-stimulated, relative to unstimulated-control cells (mean ± SD).
The activation of the NDY1-miR-101-EZH2 axis by FGF-2 was confirmed with experiments in which FGF-2 was used to stimulate serum-starved untransfected MEFs (Fig S3C) or MEFs transfected with siNDY1 or siControl. This experiment showed that FGF-2 upregulates NDY1, represses miR-101 and upregulates EZH2 at the protein, but not at the RNA level and that knockdown of NDY1 blocks the repression of miR-101 and the upregulation of EZH2 (Fig 3B and 3C). Transduction of the siNDY1-transfected cells with anti-miR-101 restored the EZH2 levels (Fig S3D). Therefore, the block of FGF-2-stimulated EZH2-induction by siNDY1 was due to the failure of FGF-2 to repress miR-101 in these cells. In parallel experiments, MEFs transfected with siNDY1 or siControl were serum-starved and they were stimulated with FGF-2 16 hours later. ChIP assays on cells harvested 12 hours from the start of the stimulation, addressed the binding of EZH2 and RNA Pol II, and the abundance of histone H3K36me2 and histone H3K27me3 on the miR-101 locus. The results confirmed that FGF-2 represses miR-101 by reproducing the chromatin modifications induced by NDY1 overexpression on the miR-101 locus. The knockdown of NDY1 blocked all the effects of FGF-2 and confirmed that the chromatin modifications and the repression of miR-101, induced by FGF-2 depend on NDY1 (Fig 3D). The data in figure 3D also showed that knockdown of NDY1 completely abolishes EZH2 recruitment and miR-101 repression by FGF-2 and confirmed that EZH2 recruitment to the miR-101 locus depends on the upregulation of NDY1.
Experiments presented in the preceding paragraphs (Fig S1G) distinguished NDY1 from its homolog NDY2/KDM2A, by showing that the latter does not repress miR-101. Further studies confirmed that the two histone H3 demethylases are functionally distinct, by showing that FGF-2 does not induce NDY2/KDM2A and that overexpressed NDY2/KDM2A does not bind the miR-101 promoter (Fig S3F).
The induction of NDY1 by FGF-2 depends on CREB phosphorylation and activation via DYRK1A
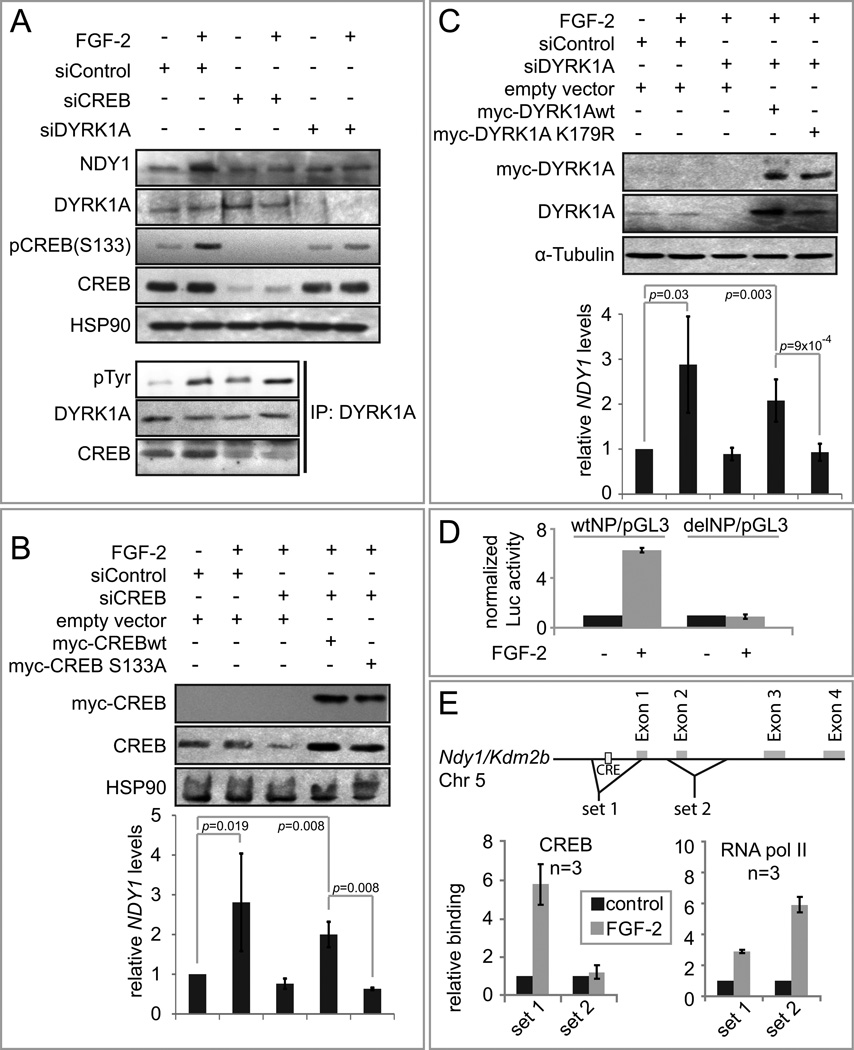
To elucidate the mechanism of the FGF-2-induced NDY1 upregulation, MEFs were treated with inhibitors for AKT (MK2206 5µM), MEK (U0126 1µM), PKA (KT5720 0.5µM), CaM kinase (KN-62 0.1µM) and PKC (Ro-31-8220 100nM) prior to stimulation with FGF-2. All inhibitors effectively inhibited their respective pathways (Fig S4B). Surprisingly however, none had any effect on the upregulation of NDY1 (Fig S4A). The promoter of ndy1 harbors a conserved cAMP response element (CRE) 20 base pairs upstream of the transcription initiation site, along with two non-canonical TATA boxes, ~860 and ~700 bp upstream of the CRE (Aerts et al., 2003; Aerts et al., 2005). Previous studies had shown that FGF-2 activates CREB by phosphorylation at Ser133 via DYRK1A (Yang et al., 2001), suggesting that FGF-2-induced NDY1 upregulation may be mediated by CREB, downstream of DYRK1A. Probing western blots of MEF lysates harvested before and 5 hours after stimulation with FGF-2 with anti-phospho-CREB and anti-CREB antibodies (Fig S4C, Upper panel) showed that FGF-2 indeed promotes CREB phosphorylation at Ser133. Probing DYRK1A immunoprecipitates with anti-phosphotyrosine, anti-DYRK1A and anti-CREB antibodies, confirmed that FGF-2 promotes DYRK1A phosphorylation and binding to CREB. Additional experiments confirmed the role of DYRK1A and CREB in the induction of NDY1 by FGF-2 by showing that the knockdown of either inhibits it (Fig 4A). Knocking down DYRK1A also interfered with the phosphorylation of CREB (Fig 4A Upper panel), while knocking down CREB blocked the induction of NDY1 (Fig 4A Upper panel), without affecting the phosphorylation of DYRK1A (Fig 4A Lower panel). We conclude that DYRK1A is an upstream regulator of CREB.
Figure 4. FGF-2 upregulates NDY1 via a DYRK1/CREB-dependent process.
(A) DYRK1A-dependent phosphorylation of CREB at Serine-133 in FGF-2-treated cells promotes the upregulation of NDY1. MEFs transfected with siDYRK1A, siCREB or siControl were serum-starved for 12 hours and then stimulated with FGF-2. (Upper panel) Cell lysates were probed with the indicated antibodies. (Lower panel) DYRK1A immunoprecipitates of the siControl or siCREB transfected cells were probed with the indicated antibodies.
(B) The knockdown of CREB interferes with the induction of NDY1 by FGF-2. Phenotypic rescue with CREBwt but not with CREB S133A. MEFs transduced with wild type CREB (myc-CREBwt), the phosphorylation-deficient CREB mutant (myc-CREB S133A) or the empty vector were transfected with siControl or siCREB and then stimulated with FGF-2. (Top panel) Nuclear extracts of these cells were probed with the indicated antibodies (Bottom panel) NDY1 mRNA levels were measured by real time RT-PCR. Data are expressed as mean ± SD.
(C) The knockdown of DYRK1A interferes with the induction of NDY1 by FGF-2. Phenotypic rescue with DYRK1Awt but not with DYRK1A S133A. MEFs transduced with wild type DYRK1A (myc-DYRK1Awt), the kinase-dead DYRK1A mutant (myc-DYRK1A K179R) or the empty vector were transfected with siControl or siDYRK1A and then stimulated with FGF-2. (Top panel) Nuclear extracts of these cells were probed with the indicated antibodies (Bottom panel) NDY1 mRNA levels were measured by real time RT-PCR. Data are expressed as mean ± SD.
(D) Ndy1 promoter activity in serum starved, FGF-2-stimulated MEFs depends on CREB. MEFs were transfected with a pGL3-based construct of a 1.1 kb wild type ndy1 promoter (wtNP/pGL3) or with an identical construct with an internal 13 bp deletion of the CREB binding site (delNP/pGL3). Data are expressed as mean ± SD.
(E) CREB binds the ndy1 promoter, in parallel with RNA Pol II. (Top) Schematic of the ndy1 promoter, showing the CREB binding site and the placement of the two sets of ChIP primers. (Bottom) ChIP assays showing the relative binding of CREB and RNA Pol II in MEFs, before and after stimulation with FGF-2. Data are expressed as mean ± SD.
To determine whether the kinase activity of DYRK1A and the phosphorylation of CREB are required for the induction of NDY1 by FGF-2, we knocked down the endogenous DYRK1A or CREB in MEFs and we replaced them with wild type DYRK1A, or CREB, or with the kinase-dead mutant of DYRK1A (K179R) and the phosphorylation site mutant of CREB (S133A). The results (Fig 4B and 4C) confirmed that the induction of NDY1 by FGF-2 indeed depends on the kinase activity of DYRK1A and the phosphorylation of CREB. To do this experiment, we introduced third base mutations in the DYRK1A and CREB constructs, which rendered them resistant to the siRNAs we used to knock down the endogenous forms of these genes without changing the amino acid sequence of the proteins they encode.
To determine whether NDY1 is a direct target of CREB in FGF-2-treated cells, we employed luciferase reporter and ChIP assays. The wild type reporter was constructed by cloning a 1.1 kb ndy1 promoter fragment upstream of the luciferase gene in the PGL3 vector (wtNP/pGL3). Deletion of the CREB binding site gave rise to the mutant reporter (delNP/pGL3). Using these constructs we showed that the luciferase gene is induced by FGF-2 only in MEFs transfected with the wild type construct (Fig 4D). ChIP assays showed that FGF-2 stimulates CREB binding to the CRE site (primers set 1), but not further downstream (primer set 2) and confirmed the role of CREB in the activation of the ndy1 promoter by FGF-2. In addition, they showed that CREB binding was associated with increased binding of RNA polymerase II (Fig 4E). Collectively, these data suggest that FGF-2 stimulation promotes transcription of NDY1 via a DYRK1A and CREB-dependent pathway (Fig S4D).
FGF-2-induced cell proliferation and migration in MEFs and FGF-2-induced in vitro tube formation in HUVECs depend on the induction of EZH2, downstream of NDY1 and miR-101
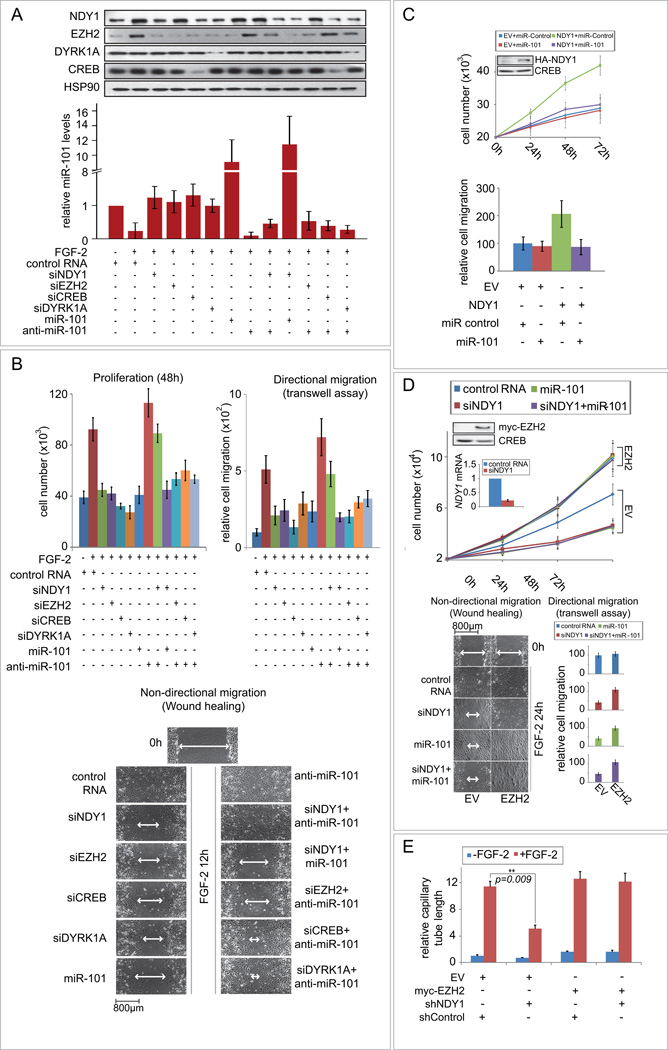
FGF-2 stimulation induces both proliferation and migration in various cell types (Cao et al., 2008; Holland and Varmus, 1998). To determine the role of NDY1 in FGF-2-driven proliferation and migration, MEFs were transfected with siNDY1 ± miR-101, or siControl ± miR-101. Twenty four hours later, they were serum-starved for 12 hours, and then they were stimulated with FGF-2. Transfection of miR-101 or siNDY1 ± miR-101 blocked both the downregulation of miR-101 and the upregulation of EZH2 by FGF-2 (Fig S5A). The FGF-2 treatment also induced proliferation and directional, as well as random migration in MEFs transfected with siControl, but failed to induce proliferation and migration in cells transfected with siNDY1, with or without miR-101 and in cells transfected with siControl and miR-101 (Fig S5B). To determine the role of the NDY1 regulators and targets in FGF-2-driven EZH2 induction and cell proliferation and migration, we repeated the experiment in MEFs transfected with siDYRK1A, siCREB, siNDY1, miR-101, or siEZH2, alone or in combination with anti-miR-101. MEFs transfected with siNDY1 ± miR-101, or siControl ± miR-101 were used as controls. The results showed that whereas FGF-2 represses miR-101 and upregulates EZH2 in siControl-transfected cells, it fails to do so in cells transfected with siDYRK1A, siCREB, siNDY1, siEZH2. Anti-miR-101 on the other hand, reduced the expression of miR-101 in all cells. As expected, anti-miR-101 also promoted the induction of EZH2 by FGF-2 in all cells, with the exception of cells transfected with siEZH2 (Fig 5A). Moreover, changes in the expression of EZH2 induced by the knockdowns, or anti-miR-101 correlated perfectly with the effects of these treatments on cell proliferation and directional and non-directional cell migration (Fig 5B). These data confirmed that the induction of NDY1 and the resulting downregulation of miR-101 are necessary for the induction of cell proliferation and migration by FGF-2. Overexpression of HA-NDY1 in serum-starved MEFs in the absence of FGF-2 stimulation, also induced cell proliferation and migration and confirmed that the NDY1-miR-101-EZH2 pathway is not only necessary but also sufficient for the proliferative and migratory phenotype of FGF-2 (Fig 5C).
Figure 5. FGF-2-induced cell proliferation and migration in MEFs, and in vitro tube formation in HUVECs, depend on the NDY1-miR-101-EZH2 pathway.
(A) The induction of EZH2 in FGF-2-stimulated cells depends on the induction of NDY1 by its upstream regulators in the FGF-2 pathway and on the repression of its target, miR-101. MEFs transfected with the indicated siRNAs, miR-101 and anti-miR-101, were serum-starved for 12 hours, and then stimulated with FGF-2. (Top panel) Nuclear cell lysates were probed with the indicated antibodies. (Bottom panel) miR-101 levels were measured by real time RT-PCR 48 hours after FGF-2 stimulation. Data are expressed as mean ± SD.
(B) Cell proliferation and directional and non-directional migration of FGF-2-stimulated cells depend on the activation of the NDY1-miR-101-EZH2 pathway. (Left panel) MEFs transfected with siControl were cultured in serum-free media with or without FGF-2. Parallel cultures of MEFs transfected with the indicated siRNAs, miR-101, or anti-miR-101 were grown in the presence of FGF-2. Cell numbers were measured at the indicated time point and the data are expressed as mean ± SD. (Middle panel) Directional migration toward FGF-2 was measured in the same cells as in A. Relative migration is the ratio of siRNA ± miR-101 or anti-miR-101-transfected cells migrating toward FGF-2 over the siControl-transfected cells migrating toward FGF-2-free media ×100. Data are expressed as mean ± SD. (Right panel) Wound healing assays were carried out on a monolayer of confluent cultures of the MEFs used in the experiment in A. The images show the wounded areas 12 hours after wounding.
(C) The repression of miR-101 is required for the stimulation of cell proliferation and migration by NDY1. (Left panel) MEFs transduced with pBabe-HA-NDY1, or empty vector were transfected with miR-control or mir-101. Cell numbers were measured at the indicated time points after FGF-2 stimulation. Data are expressed as mean ± SD. (Right panel) Directional migration, of the cells in the left panel. Relative migration was calculated as in B, right panel.
(D) The knockdown of NDY1 and the overexpression of miR-101 inhibit FGF-2-induced cell proliferation and migration in wild type MEFs but not in MEFs engineered to express EZH2. (Left panel) MEFs transduced with pbabe-myc-EZH2 or EV were transfected with siControl, siNDY1, miR-101 or siNDY1 ± mir-101. Cell numbers were measured at the indicated time points after FGF-2 stimulation and the Data are expressed as mean ± SD. (Middle panel) Wound healing assays were carried out on a monolayer of confluent cultures of the MEFs in the experiment in A. The images show the wounded areas at 24 hours after wounding. (Right panel) Directional migration toward FGF-2 was measured in the same cells as in the middle panel. Relative migration was calculated as in B and C (mean ± SD).
(E) HUVECs transduced with pBabe-myc-EZH2 or EV were transduced with pLKO.1-shNDY1 or pLKO.1-shControl and stimulated with FGF-2. Tube formation assay was performed on Cultrex-RGF-BME (Reduced Growth Factor Basement Membrane Extract) in the presence or absence of FGF-2 (20ng/ml). Quantitation of the in vitro tube formation was performed with the Angioquant software. Relative tube formation is the ratio of total capillary tube length in experimental cultures over the total capillary tube length in EV cells cultured in the absence of FGF-2. Data are expressed as mean ± SD. For more information, see also figure S5.
To determine whether the induction of EZH2 has a causative role in the induction of cell proliferation and cell migration by FGF-2, EZH2 and empty vector-transduced early passage MEFs were transfected with siNDY1 ± miR-101 or siControl ± miR-101, serum starved and stimulated with FGF-2. The results showed that whereas siNDY1 ± miR-101 inhibited the proliferation and the directional and random FGF-2-driven migration of EV cells, they had no effect on the proliferation and motility of EZH2-expressing cells (Fig 5D). These data confirmed the causative role of EZH2 in FGF-2-driven cell proliferation and cell migration.
FGF-2 and its downstream target EZH2 play a major role in angiogenesis (Cao et al., 2008; Lu et al., 2010), raising the question whether the FGF-2-driven NDY1-miR-101-EZH2 pathway is responsible for the angiogenic properties of FGF-2. To address this question, nuclear lysates of serum-starved HUVECs were probed with antibodies against NDY1, EZH2 and CREB, 12 hours after stimulation with FGF-2. RNA isolated from the same cells was analyzed by real time RT-PCR for the abundance of miR-101. The results (Fig S5C) confirmed that FGF-2 activates the NDY1-miR-101-EZH2 pathway in these cells.
To determine the role of the FGF-2-activated NDY1-miR-101-EZH2 pathway in FGF-2-driven in vitro tube formation, we transduced HUVECs with pBabe-myc-EZH2 or with the empty vector. Both myc-EZH2 and EV-transduced cells were then super-infected with lentiviral shNDY1 or shControl giving rise to the four types of cultures shown in figures 5E, S5C and S5D. The transduced HUVECs were then seeded on Reduced-Growth-Factor-Basement-Membrane-Extract ± FGF-2 ± the angiogenesis inhibitor sulphoraphane and they were scored for in vitro tube formation 6 hours later. The results showed that the knockdown of NDY1 interferes with tube formation and that myc-EZH2 reverses the inhibitory effect of the NDY1 knockdown (Fig S5E, Right panel and Fig 5E). We conclude that FGF-2-induced in vitro tube formation is mediated by NDY1 and its downstream target EZH2.
The NDY1-miR-101-EZH2 pathway is active in tumor cell lines expressing high levels of FGF-2 and its activation is FGF-2-dependent
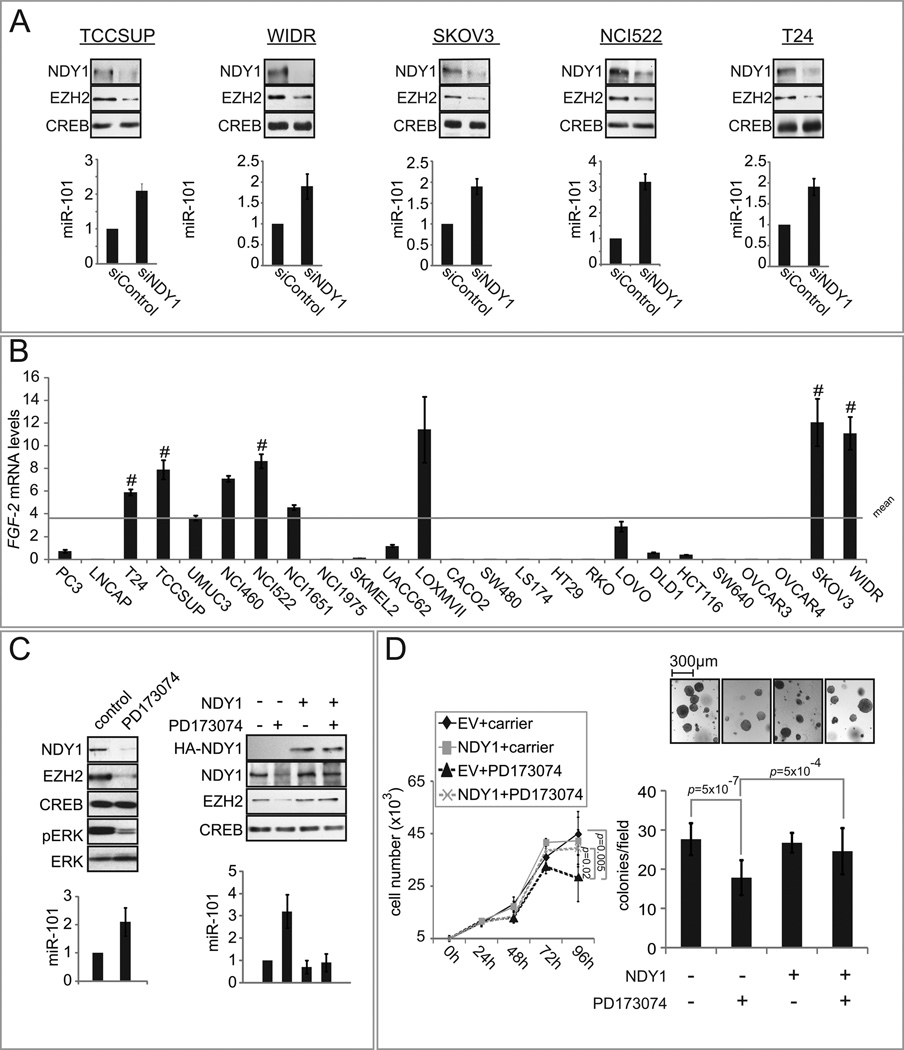
To determine whether the FGF-2-activated NDY1-miR-101-EZH2 pathway has a direct role in human cancer, we examined its’ activity in a set of twenty five human cancer cell lines all of which express NDY1 (Data not shown). All cell lines were transfected with siNDY1 or siControl. Probing transfected cell nuclear extracts, collected 48 hours later, with anti-NDY1, anti-EZH2 and anti-CREB antibodies and analyzing RNA from the same cells for the abundance of miR-101, identified five cell lines in which the knockdown of NDY1 upregulates miR-101 and downregulates EZH2 (Fig 6A). To determine whether the activation of NDY1-miR-101-EZH2 pathway in the five cell lines was driven by FGF-2, we examined the expression of FGF-2 in all twenty-five cell-lines by real time RT-PCR. The results (Fig 6B) showed that the expression of FGF-2 was above the mean value in all five and in two additional cell lines out of the total of twenty-five. The correlation between the activation of the pathway and the expression of FGF-2 was statistically significant (P<0.01 by the one-sample t-test).
Figure 6. The activity of the NDY1-miR-101-EZH2 pathway in cancer cell lines depends on FGF-2.
(A) Twenty five human tumor cell lines cells were transfected with siNDY1 or siControl. The knockdown of NDY1 in five of the transfected lines (TCCSUP, WIDR, SKOV3, NCI522 or T24) upregulated miR-101 and downregulated EZH2. (Upper panel) Nuclear lysates were probed with the indicated antibodies. (Lower panel) miR-101 levels were measured in the same cells, using real time RT-PCR. Data are expressed as mean ± SD.
(B) FGF-2 mRNA levels were measured with real time RT-PCR. Data are expressed as mean ± SD.
(C) WIDR cells transduced or not transduced with pBabe-HA-NDY1 were treated with PD173074 or carrier for 24 hours. (Upper panels) Nuclear and cytoplasmic cell lysates were probed with indicated antibodies. (Lower panels) miR-101 levels were measured in the same cells, using real time RT-PCR. Data are expressed as mean ± SD.
(D) The inhibition of WIDR cell accumulation over time in attached and soft agar cultures treated with PD173074 is rescued by wild type NDY1. (Left panel) WIDR cells transduced with pBabe/HA-NDY1 or the empty vector (EV) were cultured in the presence of the PD173074 or carrier (DMSO). Cell numbers were monitored at the indicated time points and the data are expressed as mean ± SD. (Right panel) 2×104 WIDR cells transduced with pBabe/HA-NDY1 or the empty vector were cultured in soft agar in the presence or absence of PD173074. Colony numbers per plate (mean ± SD) were counted at 10 days. The statistical significance of the data in both panels was assessed with the two-sample t-test.
The preceding findings suggested that the activation of the NDY1-miR-101-EZH2 pathway in tumor cell lines correlates with the expression of FGF-2 and that the expression of FGF-2 correlates with the activation of the pathway. This raised the question whether FGF-2 plays a causative role in the activation of this pathway. To address this hypothesis, two cell lines expressing high levels of FGF-2 (WIDR – Fig 6C and TCCSUP – Fig S6) were transduced with a pBabe retroviral construct of HA-NDY1 or with the empty vector. Subsequently, they were treated with the FGFR1/FGFR3 inhibitor PD173074 (1µM) or with carrier. Probing western blots of cell lysates harvested 24 hours later with the indicated antibodies and analyzing RNA from the same cells for the expression of miR-101 by real time RT-PCR (Fig 6C and S6A) showed that PD173074 inhibits the phosphorylation of ERK, as expected. In addition, it inhibits the expression of NDY1, upregulates miR-101 and downregulates EZH2. Since the ectopic expression of HA-NDY1 blocks the effects of PD173074 on this pathway (Fig 6C and S6A, right), we conclude that the effects of the FGFR inhibitor on the expression of miR-101 and EZH2 are mediated by NDY1.
To determine the role of the FGF-2-NDY1-miR-101-EZH2 pathway in transformation, WIDR cells transduced with an HA-NDY1 retroviral construct or with the empty vector, were plated in attached cultures or in soft agar. Plated cells were treated with PD173074 or with vehicle and they were monitored for cell accumulation over time. The results confirmed that PD173074 inhibits cell proliferation in attached cultures and transformed colony formation in soft agar in cells transduced with the empty vector, but not in cells transduced with NDY1 (Fig 6D). Since earlier observations provided evidence for a link between cell proliferation/soft agar growth and oncogenicity (Land et al., 1983), we interpret the preceding data to suggest a role of the NDY1-miR-101-EZH2 pathway in oncogenesis.
Activation of the NDY1-miR-101-EZH2 pathway by FGF-2 in human carcinomas or by cell autonomous mechanisms in MoMuLV-induced rat T cell lymphomas
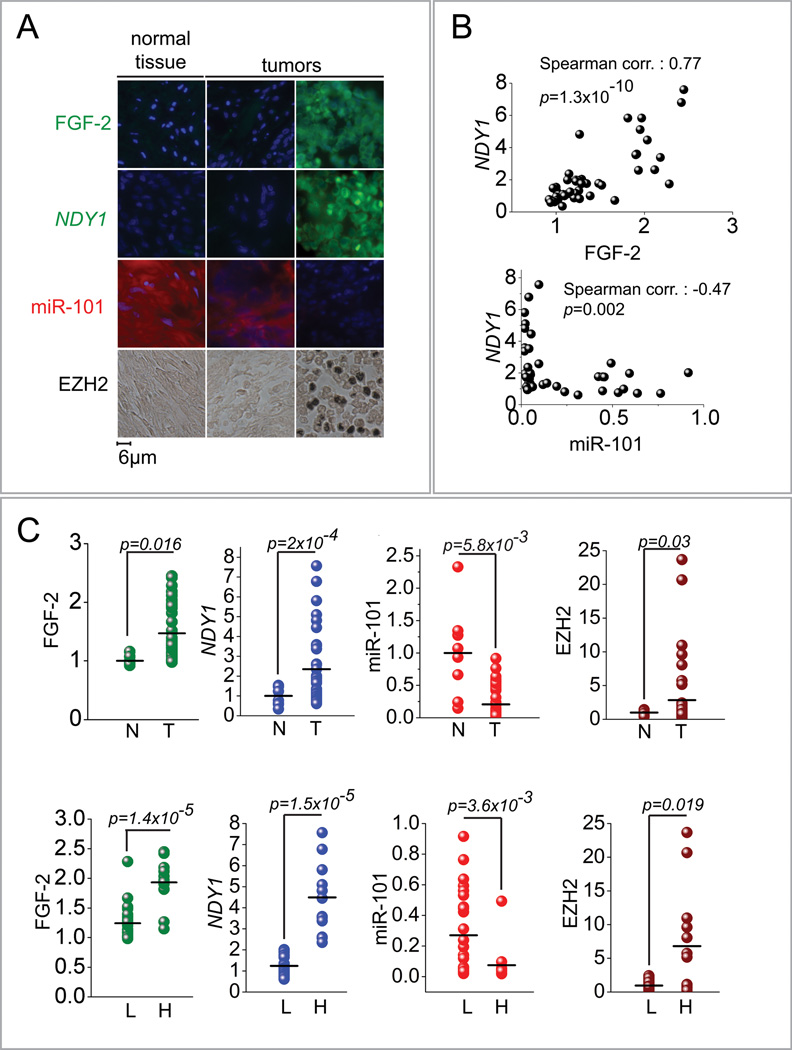
EZH2 is upregulated in a variety of human tumors (Gil et al., 2005). In some of these tumors its upregulation is due to deletions of miR-101, while in others, such as bladder carcinomas, it has been linked to the transcriptional repression of miR-101 (Friedman et al., 2009; Varambally et al., 2008). To determine whether NDY1 contributes to the repression of miR-101 and the upregulation of EZH2 in human tumors, we first examined a group of three lung carcinomas by western blotting, immunohistochemistry and in situ hybridization for the expression of NDY1, EZH2 and miR-101 (Fig S7A, S7B and S7C). The preliminary experiment conducted in this small group of tumor samples suggested that the NDY1-miR-10-EZH2 pathway may be active in these tumors. In addition, it allowed us to establish conditions for the screening of a tissue array of 40 bladder carcinomas and eight normal bladder specimens. A sample of the data showing the expression of FGF-2, NDY1, miR-101 and EZH2 in two tumors and one normal bladder specimen is shown in figure 7A. The relative expression levels of FGF-2, NDY1, miR-101 and EZH2 in all the samples were determined by quantification of the intensity of immunofluorescence or the intensity of the immunohistochemical reaction, using the Histogram feature of the Adobe Photoshop program. Plotting the relative levels of FGF-2 against the relative levels of NDY1 revealed an excellent correlation between the two (p=1.3×10−5) (Fig 7B, upper panel). Moreover, plotting the relative levels of NDY1 against the relative levels of miR-101 (Fig 7B, lower panel) revealed an excellent non-linear inverse correlation between the two (p=0.002). Further analyses of the quantitative data revealed that bladder carcinomas tend to express higher levels of FGF-2 and NDY1 than normal bladder tissues and that the higher levels of NDY1 in the tumors correlate with lower levels of miR-101 and higher levels of EZH2 (Fig 7C, upper panel). The same correlations were observed when we compared tumors expressing high and low levels of NDY1 (Fig 7C bottom panel). These data confirmed that in a significant fraction of bladder carcinomas expressing high levels of EZH2, the upregulation of EZH2 is due to the activation of the FGF-2-NDY1-miR-101-EZH2 pathway.
Figure 7. NDY1-miR-101-EZH2 pathway is active in primary tumors.
(A) Immunofluorescence/Immunohistochemistry (FGF-2 and EZH2) and in-situ hybridization (NDY1 and miR-101) in transitional cell bladder carcinomas. Representative images out of a 48-core (40 carcinomas/8 normal tissue samples) bladder tissue array.
(B) (Upper panel) The expression of NDY1 exhibits a linear correlation with the expression of FGF-2. Correlation coefficient: 0.77 and significance p=1.3×10−7 by Spearman correlation analysis. (Lower panel) The expression of NDY1 and miR-101 in bladder carcinomas exhibit a non-linear inverse correlation. Correlation coefficient: −0.47 and significance: P=0.002 by Spearman correlation analysis.
(C) (Upper panel) Comparison of the expression levels of FGF-2, NDY1, miR-101 and EZH2 in the normal bladder tissue samples and the bladder carcinomas in the bladder carcinoma tissue array. Expression was measured as described in the Experimental Procedures. Statistical significance was calculated using the unpaired Student’s t test. (Lower panel) Comparison of the expression levels of FGF-2, miR-101 and EZH2 in bladder carcinomas expressing high and low levels of NDY1. Expression was measured as described in the Experimental Procedures. Statistical significance was calculated using the unpaired Student’s t test.
Whereas in human bladder carcinomas the NDY1-miR-101-EZH2 pathway appears to be activated by FGF-2, in other tumors, such as MoMuLV induced rodent T cell lymphomas, it may be activated by direct cell autonomous mechanisms. Our earlier studies had indeed shown that in these tumors NDY1 is induced by provirus DNA integration (Pfau et al., 2008). Probing tumor cell lysates with an anti-EZH2 antibody and analysis of tumor cell RNA for the expression of NDY1 and miR-101 by real time RT-PCR, revealed an inverse correlation between NDY1 and miR-101, as well as between miR-101 and EZH2 (Fig S7D) and provided support to the hypothesis that the cell autonomous induction of NDY1 activates the NDY1-miR-101-EZH2 pathway.
The preceding data collectively support the hypothesis that the upregulation of EZH2 in the majority of bladder carcinomas is due to the upregulation of NDY1 upstream of miR-101. Although in some tumors the activation of this pathway may be cell-autonomous, in human bladder carcinomas its activation appears to depend primarily on FGF-2.
DISCUSSION
Histone demethylases and other chromatin modifying enzymes have been linked to stem cell renewal and differentiation. Some of them are also known to contribute to oncogenesis (Kampranis and Tsichlis, 2009). However, little is known to-date on the integration of these enzymes into the signaling machinery. In this report, we presented evidence that the histone H3 demethylase NDY1 is significantly upregulated not only in MoMuLV-induced rodent lymphomas, but also in bladder carcinomas and in tumor cell lines derived from different types of human cancer. We also showed that the upregulated NDY1 triggers the recruitment of basally expressed EZH2, which binds and represses miR-101 in concert with NDY1. The repression of miR-101 leads to the upregulation of its targets, including EZH2, which promotes cell proliferation, cell migration and angiogenesis. The upregulation of EZH2 also stabilizes the repression of miR-101.
The induction of NDY1 in tumor cells may be cell-autonomous, or it may be driven by FGF-2, via DYRK1A-mediated CREB phosphorylation and activation (Fig S4D). FGF-2 and, to a lesser degree, VEGF were unique in their ability to upregulate NDY1 and to induce EZH2 by repressing miR-101. The interdependence between NDY1, miR-101 and EZH2 in FGF-2-stimulated cells was confirmed by the knockdown of NDY1. The induction of NDY1 by FGF-2 via CREB phosphorylation and activation, downstream of DYRK1A was confirmed by DYRK1A or CREB knockdown experiments, followed by the rescue of the knockdown phenotype with wild type DYRK1A or CREB but not with the kinase-dead DYRK1A K179R or CREB S133A.
The concerted binding of NDY1 and EZH2 to the miR-101 promoter is triggered by the upregulation of NDY1. EZH2 did not bind when overexpressed in the absence of NDY1 overexpression, while NDY1 bound the miR-101 promoter when overexpressed in the absence of EZH2, but failed to repress transcription. The binding of NDY1 and EZH2 to the miR-101 promoter was shown to correlate with a decrease in the abundance of H3K36me2 marks and an increase in the abundance of H3K27me3 marks. Moreover, it did correlate with the binding of BMI1, a component of the PRC1 complex, and with a decrease in the binding of RNA Pol II. These data combined, suggest that NDY1 represses gene expression by coupling histone H3K36me2 demethylation with histone H3K27 trimethylation and perhaps BMI1-mediated histone ubiquitination.
The JmjC domain mutant NDY1 H283Y, which lacks demethylase activity, binds the miR-101 promoter, but does not demethylate H3K36me2 and does not recruit EZH2. These experiments strongly suggest that H3K36me2 may hinder EZH2 binding to chromatin and that NDY1 promotes the recruitment of EZH2 via H3K36me2 demethylation. EZH2 was shown recently to bind its target genes in concert with the enzymatically inactive Jumonji domain protein Jmj or JARID2. The concerted binding of the two proteins was attributed to their strong interaction (Herz and Shilatifard, 2010). Future studies will address whether NDY1 and JARID2 synergize or compete with each other for EZH2 colocalization and whether the abundance of H3K36me2 affects the concerted binding of EZH2 and JARID2.
The role of FGF-2 in cancer is well established. However, despite the progress that has been made in recent years in this area (MacFarlane and Murphy, 2010; Schlessinger, 2004), our understanding of the molecular mechanisms by which FGF-2 contributes to oncogenesis is limited. The data presented in this report address this issue by showing that FGF-2 induces NDY1 and activates the novel NDY1/EZH2-miR-101-EZH2 pathway. The hypothesis that the effects of FGF-2 in oncogenesis are mediated by the FGF-2-activated NDY1/EZH2-miR-101-EZH2 pathway is supported by the following observations. First, the expression of FGF-2 correlates with the activity of this pathway in a set of 25 human tumor cell lines and the FGFR inhibitor PD173074 blocks the activation of the pathway. Second, inhibition of FGFR with the small molecule inhibitor PD173074 inhibits empty vector-transduced, but not NDY1-transduced WIDR cells from proliferating in attached cultures and from forming colonies in soft agar. Third, the cell autonomous activation of this pathway phenocopies FGF-2.
In summary, data presented in this report, identify a novel pathway, NDY1/EZH2-miR-101-EZH2, which promotes normal and tumor cell proliferation, survival and migration as well as angiogenesis and is active in a significant fraction of bladder carcinomas. The same pathway may promote pluripotency and self-renewal in various types of stem cells. Activation of the pathway is triggered by the induction of NDY1, which occurs either via cell autonomous mechanisms or in response to FGF-2. The upregulated NDY1 recruits EZH2 and the two act in concert to repress miR-101. The upregulation of EZH2 caused by the miR-101 repression stabilizes the pathway. Given that the oncogenic program of FGF-2 depends on the FGF-2-NDY1/EZH2-miR-101-EZH2 pathway, the data presented in this report identify a set of potential new molecular targets for therapeutic intervention in FGF-2-driven human tumors.
EXPERIMENTAL PROCEDURES
Cell culture
MEFs were isolated from day E13.5 mouse embryos and they were cultured as previously described (Pfau et al., 2008). Human cancer cell lines and culture conditions are listed in Supplement. Stimulation with growth factors and treatment with inhibitors of signaling molecules, were performed as described in the Supplement.
siRNAs for mouse and human NDY1, CREB, DYRK1A and EZH2, pre-miR-101, anti-miR-101 and control RNAs as well as shRNAs for human NDY1 and mouse EZH2 are listed in the Supplement.
Retroviral and lentiviral packaging and infection with the packaged viruses were carried out using standard methods, as outlined in the Supplemental Experimental Procedures.
Cloning and site-directed mutagenesis
Open reading frames (ORFs) for mouse DYRK1A and CREB were purchase form Open Biosystems (cat no. MMM1013-99828657, MMM1013-64862). The ORFs were transferred to a lentiviral-based expression vector, as outlined in the Supplement.
Immunoblotting
Cytoplasmic and nuclear extracts were isolated with the Nuclear and Cytoplasmic extraction reagent kit (Thermo Scientific, cat no. 78833). Total cell lysates were isolated using a Triton X-100 lysis buffer (see Supplement). Western blots of cell lysates resolved by electrophoresis in SDS-PAGE were probed with the antibodies listed in the Supplement.
Real-time RT- PCR for miR-101 and mRNAs
Total RNA was isolated from normal and tumor cells with Trizol (Invitrogen, cat no. 15596-026), and it was analyzed by real-time RT-PCR for the expression of miR-101 and the indicated mRNAs, as described in the Supplement. The levels of miR-101 were normalized based on the levels of U6 small nuclear RNA (internal control). The mRNA levels were normalized based on the levels of GAPDH (internal control). The primer sets used, are listed in the Supplement.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed using the Chromatin Immunoprecipitation assay kit (Millipore, cat no. 17–295) (see Supplement).
Luciferase assay
The ndy1 promoter region was analysed with the Tucan2 software for the presence of conserved transcription-factor binding sites (Aerts et al., 2003; Aerts et al., 2005). MiR-101 and ndy1 promoter regions (1kb each) were cloned by PCR from genomic MEF DNA, using the primers listed in the Supplemental Experimental Procedures. The fragments was cloned into the pGL3-basic firefly luciferase reporter gene vector (Promega, cat no. E1751). Luciferase assays were carried out as described in the Supplement.
Cell proliferation assays
To monitor the number of live cells in cell culture, we employed the 3-[4, 5-dimethylthiazol-2-yl]-2, 5-dimethyltetrazolium-bromide (MTT, Invitrogen, cat no. M6494) assay, as described previously (Polytarchou et al., 2008). For details, see Supplement. Results were always confirmed by direct cell counting, using a hemocytometer.
Cell migration assays
Directional migration: Transwell filter assay
Migration assays were performed using 24-well microchemotaxis chambers with uncoated polycarbonate membranes (pore size 8 µm, Costar, cat no. 3422). Cells migrating through the filter toward FGF-2 were fixed in paraformaldehyde and counted, using a grid and an Optech microscope at 20× magnification. For details, see Supplement.
Nondirectional migration: Wound healing assay
Cells were plated on 6-well plates in DMEM containing 10% FBS. Wounds were inflicted to confluent cell monolayers with a plastic pipette tip, following 16 hours of serum starvation. Cell migration into the wound in serum-free cultures, ± FGF-2, was recorded 12 and 24 hours later. For details, see Supplemental Experimental Procedures.
In vitro tube formation assay
The in vitro tube formation assay was performed using the In vitro Angiogenesis Assay Kit (Trevigen, cat no. 3470-096-K) (see Supplement). The total length of capillary tubes in the total area of each well was measured using the Angioquant image analysis software (Niemisto et al., 2005). All images were captured and processed using identical settings.
Soft agar assay
2×104 WIDR cells transduced with pBabe-puro or pBabe-puro/HA-NDY1 were cultured in 6-well plates in soft agar (0.4%agarose, with a 0.8% agarose underlay, Sea Plaque Agarose), ± PD173074 (0.1µM). Anchorage-independent growth was assayed by scoring the number of colonies per field 10 days later (microscopic counting of >50µm colonies). For more information see also Supplement.
In situ hybridization
For in situ hybridization, a Mircury LNA Detection probe for mmu-miR-101, labeled at the 5'-end with DIG (Exiqon, cat no. 38475-01), and an NDY1 probe, labelled with 56-FAM (Exiqon, LNA probe, 5′ TTCCCAGTCCATCCTTTTCTCG 3′) were used according to the manufacturer’s instructions, with modifications described in the Supplemen. Images were obtained using a Nikon Eclipse 80i microscope and a Spot charge-coupled device camera (Diagnostic Instruments). All images were captured and processed using identical settings.
Immunohistochemistry
Immunohistochemical detection of proteins in paraffin embedded tissues was performed as described previously (Iliopoulos et al., 2009), with minor modifications described in the Supplement.
Tumors
A bladder carcinoma tissue array, containing a total of 48 cores (40 tumors/8 normal tissues) was purchased from US. Biomax (cat no. BL481). De-identified lung adenocarcinoma specimens were obtained from the Tufts Medical Center tumor bank (Boston, MA) following institutional IRB approval. MoMuLV-induced rat T-cell lymphomas were previously described (Pfau et al., 2008).
HIGHLIGHTS.
Pathway linking FGF-2 with epigenetic regulation of gene expression
NDY1 upregulation triggers the concerted binding of EZH2 and NDY1 to the miR-101 locus
Activation of this pathway is essential for the biology elicited by FGF-2
The pathway is active in normal cells, tumor cell lines and primary tumors
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant (R01 CA109747) from the National Cancer Institute. We wish to thank Dr Michael Comb (Cell Signaling Technologies Inc.) for making available to us a non-commercially available antibody. Also, we wish to thank Dr Phil Hinds and Dr Guo-fu Hu for critical review of the manuscript. Finally, we wish to thank all the members of the PNT laboratory for supporting this work with their intellectual and technical expertise. CP was a fellow of the Leukemia and Lymphoma Society Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aerts S, Thijs G, Coessens B, Staes M, Moreau Y, De Moor B. Toucan: deciphering the cis-regulatory logic of coregulated genes. Nucleic Acids Res. 2003;31:1753–1764. doi: 10.1093/nar/gkg268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts S, Van Loo P, Thijs G, Mayer H, de Martin R, Moreau Y, De Moor B. TOUCAN 2: the all-inclusive open source workbench for regulatory sequence analysis. Nucleic Acids Res. 2005;33:W393–W396. doi: 10.1093/nar/gki354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett. 2006;580:2869–2874. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- Fahmy RG, Khachigian LM. Suppression of growth factor expression and human vascular smooth muscle cell growth by small interfering RNA targeting EGR-1. J Cell Biochem. 2007;100:1526–1535. doi: 10.1002/jcb.21145. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Liang G, Liu CC, Wolff EM, Tsai YC, Ye W, Zhou X, Jones PA. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- Gil J, Bernard D, Peters G. Role of polycomb group proteins in stem cell self-renewal and cancer. DNA Cell Biol. 2005;24:117–125. doi: 10.1089/dna.2005.24.117. [DOI] [PubMed] [Google Scholar]
- Hara F, Samuel S, Liu J, Rosen D, Langley RR, Naora H. A homeobox gene related to Drosophila distal-less promotes ovarian tumorigenicity by inducing expression of vascular endothelial growth factor and fibroblast growth factor-2. Am J Pathol. 2007;70:1594–1606. doi: 10.2353/ajpath.2007.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Shilatifard A. The JARID2-PRC2 duality. Genes Dev. 2010;24:857–861. doi: 10.1101/gad.1921610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampranis SC, Tsichlis PN. Histone demethylases and cancer. Adv Cancer Res. 2009;102:103–169. doi: 10.1016/S0065-230X(09)02004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci. 2008;11:1137–1139. doi: 10.1038/nn.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Lu M, Tian X, Han Z. Molecular mechanisms involved in self-renewal and pluripotency of embryonic stem cells. J Cell Physiol. 2007;211:279–286. doi: 10.1002/jcp.20978. [DOI] [PubMed] [Google Scholar]
- Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane L, Murphy P. FGF2 (fibroblast growth factor 2 (basic)) In Atlas Genet Cytogenet Oncol Haematol. 2010 [Google Scholar]
- Niemisto A, Dunmire V, Yli-Harja O, Zhang W, Shmulevich I. Robust quantification of in vitro angiogenesis through image analysis. IEEE Trans Med Imaging. 2005;24:549–553. doi: 10.1109/tmi.2004.837339. [DOI] [PubMed] [Google Scholar]
- Pfau R, Tzatsos A, Kampranis SC, Serebrennikova OB, Bear SE, Tsichlis PN. Members of a family of JmjC domain-containing oncoproteins immortalize embryonic fibroblasts via a JmjC domain-dependent process. Proc Natl Acad Sci U S A. 2008;105:1907–1912. doi: 10.1073/pnas.0711865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polytarchou C, Pfau R, Hatziapostolou M, Tsichlis PN. The JmjC domain histone demethylase Ndy1 regulates redox homeostasis and protects cells from oxidative stress. Mol Cell Biol. 2008;28:7451–7464. doi: 10.1128/MCB.00688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsos A, Pfau R, Kampranis SC, Tsichlis PN. Ndy1/KDM2B immortalizes mouse embryonic fibroblasts by repressing the Ink4a/Arf locus. Proc Natl Acad Sci U S A. 2009;106:2641–2646. doi: 10.1073/pnas.0813139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedlocha A, Sorensen V. Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr Top Microbiol Immunol. 2004;286:45–79. doi: 10.1007/978-3-540-69494-6_3. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen H, Parker B, Rubin E, Zhu T, Lee JS, Argani P, Sukumar S. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66:9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Ahn YS, Chung KC. Protein kinase Dyrk1 activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells. J Biol Chem. 2001;276:39819–39824. doi: 10.1074/jbc.M104091200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.