Abstract
Background. A 23-valent unconjugated pneumococcal polysaccharide vaccine (23vP), routinely administered at the age of 65, has limited effectiveness, and revaccination induces attenuated antibody responses. It is not known whether pneumococcal polysaccharide-protein conjugated vaccines (PCV), although highly effective in infants, offer any immunological advantages over 23vP in adults.
Methods. We immunized adults with schedules combining both PCV and 23vP and investigated B-cell responses to establish whether PCV7 (a 7-valent PCV) induced T-dependent responses in adults, to assess the role of memory B cells in 23vP-induced antibody hyporesponsiveness, and to identify the B-cell subtypes involved.
Results. A single dose of PCV7 induced significant increases in serotype-specific memory B-cell populations in peripheral blood indicating a T-dependent response. Conversely, immunization with 23vP resulted in a decrease in memory B-cell frequency. Furthermore, memory B-cell responses to subsequent immunization with PCV7, when given after 23vP, were attenuated. Notably, B1b cells, a subset important in protecting mice against pneumococci, were also depleted following immunization with 23vP in humans.
Conclusions. This study indicates that PCV7 may have an immunological advantage over 23vP in adults and that 23vP-induced depletion of memory and B1b-cell subsets may provide a basis for antibody hyporesponsiveness and the limited effectiveness of 23vP.
Clinical Trials Registration. ISRCTN: 78768849.
Pneumococcal infection is a leading cause of morbidity and mortality in the elderly [1]. In the United States and the United Kingdom a polysaccharide vaccine covering 23 pneumococcal serotypes (23vP) is used routinely at the age of 65. However, meta-analyses show that 23vP has limited effectiveness against invasive pneumococcal disease [2]. Some reports suggest that protection is short-lived, which is in keeping with the rapid waning of antibody concentrations from the peak concentrations measured 1 month after vaccination [3–5]. The utility of additional doses of 23vP to sustain immunity in the elderly is limited, as antibody concentrations after subsequent doses of 23vP are similar or lower than after primary vaccination. This phenomenon, which has been termed “hyporesponsiveness,” has also been described following meningococcal polysaccharide vaccines [6, 7]. We have proposed that hyporesponsiveness is due to the depletion of the peripheral memory B-cell pool by plain polysaccharide antigens that drive memory B cells into terminal differentiation, without replenishing the memory B-cell pool, but there is no direct evidence for the existence of this phenomenon with pneumococcal vaccines or in the elderly population [8].
Polysaccharide antigens are postulated to stimulate splenic marginal zone B (MZB) cells, which do not mature until the second year of life [9]; therefore, the purified polysaccharide contained in 23vP, a T-independent antigen, is poorly immunogenic in young children. Chemical conjugation of pneumococcal polysaccharide to a carrier protein creates a T-dependent vaccine (pneumococcal conjugate vaccine [PCV]) that generates higher affinity antibodies, immunological memory, and induces responsiveness to booster doses of vaccine, resulting in a vaccine that is both immunogenic and highly effective from early infancy [10]. Because the splenic marginal zone is immature in early life, MZB cell responses are not present, and it is postulated that the conjugated polysaccharides in PCV are processed by the follicular origin (FO) B cells at that age [11]. Despite the immunological advantages of PCV in early childhood, both PCV7 (a 7-valent PCV) and 23vP induce similar antibody concentrations in adults [12], and it is therefore unclear whether the conjugate vaccine has any immunological advantage over 23vP or whether the same B-cell subsets are involved in the response.
In the present study, we enumerated the frequency and identified the phenotype of the serotype-specific B cells in the peripheral blood of older adults following immunization with combinations of PCV7 and 23vP to investigate the effects of these vaccines on B-cell populations.
METHODS
Participants and Study Design
A phase 4, open-label, randomized, parallel trial was conducted in Oxford, United Kingdom, involving adults aged 50–70 years, as described elsewhere [12]. Written informed consent was obtained from the participants before enrollment. Ethical approval was obtained from the Oxfordshire Research Ethics Committee 06/Q1604/121.
Participants were randomized to receive PCV7-PCV7-23vP or 23vP-PCV7-PCV7 or PCV7-23vP-PCV7 with vaccines given 6 months apart. Blood was sampled prior to and after (7 days and 1 month) vaccination.
Vaccines
The pneumococcal conjugate vaccine (PCV7; Prevenar, Wyeth Vaccines; batch numbers ND05370, NE31130, NG12460) consisted of Streptococcus pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F saccharides (2 μg of all serotypes except 4 μg of 6B) conjugated to a CRM197 carrier protein with aluminum phosphate as an adjuvant. The pneumococcal plain polysaccharide vaccine (23vP; Pneumovax II, Aventis Pasteur MSD; batch numbers 20218, 25305, 22995) consisted of S. pneumoniae serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F (25 μg for each serotype). Both vaccines were given as 0.5-mL solutions intramuscularly using a 23G 25-mm needle.
B-Cell Enzyme-Linked Immunosorbent Spot Assay
Preparation of Peripheral Blood Mononuclear Cells
A maximum volume of 18 mL of heparinized blood was available for the separation of peripheral blood mononuclear cells (PBMCs). The blood was diluted 1:2 with RPMI 1640 (Sigma-Aldrich) to which penicillin-streptomycin solution (Sigma-Aldrich) and 200 mM l-glutamine (Sigma-Aldrich) had been added at a dilution of 1:100 (complete medium). PBMCs were then separated by density gradient centrifugation over Lymphoprep (Axis-Shield). PBMCs were washed once in complete medium before being seeded directly onto enzyme-linked immunosorbent spot assay (ELISpot) plates or being placed into cell culture.
Preparation of ELISpot Plates
Multiscreen IP 96-well filter plates, (Millipore) were coated with either 10 μg/mL (serotypes 4, 9V, 14, 18C, and 19F) or 20 μg/mL (serotypes 6B and 23F) of purified pneumococcal polysaccharide (LGC Promochem) conjugated to methylated human albumin (UK National Institute for Biological Standards and Control), 10 μg/mL diphtheria toxoid tetanus toxoid (Statens Serum Institut) or phosphate-buffered saline (PBS) alone. Prior to cells being seeded onto the plates, all wells were blocked with newborn bovine serum (NBBS).
Detection of Plasma Cells
Washed PBMCs were seeded onto ELISpot plates with 200 000 cells placed in each well; at least 3 wells for each antigen were seeded. The plates were incubated overnight at 37°C in 5% carbon dioxide and 95% humidity. The cells were washed in PBS-Tween and bound immunoglobulin G (IgG) antibody was detected using an alkaline phosphatase conjugate and AP-conjugate substrate kit (nitroblue tetrazolium + 5-bromo-4-chloro-3-indolyl phosphate in dimethylformamide; Bio-Rad).
Detection of Memory B Cells
PBMCs prepared as described above were resuspended in RPMI with 10% NBBS and placed in 96-well round bottom culture plates (Costar) at a final concentration of 2 × 106 cells/mL. The cells were cultured with an additional 100 μL of RPMI with 10% NBBS, and (final well concentrations) Staphylococcus aureus Cowan strain (SAC) at 1:5000 dilution of the Pansorbin cell suspension (Calbiochem-Novabiochem), pokeweed mitogen at 1:6000 dilution (Sigma-Aldrich), and CpG oligonucleotide at a 1:40 dilution (ODN-2006; TCG-TCG-TTT-TGT-CGT-TTT- GTC-GTT, InvivoGEN). The cells were incubated for 6 days at 37°C in 5% carbon dioxide and 95% humidity, after which time they were washed in PBS-Tween and seeded onto ELISpot plates and processed as described above for the detection of plasma cells.
ELISpot Counting
Spots were counted using the AID ELISpot Reader ELR02 and software version 3.3 (Cadama Medical). Identical settings were used for all plates and antigens. All plates were manually verified and any artifacts removed; the operator was blinded to the sample being counted.
Phenotypic Analysis by Flow Cytometry
B cells were purified (94%–98%) from fresh PBMCs, following Fc Receptor (FcR) blocking, using anti-CD19 microbeads and AutoMACS (Miltenyi Biotec). The B-cells were labeled with combinations of anti-CD21–fluorescein isothiocyanate (FITC) (Serotec), anti–CD5-APC, anti–CD23-PE, anti–CD27-PE, anti–IgM-PECy5, and anti–IgG-PE (BD Biosciences). Specificity of the B-cells was determined using biotinylated pneumococcal capsular polysaccharides labeled with anti–Biotin-FITC (Miltenyi Biotec). The cell data were acquired and analyzed using a BD FACSCalibur and CellQuest software.
Plasma cell populations were identified by intracellular labeling of CD19-sorted B cells for immunoglobulin (Ig). B-cells were permeabilized using BD Cell Fix/Perm Kit (BD Biosciences). The population was identified using CD38-PE(hi), CD20-APC(lo), CD19-PECy5(+) and IgA, IgG, or IgM-FITC(+).
Statistical Analysis
The primary outcome was based on antibody response, which has been published separately (12). The secondary outcomes relating to B-cells were to characterize the phenotype of the B-cells involved in the response to immunization with 23vP and PCV7; to assess the memory B-cell response to a single dose of PCV7 and 23vP; and to assess the effect of prior immunization with 23vP on the memory B-cell response to 1 or 2 doses of PCV. The sample size for memory B-cell analysis was determined by 2 interim analyses, the first performed after the first 150 participants completed the second visit and the second after the same participants had completed the fourth visit. Phenotypic analysis was performed on blood sampled at the fourth study visit when sufficient blood was available. A subset of participants who agreed to an additional venipuncture had a blood sample drawn 7 days after their last vaccination. The final data set available was determined by the practical constraints including the volume of blood collected and the assay failure.
B-cell numbers were log-transformed to obtain normality for analysis, but untransformed numbers with median values are presented as descriptive data. Analyses were performed by adjusting for baseline frequencies. The P values were adjusted for multiple comparisons using the false discovery rate method [13]. Significance was set at α < .05%. Data were analyzed using Stata software, version 9.1 (StataCorp).
RESULTS
Samples from at least 150 individuals at each study time point were obtained for B-cell studies. Approximately 1050 individual samples were analyzed. In addition, 18 samples were obtained at 7 days after the final vaccination for analysis of plasma cell response.
Memory B Cells After a Single Dose of 23vP or PCV7
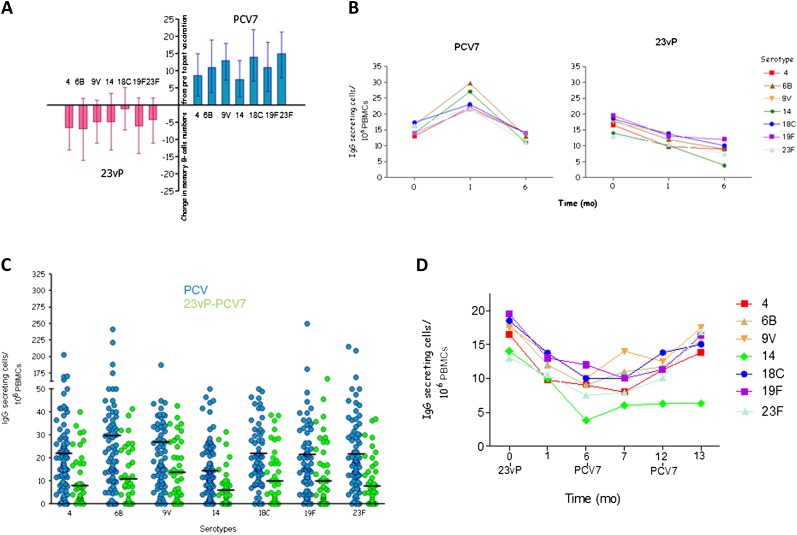
Prior to vaccination, 86% of participants evaluated had detectable memory B-cells that secreted antibody to at least 1 of the 7 serotypes tested, despite no history of previous pneumococcal vaccination. Twenty-eight days after vaccination there was an increase in the frequency of memory B-cells detected in those who had received PCV7. In contrast, participants who received 23vP showed a decrease in the memory B-cell frequency from baseline (Figure 1A). Six months after vaccination, the memory B-cell frequency in those who had received PCV7 returned to baseline, whereas in those who had received 23vP a further decline was shown (Figure 1B).
Figure 1.
Downregulation of memory B cells by the 23-valent unconjugated pneumococcal polysaccharide vaccine (23vP) and subsequent recovery by a 7-valent pneumococcal conjugate vaccine (PCV7). A, Participants were vaccinated with either 1 dose of 23vP (red) or 1 dose of PCV7 (blue). Calculation of the mean change in serotype specific memory B-cells (MBC) from before to after vaccination showed an increase for all serotypes after PCV7 but a decrease after 23vP. MBC responses significantly differed between the vaccines for serotypes 4 (P = .045), 9V (P = .042), 18C (P = .045), 19F (P = .035), and 23F (P = .0149) B, The decline in MBCs seen after 23vP continued for 6 mo after vaccination. C, When participants who had received 23vP were given a dose of PCV7 6 mo later, there was an increase in their MBCs, but the number of MBCs after 23vP-PCV7 (green) remained lower than after a single dose of PCV7 (blue) alone. Horizontal bar represents median value, and each spot represents 1 sample (serotype 4, P = .038; serotype 6B, P = .006; serotype 9V, P = .003; serotype 14, P = .0009; serotype 18C, P = .007; serotype 19F, P = .008; serotype 23F, P = .0009). D, After a second dose of PCV7, memory B-cell numbers recovered to baseline values. All comparisons were made using analysis of covariance and P values adjusted using a false recovery method. Abbreviation: PBMCs, peripheral blood mononuclear cells.
Memory B-Cell Responses After Primary Vaccination With 23vP or PCV7
Memory B-cell (MBC) responses to PCV7 given 6 months after 23vP were significantly lower than after a single dose of PCV7 for all serotypes except serotype 14 (Figure 1C), but recovery of MBC levels was seen after the second dose of PCV7 (Figure 1D). When 23vP was administered after 1 or 2 doses of PCV7, a decrease in MBC was recorded.
Phenotypic Analysis
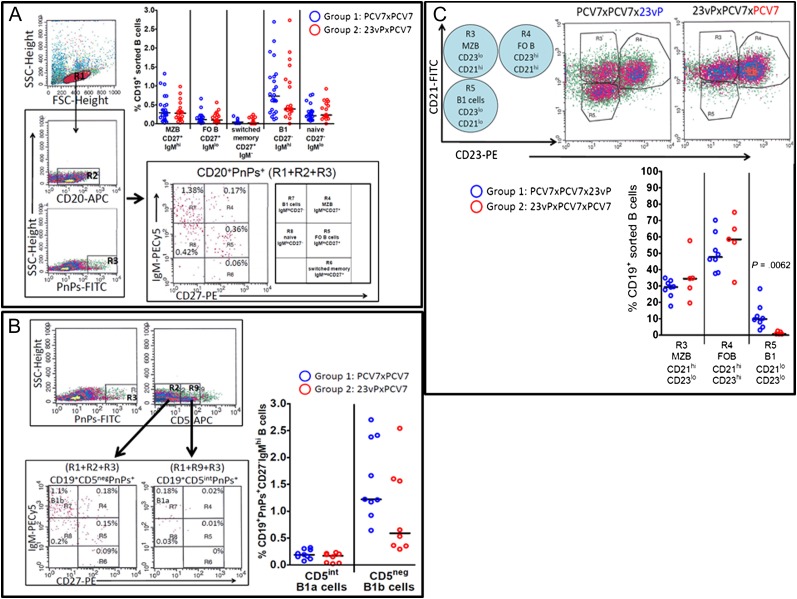
The proportion of pneumococcal polysaccharide (PnPs), specific MZB (CD19+CD20+CD27hiIgMhi), and FO B-cells (CD19+CD20+CD27hiIgMlo) in peripheral blood 1 month after the second vaccination in the series did not differ between the 2 groups (PCV7-PCV7, n = 24 vs 23vP-PCV7, n = 19; Figure 2A). However, there were fewer PnPs-specific switched MBCs (CD19+CD20+CD27hiIgM−) in the 23vP-PCV7 group than in the PCV7-PCV7 group. There were also fewer PnPs-specific cells of the B1-cell phenotype (CD19+CD20+CD27−IgMhi) in the 23vP-PCV7 group (Figure 2A). The B1-cell subset was further analyzed for expression of CD5 to differentiate between B1a (CD5int) and B1b (CD5neg) cells (Figure 2B). The B1a population (CD19+PnPs+CD5intCD27−IgMhi) did not differ between the 2 groups. However, fewer B1b-cells (CD19+PnPs+CD5negCD27−IgMhi) were seen in those who had received 23vP-PCV7 (n = 8; Figure 2B).
Figure 2.
Depletion of polysaccharide-specific B1b-cells in 23-valent unconjugated pneumococcal polysaccharide vaccine (23vP)–primed individuals. A, Subjects were randomized to receive 7-valent pneumococcal conjugate vaccine (PCV7)-PCV7 (group 1; blue circles, n = 24) or 23vP-PCV7 (group 2; red circles, n = 19). CD19+ purified B-cells were obtained 28 d after the second vaccine dose. Following gating on CD20+ pneumococcal polysaccharide (PnPs+) B-cells, the proportions of naive (CD27−IgMlo), B1 (CD27−IgMhi), MZB (CD27hiIgMhi), FO (CD27+IgMlo), and switched memory B-cells (CD27+IgM−) that bound PnPs–fluorescein isothiocyanate (FITC) were determined. The data in the graph show the percentage of PnPs-specific B-cells within each of the subsets for each vaccine group, and the bar represents the median. B, B1b-cell but not B1a-cell frequency was reduced in 23vP-primed individuals. Purified CD19+ B-cells were labeled with anti-CD5, and the frequency of B1a (CD19+PnPs+IgMhiCD27−CD5int) vs B1b (CD19+PnPs+IgMhiCD27−CD5neg) cells were determined for group 1 (blue, n = 9) and group 2 (n = 8). The data represent the percentage of CD19+PnPs+CD27−IgMhi B cells that were CD5int or CD5neg, and the bar shows the median (CD5 bright cells were residual T cells remaining after the sort). C, Boosting with 23vP induced a large B1-cell efflux into peripheral blood on day 7: PCV7-primed individuals received a 23vP booster (group 1: n = 8, blue circles) whereas those primed with 23vP-PCV7 were boosted with PCV7 (group 2: n = 5, red circles). Purified CD19+ B cells were then obtained on day 7 postboost, and the frequency of CD19+ marginal zone B-cells (CD23−CD21hi), follicular origin B-cells (CD23+CD21−), and B1 cells (CD23−CD21−) present in peripheral blood mononuclear cells (PBMCs) on day 7 postboost in each group were compared. The graph represents the percentage of CD19+ B-cells within each subset, and the line shows the median value.
In order to assess the early T-independent versus T-dependent B-cell response, blood was sampled 7 days following the final vaccine in each group, either PCV (23vP-PCV-PCV, n = 5) or 23vP (PCV-PCV-23vP, n = 8) (Figure 2C). When either 23vP or PCV7 was given as the final dose in the series, the production of MZB cells (R3-CD21hiCD23lo) was similar between the groups (Figure 2C). However, PCV7 induced a greater number of FO B-cells (R4-CD21loCD23hi), the putative precursors of switched MBC (Figure 2C). Conversely, 23vP induced a significantly higher percentage of B1-cells (R5-CD21loCD23lo; P = .0062, Figure 2C).
Plasma Cell Response
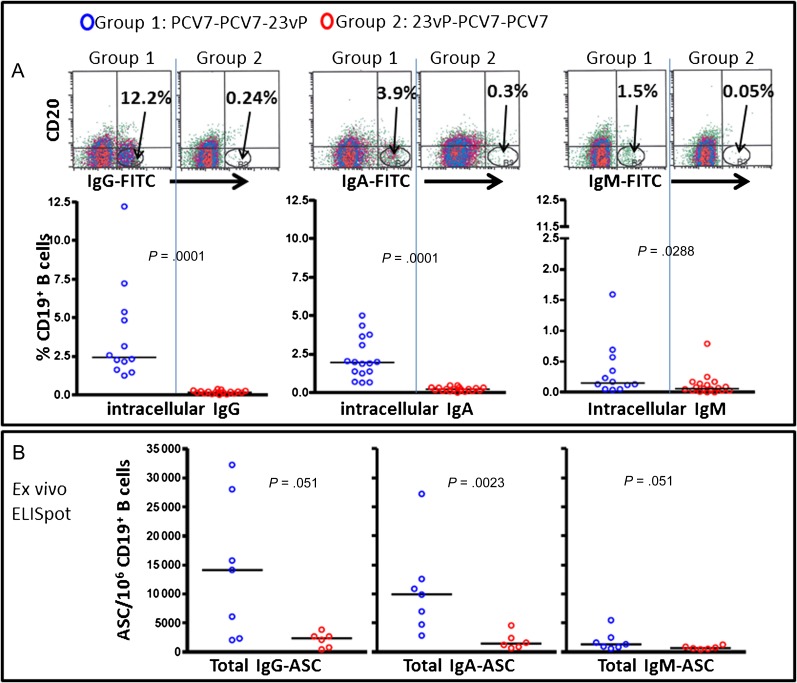
There were significantly higher frequencies of intracellularly labeled IgG+, IgA+, and IgM+ plasma cells (CD19+CD20−CD38hi) following a final vaccine dose of 23vP than PCV7 (P = <.0001, P = <.0001, and P = .0288, respectively) (Figure 3A). Similarly, the total number of IgG-, IgA-, and IgM-secreting plasma cells (ASCs) in peripheral blood on day 7 was significantly higher after boosting with 23vP when compared with PCV7 (P = .051, P = .0023, and P = .051, respectively; Figure 3B).
Figure 3.
Boosting of 7-valent pneumococcal conjugate vaccine (PCV7)–primed individuals with the 23-valent unconjugated pneumococcal polysaccharide vaccine (23vP) induced a large efflux of plasma cells 7 d postboost. Purified CD19+ B-cells were obtained 7 d after boosting with 23vP (group 1, blue) or PCV7 (group 2, red). A, Plasma cells were identified using the phenotype (CD19+CD20lo/-CD38hi) and intracellular immunoglobulin G (IgG), immunoglobulin A (IgA), or immunoglobulin M (IgM) expression was determined. Example density plots showing the plasma cell populations (IgG, IgA, and IgM) for each group are given, and the graphs show the frequencies (with median) as a percentage of CD19+ B-cells for group 1 (n = 12) and group 2 (n = 18). B, B-cells isolated from some of the same individuals were also seeded directly onto enzyme-linked immunosorbent spot assay (ELISpot) plates to detect the total number of IgG-, IgA-, and IgM-secreting cells for group 1 (n = 7) and group 2 (n = 5).
DISCUSSION
This is the first study to our knowledge to demonstrate that pneumococcal conjugate and polysaccharide vaccines produce distinct B-cell responses in adults and to establish potential cellular mechanisms responsible for polysaccharide-induced hyporesponsiveness.
The detection of memory B-cells following immunization with PCV7 in older adults in this study indicates that this vaccine induces a T-dependent response in this age group and therefore may have an immunological advantage over 23vP, which does not. It may be that repeated doses of PCV7 allow immunity to be sustained. Although the use of repeated doses of 23vP in adults to sustain immunity cannot be supported owing to the induction of attenuated responses to subsequent pneumococcal vaccine, it is likely that carrier protein–derived peptides, presented in the context of major histocompatibility complex class II by B-cells to cognate T cells, are responsible for driving the response through germinal centers, resulting in MBC production. It has also been postulated that conjugation of polysaccharides reduces the repetitive nature of their epitopes [14] limiting the T-independent stimulus through the B-cell receptor, leading to the preferential selection of B-cells for entry into germinal centers rather than driving extrafollicular proliferation. A recent study, however, during which older adults were revaccinated with 23vP 10 years after their last dose, demonstrated that the antibody response to 23vP was preserved and was not hyporesponsive [15]. We propose that this relates to recovery of memory B-cells over time.
The lack of memory B-cell production following immunization with 23vP is consistent with its T-independent nature. Polysaccharide antigens are believed to predominantly activate MZB cells in extrafollicular reactions that do not lead to MBC induction. In this study it was demonstrated that immunization with 23vP resulted not only in a lack of MBC induction but a decrease in the frequency of peripheral MBCs compared with prevaccination levels. This finding is in keeping with the hypothesis that polysaccharide antigens drive preexisting switched memory B cells into terminal differentiation and are unable to replenish the MBC pool [8].
The depletion of the peripheral MBC pool after immunization with 23vP may result in a lower frequency of peripheral B-cells being available to respond to subsequent doses of pneumococcal vaccine (or indeed natural antigen) as seen in this study. This phenomenon may explain why attenuated antibody responses are produced to subsequent doses of pneumococcal vaccination in individuals previously immunized with 23vP. Depletion of memory B-cells and antibody hyporesponsiveness induced by 23vP may in part explain the limited effectiveness and short-term immunity associated with this vaccine in the elderly [3, 4]. These results contrast with those reported by Baxendale et al [16], who found no differences in memory B-cell responses between these 2 pneumococcal vaccines in adults. However, the small sample size in the 23vP group in the previous study and methodological differences in the in vitro B-cell assay make it impossible to directly compare the findings. However, both studies clearly demonstrate that adults have serotype-specific memory B-cells detectable prior to pneumococcal immunization.
The significance of the large plasma response induced by 23vP when given as the second vaccine in the series is not clear. It may be that 23vP induces a large plasma cell response by virtue of its high polysaccharide content. Alternatively, it may be that the initial dose of PCV7 has led the production of MBCs meaning that there is a greater pool of B-cells available to respond to 23vP, resulting in a large plasma cell response.
The B-cell subsets involved in the response to pneumococcal vaccines in adults are yet to be clarified. The peripheral B-cell compartment consists of 2 main subsets—B1 and B2 cells. The B2 subset consists of MZB cells and FO B cells (which are the precursors to switched MBCs). Classically, polysaccharide antigens are thought to activate MZB cells, whereas protein antigens activate FO B cells [17, 18]. The B1 subset, less well characterized in humans than in rodents, is believed to originate from the fetal liver and adult bone marrow and be involved in the early, nonadaptive phase of the immune response [19]. In this study we demonstrate no differences in the frequency of serotype-specific MZB cells 1 month or 7 days after vaccination with 23vP or PCV7 when given as the second vaccine in the series, suggesting that both vaccines are equally able to stimulate the marginal zone subset, perhaps because the PCV7 formulation also contains unconjugated polysaccharide, which is responsible for MZB-cell activation. However, assessment of changes in the frequency of this subset in the peripheral compartment might be an insensitive measure as the majority of these cells are located in the spleen. When the frequencies of switched MBCs were compared after 2 doses of vaccine, individuals who had received 23vP (23vP-PCV7) as their primary vaccination had fewer of this cell type than those who had received 2 doses of PCV7. This difference may be explained by the depletion of the switched MBC subset by 23vP or the induction of this subset by PCV7. The latter is supported by the higher frequency of FO B cells (the precursors to switched MBCs) detected 7 days after vaccination with PCV7 (as the second vaccine in the series) when compared with 7 days after 23vP (preceded by PCV7). An unexpected finding was that in the 23vP-PCV7 group there were also significantly fewer cells of the B1b phenotype compared with the PCV7-PCV7 group 1 month after the second vaccination. In addition, 7 days after 23vP (preceded by PCV7) a significantly higher percentage of B1-cells were detected in comparison to the PCV7-PCV7 group. These data suggest that 23vP may specifically interact with the B1-cell subset leading to their activation as antibody-secreting cells and subsequent depletion, a finding that to our knowledge has not previously been described in humans. B1b-cells were found previously to play an essential role in the protection of mice against blood-borne pathogens such as S. pneumoniae and Borrelia hermsii [20, 21]. B1b-cells are part of a self-replenishing population that derives from low-frequency bone marrow precursors in adult mice [22]. B1b-cells are not thought to engage in follicular responses and therefore do not generate MBCs. Thus, terminal differentiation of large numbers of B-cells from this subset by 23vP could have a long-lasting impact on the peripheral population. This may contribute to fewer B-cells being available to respond to subsequent antigen challenge after vaccination with 23vP, as supported by our data.
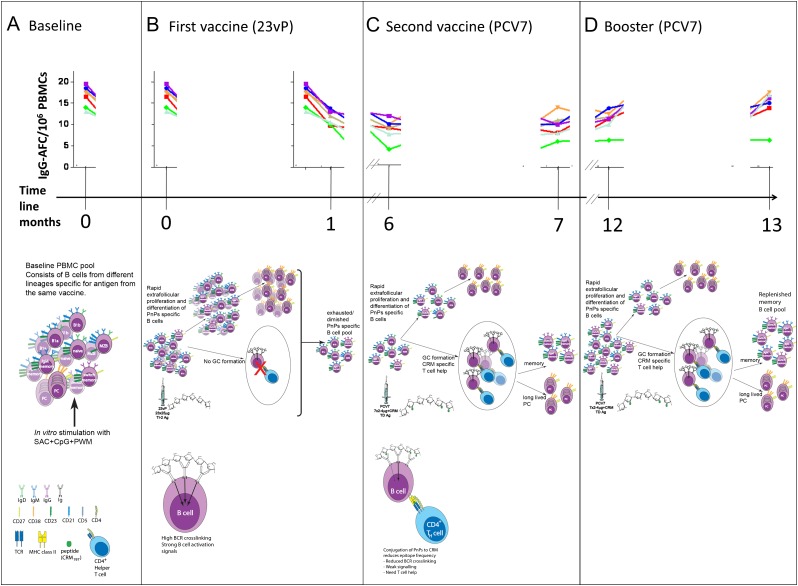
The cellular mechanisms responsible for 23vP-induced antibody hyporesponsiveness have not been fully elucidated, but using the observations outlined above, we propose a new model incorporating B1b-cells (Figure 4A–D).
Figure 4.
Priming with 23-valent unconjugated pneumococcal polysaccharide vaccine (23vP) induces depletion of peripheral blood B1b and marginal zone B (MZB) cells—a mechanism. The top section of each panel is a representation of the B-cell enzyme-linked immunosorbent spot assay (ELISpot) data obtained, for each serotype in 7-valent pneumococcal conjugate vaccine (PCV7), at each point during the time course (x-axis). The middle section of each panel describes the effects of each immunization on the B-cell compartment. The bottom sections of panels B and C show the interaction of multivalent, high-epitope-density T-independent antigens vs low-epitope-density T-dependent antigens with a responsive B cell. A, Prior to immunization, the baseline composition of the peripheral B-cell pool is a mix of preexisting memory B cells, B1 cells, and MZB cells that can be stimulated to form immunoglobulin G (IgG)-ASCs following in vitro stimulation with mitogens, and these ASCs are detected in B-cell ELISpots at day 0. B, The polysaccharides in 23vP consist of multivalent (repeating) epitopes that are able to cross-link many B-cell receptors (BCRs) on a single cell providing a strong activation signal. Thus, the high dose of multivalent polysaccharides from 23 serotypes in 23vP may induce exhaustion of the peripheral B-cell pool by preferentially driving extrafollicular proliferation of B1b, memory B, MZB, and naive B cells via cross-linking BCRs, inducing a large plasma cell response. The lack of T-cell recruitment and germinal center formation prevents replenishment of the memory B-cell pool. This is demonstrated by the reduced frequency of ASCs detected in the ELISpot 1 mo postimmunization. C, Reduced frequency of polysaccharide specific B cells is still evident 6 mo later, and a subsequent dose of PCV7 is unable to induce increased frequencies of polysaccharide-specific B cells. The conjugated polysaccharide has reduced epitope density compared with the plain polysaccharide and cross-links fewer BCRs. This results in a weaker signal, and thus T-cell help is required for the B cell to proliferate. This occurs in the germinal center and results in formation of new memory B cells. However, the hyporesponsiveness observed in the ELISpot assay shows that 1 dose of PCV7 is not enough to overcome the effects of 23vP. D, The booster dose of PCV7 induces an increase in memory B cells detected by ELISpot demonstrating that by month 13 the hyporesponsiveness induced by 23vP has been overcome for the 7 serotypes in PCV7 and that the memory B-cell pool has been replenished for these serotypes. Abbreviation: PMBC, peripheral blood mononuclear cell.
The clinical significance of hyporesponsiveness in humans is unknown. Antibody hyporesponsiveness or immune tolerance to repeated vaccination with polysaccharide antigens was first described in mice by Felton and Bailey in 1926 [23]. They showed that administration of large amounts of serotype-specific polysaccharide to mice rendered them more susceptible to infection with that serotype and unresponsiveness to vaccination with that serotype. Moreover, the role of B1b-cells in the protection of mice against S. pneumoniae, which we found were depleted by 23vP, has been established [21]. These observations in mice, taken together with our finding that the same B-cell subsets are affected by plain polysaccharides in humans, is of concern. The amount of polysaccharide used for immunization has been shown to influence the induction of immune tolerance. 23vP contains at least 575 μg of polysaccharide, whereas PCV7 contains <20 μg; therefore, 23vP-induced hyporesponsiveness may be related to its high polysaccharide content. Furthermore, polysaccharide antigen has been shown to persist for prolonged periods of time in vivo [23]. Indeed, retention of pneumococcal polysaccharide in mice has been described for up to 18 months following immunization, depending on the serotype tested [24] and could lead to depletion or exhaustion of the B-cell pool due to chronic antigen stimulation. The generation of MBCs by PCV7 may potentially overcome antibody hyporesponsiveness by expanding the B-cell pool available to respond to subsequent antigen challenge. Despite the recovery of MBCs, depleted by the first dose of 23vP, using 2 doses of PCV7 antibody responses remained attenuated during this 12-month study [12], although recovery has been demonstrated with longer duration between doses with group C meningococcal vaccines for young adults [25]. However, these observations do suggest that memory B cells, depleted by polysaccharide vaccine, could be recovered by immunization with PCV.
In the original study from which these data were obtained, all participants received 2 doses of PCV7 and 1 dose of 23vP over a 12-month period [12]. The immunogenicity produced by the 3-dose vaccine schedule did not significantly improve on a single dose of PCV7. In addition to the lack of superiority, such a schedule may have also been problematic in terms of both compliance and cost, which need to be carefully considered when designing vaccine schedules.
In summary, we show that PCV7 induces MBC production, whereas 23vP causes depletion in the peripheral MBC population. The latter may explain antibody hyporesponsiveness induced by 23vP to subsequent vaccination, which may contribute to the limited and poorly sustained effectiveness of the vaccine. Induction of MBC by PCV7 provides the possibility that it could be used in individuals previously immunized with 23vP to overcome hyporesponsiveness, but the timing and dosing required have not been determined. With the availability of PCVs covering 10 and 13 serotypes, the possibility of improved protection against pneumococcal disease in the elderly must be explored in efficacy trials as soon as possible. The data provided in this study emphasize the immunological disadvantage of the current vaccine, 23vP, given to vast numbers of older adults [26].
Notes
Author contributions.
Obtaining funding: A. J. P., P. B., and D. M.; study concept and design: A. J. P.; study supervision: A. J. P., P. B., D. M., B. A.; acquisition of data: R. L., E. A. C., E. A. B., J. B.; analysis and interpretation of data: R. L., E. A. C., A. J. P., T. P., and L.-M. Y.; drafting of manuscript: R. L. and E. A. C.; critical revision of manuscript for important intellectual content: R. L., E. A. C., A. J. P., B. A., T. P., D. M., P. B., and L.-M. Y.; statistical analysis: L.-M. Y.
Acknowledgments.
The authors are grateful to all the study participants, without whom this study would have not been possible, and to the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, the NIHR Thames Valley Comprehensive Local Research Network, and Wyeth Vaccines for funding. The authors are also grateful to Diane Kirby, Sarah Kelly, Mushiya Mpelembue, and Maheshi Ramasamy for conducting study visits; Tessa John and Matthew Snape for their advice on study management; Health Protection Agency Laboratory, Manchester, for multiplex antibody analysis; Stephen Lockhart, William Gruber, and Michael Pride at Wyeth Vaccines for helpful discussions about the study, and Hilary Watt for review of the manuscript. A. J. P. is a Jenner Institute Investigator and James Martin Senior Fellow.
Financial support.
This work was supported by Wyeth Vaccines and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre with support from the NIHR Thames Valley Comprehensive Local Research Network. Neither Wyeth nor Oxford Biomedical Research Centre were involved in the study design; study conduct; collection, management, analysis, or interpretation of these data; or preparation or approval of the manuscript.
Potential conflicts of interest.
A. J. P. has conducted clinical trials on behalf of Oxford University sponsored by manufacturers of pneumococcal vaccines and does not accept any personal payments from vaccine manufacturers; grants for support of educational activities are paid to an educational/administrative fund held by the Department of Paediatrics, Oxford University. R. L. has received financial assistance from Wyeth Vaccines and GlaxoSmithKline to attend scientific meetings. All other authors: no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Melegaro A, Edmunds WY, Pebody R, Miller E, George R. The current burden of pneumococcal disease in England and Wales. J Infect. 2006;52:37–48. doi: 10.1016/j.jinf.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Haas A, Scott P, Stuck A, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro ED, Berg AT, Austrian R. The protective efficacy of the polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–60. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 4. Department of Health, UK. Joint committee on vaccination and immunisation. http://www.advisorybodies.doh.gov.uk/jcvi/mins-pneumococcal-070907.htm. Accessed 31 July 2010.
- 5.Musher DM, Manoff SB, Liss C, et al. Safety and antibody response, including antibody persistence for 5 years after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–24. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 7.MacLennan J, Obaro S, Deeks J. Immunological memory 5 years after meningococcal A/C conjugate vaccination in infancy. J Infect Dis. 2001;183:97–104. doi: 10.1086/317667. [DOI] [PubMed] [Google Scholar]
- 8.Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- 9.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy: possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–6. [PubMed] [Google Scholar]
- 10.Black S, Shinefield H, Baxter R, et al. Impact of the use of heptavalent pneumococcal conjugate vaccine on disease epidemiology in children and adults. Vaccine. 2006;24(Suppl 2):S2-79–80. doi: 10.1016/j.vaccine.2005.01.132. [DOI] [PubMed] [Google Scholar]
- 11.Weller S, Reynaud CA, Weill JC. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 2005;26:85–9. doi: 10.1016/j.it.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus R, Clutterbuck E, Yu LM, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis. 2011;52:736–42. doi: 10.1093/cid/cir003. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Yekutieli D. False discovery rate-adjusted multiple confidence intervals for selected parameters. J Am Stat Assoc. 2005;100:71–81. [Google Scholar]
- 14.Paus D, Giang Phan T, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal centre B cell differentiation. J Exp Med. 2006;203:1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musher DM, Manoff SB, McFetridge RD, et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccines. 2011;7:1–9. doi: 10.4161/hv.7.9.15996. [DOI] [PubMed] [Google Scholar]
- 16.Baxendale HE, Keating SM, Johnson M, Southern J, Miller E, Goldblatt D. The early kinetics of circulating pneumococcal-specific memory B cells following pneumococcal conjugate and plain polysaccharide vaccines in the elderly. Vaccine. 2010;28:4763–70. doi: 10.1016/j.vaccine.2010.04.103. [DOI] [PubMed] [Google Scholar]
- 17.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-dependent (TI-2) antigens. Eur J Immunol. 1985;15:508–12. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 18.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire: marginal zone and B1 B-cells as part of a natural immune memory. Immunological Rev. 2000;175:70–9. [PubMed] [Google Scholar]
- 19.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan ICM. Sites of specific B-cell activation in primary and secondary response to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 20.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique function roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Montecino-Rodriguez EH, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 23.Felton LD, Bailey G. Biological significance of the soluble specific substances of pneumococci. J Infect Dis. 1926;38:131–44. [Google Scholar]
- 24.Felton LD, Kauffmann G, Prescott B, Ottinger B. Studies on the mechanism of the immunological paralysis induced in mice by pneumococcal polysaccharides. J Immunol. 1955;74:17–26. [PubMed] [Google Scholar]
- 25.Richmond P, Kaczmarski E, Borrow R, et al. Meningococcal C vaccine induces immunological hyporesponsiveness in adults that can be overcome by meningococcal C conjugate vaccine. J Infect Dis. 2000;181:761–4. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 26.Hak E, Sanders EA, Verheij TJ, et al. Rationale and design of CAPITA: a RCT of 13-valent conjugated pneumococcal vaccine efficacy among older adults. Neth J Med. 2008;66:378–83. [PubMed] [Google Scholar]