Abstract
Background. CD4− Vγ2Vδ2 T cells are depleted during human immunodeficiency virus (HIV) infection but can recover to near normal levels in patients who spontaneously control viremia in the absence of therapy. By contrasting Vγ2Vδ2 T-cell numbers, phenotype, and T-cell receptor (TCR) repertoire, we investigate the dynamic tension between active immunity and progressive T-cell destruction during persistent viremia.
Methods. Peripheral blood Vγ2Vδ2 T-cell levels and phenotypes were characterized by flow cytometry. Lymphoproliferation assays measured functional responses. Spectratyping characterized damage to the TCR repertoire.
Results. Levels, responses to antigen and the proportion of T effector memory Vγ2Vδ2 T cells in patients with persistent viremia, were intermediate between patients with natural virus suppression (NVS) and patients receiving antiretroviral therapy. Damage to the TCR γ-2 chain repertoire and depletion of CD56+ Vγ2Vδ2 T cells were more pronounced in viremic patients, compared with antiretroviral therapy recipients and patients with natural virus suppression.
Conclusions. Characteristics of Vγ2Vδ2 T cells in viremic patients reflect both active responses (increasing cell numbers, better antigen responses, and higher proportion of effector memory cells) and ongoing damage (repertoire changes and loss of CD56+ cells). Unlike patients who control viremia to undetectable levels, Vγ2Vδ2 T cells are diminished during persistent viremia and may eventually be lost because of progressive destruction of the TCR repertoire.
Human immunodeficiency virus (HIV) infection leads commonly to chronic CD4 cell depletion with opportunistic infections, cancer, coronary disease, or metabolic complications. The spectrum of outcomes after HIV infection include extremely early onset of disease with rapid, fatal progression, which was attributed to a lack of virus-specific immune response [1]; slow disease progression [2–4] with stable CD4 cell counts over years and persistent viremia in the absence of antiretroviral therapy [5, 6]; strong natural virus suppression [7] with prolonged undetectable viremia (except for occasional virus spikes) without treatment; or the more common condition, in which progressing disease requires and responds well to therapy.
Our previous studies documented near complete loss of phosphoantigen-responsive Vγ2Vδ2 T cells in patients who initiated highly active antiretroviral therapy (HAART) once their CD4 cell counts were <200 cells/mm3 [8]; Vγ2Vδ2 T-cell levels in these patients with more-advanced disease were significantly lower than among controls or patients receiving antiretroviral therapy and having a CD4 cell count of ≥350 cells/mm3 [9]. Higher levels of Vγ2Vδ2 T cells and more-functional Vγ2Vδ2 T-cell responses were observed among treated patients who initiated therapy earlier [10], and the highest Vγ2Vδ2 T-cell levels were seen in natural virus suppressors who control viremia in the absence of therapy [11]. Evaluation of former plasma donors in China [12] who were infected with HIV between 1992 and 1995, provided samples during 2004–2006 for our studies, and were positive for highly related strains of clade B HIV showed a direct relationship between loss of Vγ2Vδ2 T cells and either increasing HIV load or declining CD4 cell levels. Slower rates of CD4 T-cell loss in HIV-infected Chinese former plasma donors were most apparent in subgroups distinguished by higher levels of effector memory Vγ2Vδ2 T cells [12]. Thus, the Vγ2Vδ2 T-cell levels, functional responses, and proportion of effector memory cells were related to markers of HIV disease.
We wondered how persistent viremia would affect Vγ2Vδ2 T-cell levels and function even if disease progression was slow. This is studied most easily among patients who have a detectable HIV load during routine testing because they do not receive antiretroviral therapy, designated here as persistently viremic individuals. Most members of our persistently viremic cohort were infected with HIV for 2–23 years and can be classified as long-term nonprogressors. However, the population of long-term nonprogressors is heterogeneous [13], and various mechanisms, including neutralizing antibody [14, 15], diminished spontaneous apoptosis [16], overrepresentation of protective major histocompatibility complex class I alleles [17], accumulation of T-helper 17 cells [18], or changes to natural killer (NK) cell subsets [19], have been invoked to explain slow disease progression despite persistent viremia. We studied this group to search for effects of persistent viremia on Vγ2Vδ2 T cells. We showed recently that Vγ2Vδ2 T cells can be killed by direct exposure to HIV envelope glycoprotein [20], which leads to a prediction that depletion will be most pronounced in viremic patients. However, persistently viremic patients in this study have elected not to receive antiretroviral therapy because their disease progression is slow or not apparent. If Vγ2Vδ2 T cells have a role in controlling disease but are eliminated by exposure to viral proteins, what is the status of this cell population in patients with stable disease but persistent viremia? By understanding the status of Vγ2Vδ2 T cells in persistently viremic patients, we will learn more about the impact of HIV on this cell subset and its potential role in combating HIV disease.
METHODS
Persistent Viremia and Comparator Groups
Patients in the persistently viremic cohort (hereafter, the “PV group”) had confirmed HIV infection, had no prior history of antiretroviral therapy except for prophylaxis during pregnancy, and had a detectable viral RNA load between 400 copies/mL (the lower limit of detection) and <12 000 copies/mL. Patients in the PV group were infected with HIV for up to 23 years. Informed consent was obtained from all subjects. The study protocol was approved by the Institutional Review Board at the University of Maryland, Baltimore. All peripheral blood mononuclear cells (PBMCs) used in this study were stored frozen for 2 years or less at the time they were assayed.
Results obtained with specimens from patients in the PV group patients were compared with samples from 3 comparator groups (Table 1). The first group included HIV-infected patients with normally progressing disease who were receiving antiretroviral therapy (hereafter, the “treated group”). Patients in the treated group had prolonged viral suppression (duration, ≥12 months) a CD4 cell counts >300 cells/mm3. This group was predominately African American (85%) and male (68%), had a mean age (±SD) of 46.7 ± 6.0 years, and had a mean CD4 cell count (±SD) of 512 ± 144 cells per mm3 [21]. The second comparator group consisted of 32 HIV-uninfected patients (hereafter, the “control group”); this group had a mean age (±SD) of 43.6 ± 12.5 years, 37% were male, and all were all African American [22]. The third comparator group was composed of 21 HIV-infected patients who were considered to have natural virus suppression because all had undetectable HIV loads without having received antiretroviral therapy (hereafter, the “NVS group”); all were African American [11]. Parts of the data for the control, NVS, and treated groups were drawn from published studies [11, 21]. The Vγ2Vδ2 T-cell assays (detection in PBMCs, phenotyping, repertoire analysis, and proliferation response) are standardized and have been used previously for retrospective or longitudinal analyses. Donors from the control group with stable Vγ2Vδ2 T-cell levels have been used for reference values in all of these studies. As an example, the T-cell receptor (TCR) γ-2 chain repertoire was >80% conserved over intervals of 10 years among subjects from the control group, who also showed consistent results in lymphoproliferation assays [8].
Table 1.
Demographic and Clinical Characteristics of Patients With Persistent Viremia (PV) and Patients in 3 Comparator Groups
| Characteristic | PV Group (n = 23) | NVS Groupa (n = 21) | Treated Groupa (n = 25) | Control Groupb (n = 32) |
| African American race, % | 100 | 100 | 85 | 100 |
| Age, years, mean ± SD | 45.47 ± 6.5 | 49.7 ± 8.5 | 46.7 ± 6.0 | 43.63 ± 12.5 |
| Male sex, % | 48 | 67 | 68 | 37 |
| CD4 cell count, cells/μL, mean ± SD | 705 ± 256 | 848 ± 301 | 512 ± 144 | Not donec |
Vγ2Vδ2 Proliferation
PBMCs were obtained from 23 patients in the PV group to measure proliferation responses to phosphoantigen. Cells were thawed and resuspended in Roswell Park Memorial Institute 1640 medium supplemented with 10% FBS, 2 mM l-glutamine (Invitrogen), 1 U/mL penicillin/streptomycin (Invitrogen), and 100 U/mL recombinant human interleukin 2 (IL-2; Tecin [Biological Resources Branch, National Institutes of Health, Bethesda, MD]). The phosphoantigen isopentenyl pyrophosphate (IPP; Sigma, St. Louis, MO) was added at a concentration of 15 μM to trigger Vγ2Vδ2 T-cell proliferation. Cultures were incubated for 14 days at 37°C and 5% CO2 and were replenished on days 3, 7, and 10 by adding IL-2–supplemented medium. Viable cell counts were performed using trypan blue dye exclusion. Cells were stained with anti-CD3 and anti-Vδ2 and then counted in a flow cytometer. The stimulation index (SI) represents the proportional increase in Vδ2+ cells following IPP stimulation, compared with cultures of specimens from the control group that were incubated with IL-2 alone. SI is defined as the ratio of Vδ2+ lymphocytes on day 14 of the IPP expansion to the number of Vδ2+ lymphocytes on day 14 without the IPP expansion. Proliferation assays are preformed routinely on viable, frozen PBMCs. In control studies, the coefficient of variation for SIs of longitudinal specimens from the control group (6–12 months between specimens) was ≤0.22.
Antibody Staining and Flow Cytometry
Viable frozen PBMCs from HIV-positive patients (ie, those in the NVS, PV, and treated groups) and donors from the control group were thawed, washed, and counted using Trypan dye exclusion to enumerate viable cells. Then, 3 × 105 cells were washed in Dulbecco’s phosphate-buffered saline (PBS; Invitrogen) and labeled with monoclonal antibodies at 4°C for 15 minutes. The following monoclonal antibodies were used in this procedure: fluorescein isothiocyanate (FITC)–conjugated anti-Vδ1 (Pierce Biotechnology, Rockford, IL), FITC-conjugated anti-CD3 (lot 83181; BD Pharmingen, San Diego, CA), phycoerythrin-conjugated anti-Vδ2 (clone B6; BD Pharmingen), allophycocyanin (APC)–conjugated anti-CD56 (lot 64453; BD Pharmingen, San Diego, CA), anti-CD45RA (Lot 22286; BD Pharmingen, San Diego, CA), APC-conjugated anti-CD27 (clone O323; eBioscience, San Diego CA), APC-conjugated anti-CD3 (clone UCHT1; BD Pharmingen), and the appropriate isotype controls (BD Pharmingen).
After staining, cells were washed once with PBS and fixed with 1% paraformaldehyde. At least 3 × 104 cells were examined on a FACSCalibur flow cytometer (BD Pharmingen). Flow cytometry data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
RNA Extraction, Reverse Transcription–Polymerase Chain Reaction (RT-PCR), and PCR
Total RNA was extracted from the remaining 106 cells (RNeasy Mini Kit; Qiagen, Valencia, CA); 1 μg of total RNA was then converted into complementary DNA, using the Reverse Transcription System (Promega, Madison, WI). The following primers were used to amplify Vδ2 chain sequences according to established methods [8, 9]: oligo-Vγ2 (5′-ATCAACGCTGGCAGTCC-3′), oligo-Cγ1 (5′-GTTGCTCTTCTTTTCTTGCC-3′), 5′ β-actin (5′-GTGGGGCGCCCCAGGCACCA-3′), and 3′ β-actin (5′-CTCCTTAATGTCACGCACGATTTC-3′).
Spectratype Analysis
Primer extension reactions were performed as described previously [23] to generate run-off products that were diluted and mixed with GeneScan-500 Rox size standards. After a denaturation step (5 minutes at 95°C followed by immediate quenching on ice), products were loaded on the 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA) and run for 27 minutes on performance-optimized polymer 7. Molecular size and relative frequency of extension products were determined using GeneMapper software (Applied Biosystems, Foster City, CA). To standardize the data irrespective of the run-off primer position, CDR3 length variation was expressed in terms of the total T-cell receptor γ-2 chain coding region lengths. Run-off product lengths were corrected by adding the lengths of known Vγ2 messenger RNA (mRNA) coding regions outside the run-off product. According to this calculation, the major peaks for Vγ2 chains are 990–996 nucleotides, on the basis of a corresponding run-off product length of 447 nucleotides. Spectratype data were expressed as the proportion of all T-cell receptor γ-2 chains with lengths of 990–996 nucleotides.
Statistical Analysis
The Mann–Whitney U test was used to compare Vγ2Vδ2 cell levels (the proportion of Vδ2+ in total lymphocytes), the stimulation indices (proliferation response to antigen), and the proportion of Vγ2Vδ2 effector memory T (Tem) cells (defined as cells that staining revealed to be CD27−CD45Ra−). The unpaired Student t test was used to analyze data for CD56 expression. Data were screened initially with the D’Agostino-Pearson omnibus normality test, which helped to define the analytical scheme.
RESULTS
A cohort was developed for HIV-infected patients (PV group) who do not receive antiretroviral therapy. We obtained PBMCs from 23 individuals in this cohort. Overall, patients were selected to compose a group whose age, sex, and race, regardless of CD4 cell count or HIV load, roughly matched the median values for comparator groups. The majority (21 of 23) individuals were infected for 5–23 years, had stable CD4 levels, and had persistent viremia, placing them in the group of long-term nonprogressors [24]. Two additional patients, who had been infected for 2 years and had viral loads of 1084 and 585 copies/mL, were added to improve the age and sex matching in terms of median values for each cohort and to be representative of all patients in this cohort. There were 11 males and 12 females; ages ranged from 36 to 59 years. Patients had median peripheral blood CD4 T-cell counts of 702 cells/μL (range, 202–1160 cells/μL). The median HIV load was 2194 copies/mL (range, 551–11 554 copies/mL). Data on CD4 cell count and HIV load were from tests done nearest to the date on which PBMCs were collected. All members of the PV group were self-reported to be African American and had no documented history of antiretroviral therapy except for pregnancy prophylaxis. Risk factors for infection included sexual transmission and injection drug use. In terms of age, sex, and race, the PV group was well matched to the NVS group [7], the treated group [21], and the control group [22]. It was important to match for race because baseline levels of Vγ2Vδ2 T cells are substantially lower for HIV-negative African Americans, compared with those in whites [22], and decline with age [25].
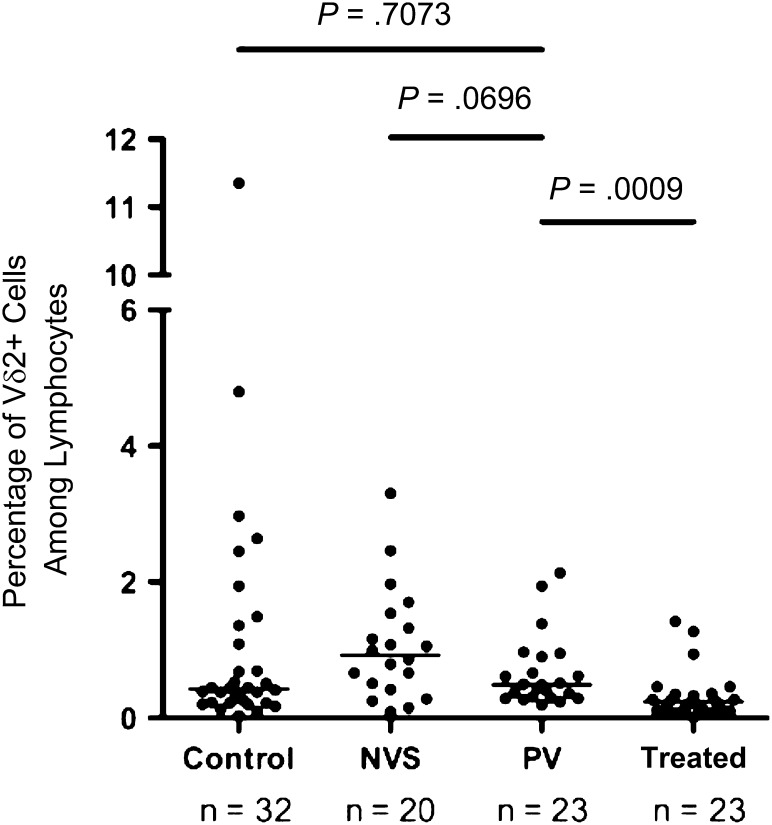
Flow cytometry was performed on PBMCs to detect the frequency of Vγ2Vδ2 T cells among circulating lymphocytes. The median frequency for Vγ2Vδ2 T cells in the PV group was 0.49% of total lymphocytes (Figure 1). Differences between median Vγ2Vδ2 T-cell frequency for the NVS group and the PV group trended to significance, with a P value of .07. The PV group was significantly different from the treated group with respect to median Vγ2Vδ2 T-cell frequency, with a P value of .001. Differences between the PV and control groups were not significant (P = .7073). Accordingly, the PV group appeared to have a lower number of circulating Vγ2Vδ2 T cells than the NVS group but a higher number than the treated group. Overall, levels of Vγ2Vδ2 T cells were greatest in patients in the NVS group, next highest among patients in the PV group, and lower among patients in the treated group.
Figure 1.
Vγ2Vδ2 T cell frequencies among patients with persistent viremia (PV; median, 0.49% of total lymphocytes [range, 0.19%–2.14%]; median count, 10.8 cells/mm3 [range, 4.9–53.0 cells/mm3]) are intermediate between values for patients with natural virus suppression (NVS) and patients treated with antiretrovirals (Treated). Flow cytometry with purified peripheral blood mononuclear cells defined the proportion of Vδ2+ T cells among all lymphocytes. Values are plotted here for individual donors. Horizontal lines represent median values. Statistical comparisons were performed using the Mann–Whitney U test; P values of <.05 were considered significant. Control, subjects without human immunodeficiency virus infection.
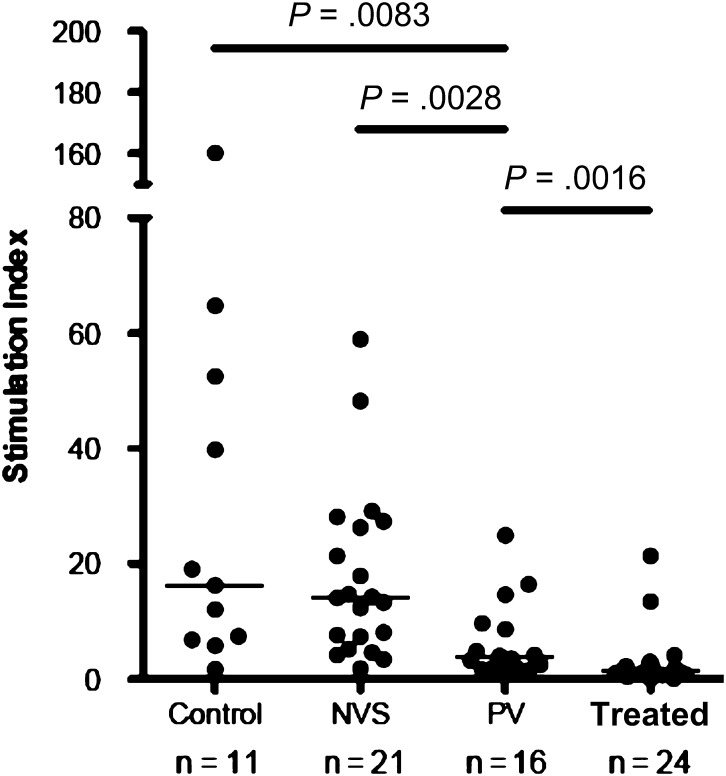
Among patients in the control group, the majority of circulating Vγ2Vδ2 T cells proliferated in response to phosphoantigens. The magnitude of response, measured as the SI for PBMCs from each of our clinical groups, showed whether antigen-responsive cells were present in the circulating lymphocyte pool (Figure 2). We found significant differences in median SIs between patients in the PV group and patients in the treated group (P = .0016), with patients in the PV group having a higher SI. In terms of phospho antigen responses, patients in the PV group ranked between patients in the NVS group and patients in the treated group, similar to the ranking that was based on Vγ2Vδ2 T-cell counts. Higher responses for patients in the NVS group [11] and patients in the PV group reflect cell populations that are already activated in vivo, possibly in response to the ongoing virus replication.
Figure 2.
Phosphoantigen-driven lymphoproliferation of Vγ2Vδ2 T cells from patients with persistent viremia (PV) was intermediate between patients with natural virus suppression (NVS) and patients treated with antiretrovirals (Treated). Plotted values are stimulation indices for individual patient samples after in vitro stimulation with isopentenyl pyrophosphate plus interleukin 2. Horizontal lines represent median values. Statistical comparisons were performed using the Mann–Whitney U test; P values of <.05 were considered significant. Control, subjects without human immunodeficiency virus infection.
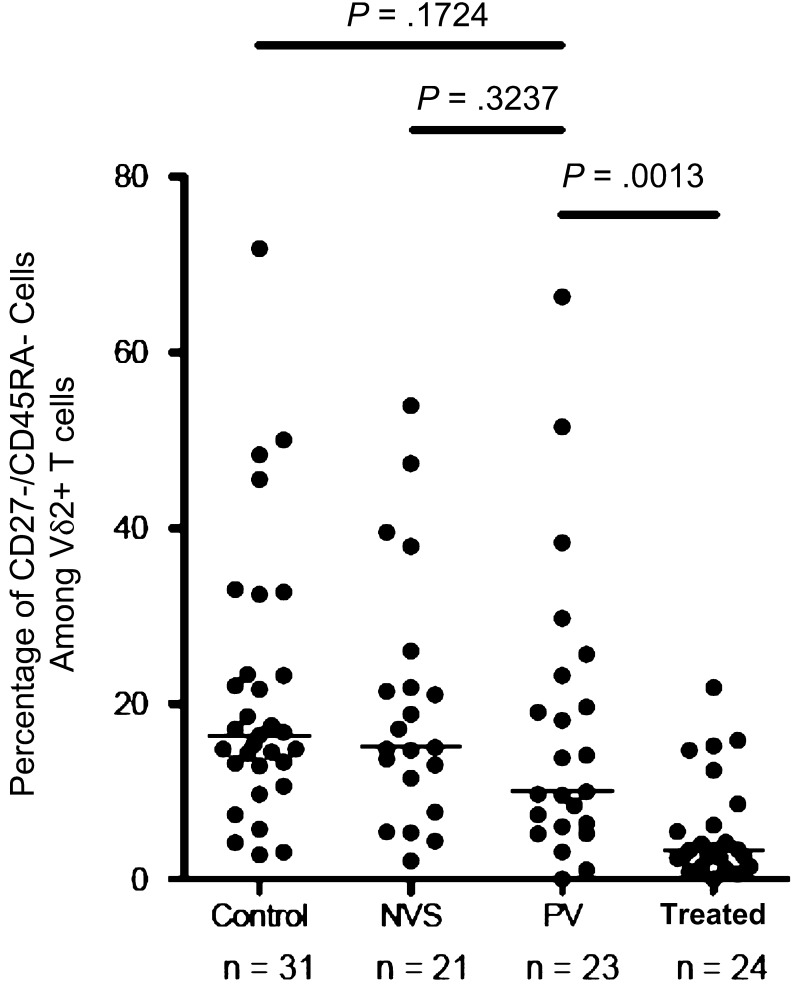
Another measure of Vγ2Vδ2 T-cell responses is the frequency of Tem cells in the circulating pool. These Tem cells are most responsive to stimulation and contain the subset of potent effector cells that includes cytotoxic effectors and cells that release proinflammatory cytokines [26, 27]. All of the HIV-positive groups had Tem values lower than those of the control group (as a proportion of total Vδ2+ T cells) (Figure 3). By measuring the proportion of Vγ2Vδ2 Tem cells, we identified a trend toward lower values for patients in the PV group, compared with patients in the NVS group, and significant differences between patients in the PV group and those in the treated group (P = .0013). In terms of cell counts, antigen responses, and accumulation of Vγ2Vδ2 Tem cells, patients in the PV group had values between those of patients in the NVS and treated groups.
Figure 3.
The proportion of effector memory Vδ2 T cells among control subjects without human immunodeficiency virus infection (Control), patients with natural virus suppression (NVS), patients with persistent viremia (PV; median, 9.9% [range, 0.0%–51.5%]), and patients treated with antiretrovirals (Treated); differences between the NVS and PV groups were not significant. T effector memory cells were identified by flow cytometry of uncultured γδ T cells after staining for CD3, Vδ2, CD27, and CD45Ra. Effector memory cells were the CD27−CD45Ra− population and are expressed as the proportion of total Vδ2+ cells. Horizontal lines represent median values. Statistical comparisons were performed using the Mann–Whitney U test; P values of <.05 were considered significant.
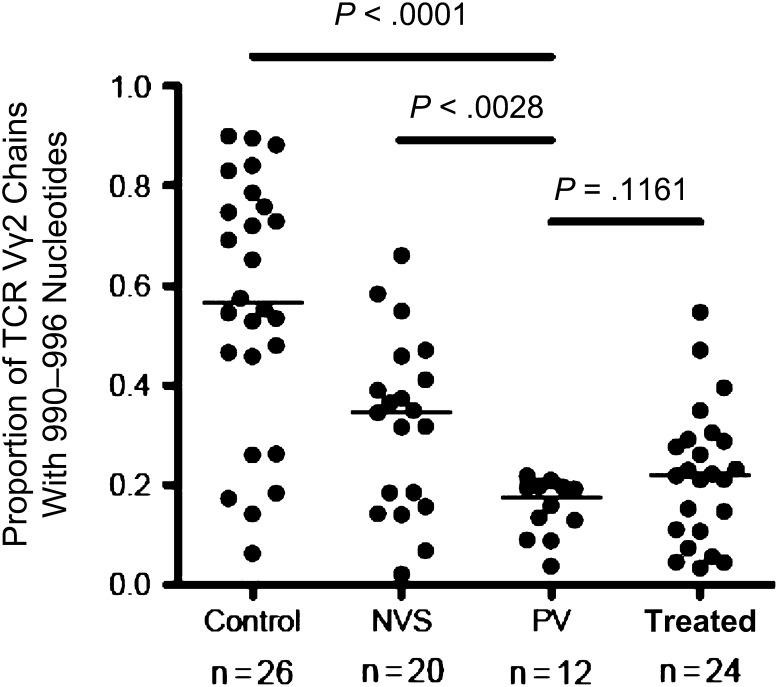
Next, we analyzed the diversity of Vγ2Vδ2 T cells in patients in the PV group by comparing the repertoire of T-cell receptor γ-2 chains. Spectratyping, a PCR-based technique, was employed to measure the distribution of γ-2 chain lengths in the circulating Vγ2Vδ2 T-cell pool. Longer chain lengths with mRNA coding regions of 990–996 nucleotides are required for stronger responses to phosphoantigen because these chains incorporate the Jγ1.2 segment [10, 23]. Loss of these long chains is a marker for HIV infection [8–10] and indicates specific depletion of phosphoantigen-responsive cells. For patients in the control group, the proportion of γ-2 chains with lengths of 990–996 nucleotides is approximately 58% (Figure 4), compared with 38% for the NVS group and 22% for the treated group. The value of 19% for patients in the PV group was lower than that for patients in the NVS group (P = .003), similar to that for patients in the treated group (P = .12), and distinct from that for patients in the control group (P < .0001). Analysis of the T-cell receptor γ-2 chain repertoire shows the impact of chronic exposure to HIV in the PV group, in which longer γ-2 chains were depleted to levels slightly lower than those in the treated group. However, higher counts of Vγ2Vδ2 T cells in the patients in the PV group partly overcame the repertoire damage to maintain strong antigen responses, which contribute to disease control.
Figure 4.
The proportion of Vγ2 chains with lengths of 990–996 nucleotides among control subjects without human immunodeficiency virus infection (Control), patients with natural virus suppression (NVS), patients with persistent viremia (PV), patients treated with antiretrovirals (Treated). The Vγ2 chains with lengths 990–996 contain the Jγ1.2 segment [23], are most responsive to phosphoantigen stimulation in vitro, and are depleted preferentially during HIV infection [9]. Horizontal lines represent median values. Statistical comparisons were performed using the Mann–Whitney U test; P values of <.05 were considered significant.
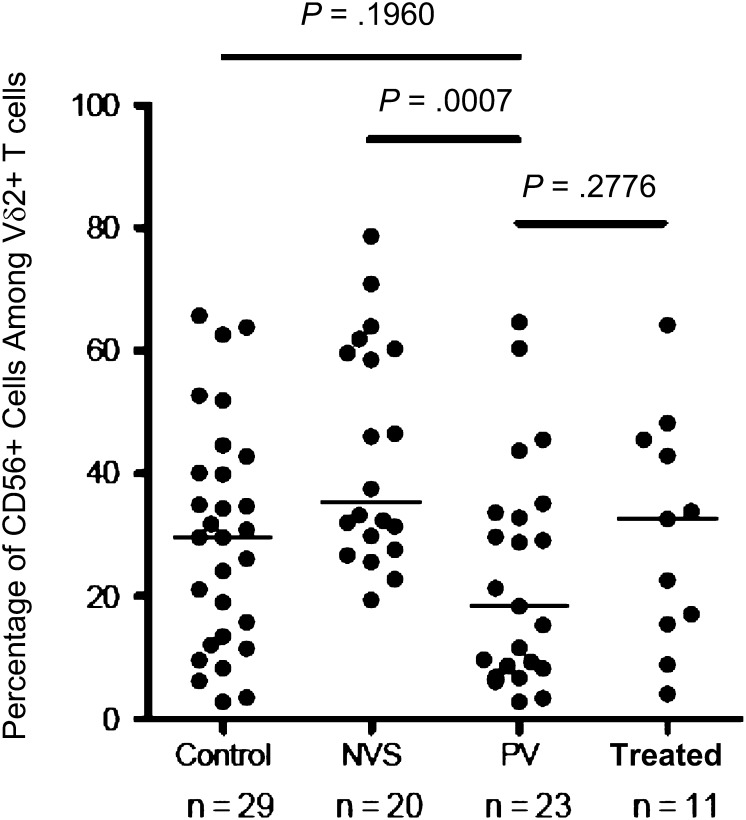
In previous studies, we found that cytotoxic Vγ2Vδ2 T cells express the cell surface glycoprotein CD56 [28, 29]. CD56+ Vγ2Vδ2 T cells are depleted preferentially in HIV disease, causing a loss of cytotoxic effector function [21, 29]. Among donors from the NVS group, we noted that CD56+ Vγ2Vδ2 T-cell levels were actually higher than those for patients in the control group [11], arguing for their active role in disease suppression. Here, we found that CD56+ Vγ2Vδ2 frequencies in patients in the PV group were significantly lower than those in patients in the NVS group (P = .0007); there were no differences in frequency between patients in the PV group and patients in the treated group (Figure 5). Differences between patients in the NVS group and patients in the PV group may reflect the impact of chronic exposure to viral protein in the latter group. Damage to the γ-2 chain repertoire and loss of CD56+ cells also showed the effects of HIV on Vγ2Vδ2 T cells in the PV group. Thus, for patients in the PV group, we found evidence for T-cell expansion and accumulation of effector memory T cells within a T-cell population that is damaged and probably less potent for effector function.
Figure 5.
The proportion of CD56+ cytotoxic precursor cells among Vγ2Vδ2 T cells is reduced significantly in patients with persistent viremia (PV; median, 18.4% [range, 2.8%–64.6%]), compared with patients with natural virus suppression (NVS), but was not different from patients treated with antiretrovirals (Treated) or control subjects without human immunodeficiency virus infection (Control). These data were obtained by flow cytometry of peripheral blood mononuclear cells without stimulation or in vitro culture. Horizontal lines represent median values. Statistical comparisons were performed using the unpaired Student t test; P values of <.05 were considered significant.
DISCUSSION
Circulating levels of Vγ2Vδ2 T cells, their proliferation responses to antigen and the proportion of cells with T effector memory phenotype for the PV group, fell between the values for the NVS and treated groups. The CD4 T-cell counts were variable among groups, and the HIV load could not be compared because of substantial virus inhibition in the treated group and lack of measurable virus in the NVS group. Despite higher Vγ2Vδ2 T-cell counts and function in the PV group than in the treated group, we found greater defects in the repertoire of T-cell receptor γ-2 chains among patients in the PV group, which we believe is the result of cell activation with chronic exposure to virus proteins. For example, we showed that HIV envelope glycoprotein, which signals through the chemokine receptor CCR5, triggers caspase activation and cell death in CD4− Vγ2Vδ2 T cells and that cell killing is enhanced after exposure to antigen [20].
By use of CD56 as a marker for Vγ2Vδ2 T-cell cytotoxicity, we found that function in patients in the PV group was again lower than that the treated group, which is also consistent with preferential loss of cytotoxic effector cells due to chronic viremia. More-extensive damage to the TCR γ-2 chain repertoire and loss of CD56+ T cells in persistently viremic individuals, compared with individuals who receive treatment, is mitigated by higher circulating Vγ2Vδ2 T-cell levels. Thus, the PV group showed an expected impact of chronic exposure to viral proteins, with γ-2 chain defects and lower CD56+ T-cell counts, but the higher Vγ2Vδ2 T-cell counts and increased proportion of T effector memory cells are evidence that this subset is part of the active immune response against HIV. Vγ2Vδ2 cells may be important for disease control, even though these cells are at risk because of chronic viral exposure.
Several functions of Vγ2Vδ2 T cells might contribute to slowing HIV disease in individuals with natural virus suppression and those with viremia. One important mechanism is the capacity for Vγ2Vδ2 T cells to activate NK cytotoxicity. The NK cells are altered in HIV disease, with significant increases in poorly cytotoxic CD56− NK cells and substantial declines of cytotoxic CD56dim NK cells. Cytotoxic, interferon γ–expressing NK cells were highest among patients who control viremia, with long-term nonprogressors intermediate between controllers and progressors [30]. Loss of cytotoxic NK cells parallels both the loss of Vγ2Vδ2 T cells and the decline in CD56 expression. We reported recently that NK cells require direct contact with and costimulation by Vγ2Vδ2 T cells to activate cytotoxicity [28, 31]. Thus, functional deficits in NK cells might be due partly to HIV-mediated damage to Vγ2Vδ2 T cells; combined changes in both subsets would impact the capacity for virus suppression and lower significantly the capacity for natural tumor surveillance [29]. Other functions of Vγ2Vδ2 T and NK cells, including CD16 (Fc receptor γ IIIa)–mediated antibody-dependent cellular cytotoxicity [32, 33], may also be important for disease control [34]. Activated Vγ2Vδ2 T cells produce soluble factors, including β-chemokines [35], which suppress HIV replication in vitro [36]. These cells also produce proinflammatory cytokines that support MHC-restricted antiviral T-cell responses. The extent of Vγ2Vδ2 T-cell depletion, in terms of reduced cell counts or loss of phosphoantigen-reactive TCR γ-2 chains, is linked to immunodeficiency and virus control.
Our emphasis on Vγ2Vδ2 T cells in HIV disease is part of an effort to develop new therapies that exploit this unique cell subset. We know that simian immunodeficiency virus disease was attenuated in macaques when Vγ2Vδ2 T cells were activated by phosphoantigen stimulation during the chronic phase of infection [37], and our data on Vγ2Vδ2 cell levels and function for multiple cohorts, including former plasma donors in China [12], demonstrated direct relationships between Vγ2Vδ2 T-cell levels, TCR γ-2 chain repertoire, and progressing HIV disease. It may be beneficial to stimulate Vγ2Vδ2 cells in HIV-positive patients, to improve at least one component of antiviral immunity [38]. Aminobisphosphonate drugs are being used in human clinical trials for cancer therapy [39, 40]. When combined with intravenous injections of IL-2, these drugs promoted expansion and activation of Vγ2Vδ2 T cells in cancer patients. Immunotherapies that are based on this drug combination have produced objective clinical responses in several forms of cancer [41–45]. Aminobisphosphonate plus IL-2 therapy in HIV-positive patients was safe in an earlier study [46], but the study was not designed to measure clinical responses or changes in outcome. Results from many sources support a strong correlation between Vγ2Vδ2 T-cell levels and HIV disease. Immunotherapy that is based on activating Vγ2Vδ2 T cells might mimic the conditions observed in patients who naturally suppress HIV and have high levels of activated cytotoxic Vγ2Vδ2 T cells that might help to regain immune control over virus replication [38, 46]. Objective markers for treatment success may include elevated levels of antigen-responsive Vγ2Vδ2 cells, increased NK cell function, and reduced viremia.
Notes
Acknowledgments.
We are grateful to Dr Maria Salvato, for critical comments; Lauren Moscato, for editorial assistance; and Rebecca Boyce, for obtaining many of the clinical specimens in support of our natural history studies of HIV disease.
Financial support.
This work was supported by the National Institutes of Health (grants CA142458 [to C. D. P.] and AI084580 [to M. M. S.]). S. B. was supported by the National Institutes of Health (grant T32GM092237) at the University of Maryland School of Medicine (Terry Rogers, PhD, principal investigator).
Potential conflicts of interests.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Demarest JF, Jack N, Cleghorn FR, et al. Immunologic and virologic analyses of an acutely HIV type 1-infected patient with extremely rapid disease progression. AIDS Res Hum Retroviruses. 2001;17:1333–44. doi: 10.1089/08892220152596597. [DOI] [PubMed] [Google Scholar]
- 2.Sheppard HW, Lang W, Ascher MS, Vittinghoff E, Winkelstein W. The characterization of non-progressors: long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS. 1993;7:1159–66. [PubMed] [Google Scholar]
- 3.Learmont J, Tindall B, Evans L, et al. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet. 1992;340:863–7. doi: 10.1016/0140-6736(92)93281-q. [DOI] [PubMed] [Google Scholar]
- 4.Levy JA. HIV pathogenesis and long-term survival. AIDS. 1993;7:1401–10. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Keet IP, Krol A, Klein MR, et al. Characteristics of long-term asymptomatic infection with human immunodeficiency virus type 1 in men with normal and low CD4+ cell counts. J Infect Dis. 1994;169:1236–43. doi: 10.1093/infdis/169.6.1236. [DOI] [PubMed] [Google Scholar]
- 6.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–16. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 7.Sajadi MM, Heredia A, Le N, Constantine NT, Redfield RR. HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS. 2007;21:517–19. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 8.Hebbeler AM, Propp N, Cairo C, et al. Failure to restore the Vgamma2-Jgamma1.2 repertoire in HIV-infected men receiving highly active antiretroviral therapy (HAART) Clin Immunol. 2008;128:349–57. doi: 10.1016/j.clim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enders PJ, Yin C, Martini F, et al. HIV-mediated gammadelta T cell depletion is specific for Vgamma2+ cells expressing the Jgamma1.2 segment. AIDS Res Hum Retroviruses. 2003;19:21–9. doi: 10.1089/08892220360473934. [DOI] [PubMed] [Google Scholar]
- 10.Bordon J, Evans PS, Propp N, Davis CE, Jr, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. J Infect Dis. 2004;189:1482–6. doi: 10.1086/382961. [DOI] [PubMed] [Google Scholar]
- 11.Riedel DJ, Sajadi MM, Armstrong CL, et al. Natural viral suppressors of HIV-1 have a unique capacity to maintain gammadelta T cells. AIDS. 2009;23:1955–64. doi: 10.1097/QAD.0b013e32832ff1ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Peng H, Ma P, et al. Association between Vgamma2Vdelta2 T cells and disease progression after infection with closely related strains of HIV in China. Clin Infect Dis. 2008;46:1466–72. doi: 10.1086/587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrere JJ, Morand-Joubert L, Mariotti M, et al. Even individuals considered as long-term nonprogressors show biological signs of progression after 10 years of human immunodeficiency virus infection. Blood. 1997;90:1133–40. [PubMed] [Google Scholar]
- 14.Montefiori DC, Pantaleo G, Fink LM, et al. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–7. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 15.Carotenuto P, Looij D, Keldermans L, de Wolf F, Goudsmit J. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS. 1998;12:1591–600. doi: 10.1097/00002030-199813000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Liegler TJ, Yonemoto W, Elbeik T, Vittinghoff E, Buchbinder SP, Greene WC. Diminished spontaneous apoptosis in lymphocytes from human immunodeficiency virus-infected long-term nonprogressors. J Infect Dis. 1998;178:669–79. doi: 10.1086/515378. [DOI] [PubMed] [Google Scholar]
- 17.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–14. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salgado M, Rallon NI, Rodes B, Lopez M, Soriano V, Benito JM. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin Immunol. 2011;139:110–4. doi: 10.1016/j.clim.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor GM, Holmes A, Mulcahy F, Gardiner CM. Natural killer cells from long-term non-progressor HIV patients are characterized by altered phenotype and function. Clin Immunol. 2007;124:277–83. doi: 10.1016/j.clim.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Pauza CD. HIV envelope-mediated, CCR5/α4β7-dependent killing of CD4-negative γδ T cells which are lost during progression to AIDS. Blood. 2011;118:5824–31. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD. Impacts of HIV infection on Vgamma2Vdelta2 T cell phenotype and function: a mechanism for reduced tumor immunity in AIDS. J Leukoc Biol. 2008;84:371–9. doi: 10.1189/jlb.1207847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairo C, Armstrong CL, Cummings JS, et al. Impact of age, gender, and race on circulating gammadelta T cells. Hum Immunol. 2010;71:968–75. doi: 10.1016/j.humimm.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/Vdelta2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 25.Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J Leukoc Biol. 2006;79:663–6. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- 26.Caccamo N, Meraviglia S, Ferlazzo V, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35:1764–72. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 27.Dieli F, Poccia F, Lipp M, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban EM, Chapoval AI, Pauza CD. Repertoire development and the control of cytotoxic/effector function in human gammadelta T cells. Clin Dev Immunol. 2010;2010:732893. doi: 10.1155/2010/732893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieillard V, Fausther-Bovendo H, Samri A, Debre P. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53:564–73. doi: 10.1097/QAI.0b013e3181d0c5b4. [DOI] [PubMed] [Google Scholar]
- 31.Maniar A, Zhang X, Lin W, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–33. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Freedman MS. CD16+ gammadelta T cells mediate antibody dependent cellular cytotoxicity: potential mechanism in the pathogenesis of multiple sclerosis. Clin Immunol. 2008;128:219–27. doi: 10.1016/j.clim.2008.03.513. [DOI] [PubMed] [Google Scholar]
- 33.Tokuyama H, Hagi T, Mattarollo SR, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs–rituximab and trastuzumab. Int J Cancer. 2008;122:2526–34. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 34.Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/IL-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy. 2012;14:173–81. doi: 10.3109/14653249.2011.623693. [DOI] [PubMed] [Google Scholar]
- 35.Tikhonov I, Deetz CO, Paca R, et al. Human Vgamma2Vdelta2 T cells contain cytoplasmic RANTES. Int Immunol. 2006;18:1243–51. doi: 10.1093/intimm/dxl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poccia F, Gougeon ML, Agrati C, et al. Innate T-cell immunity in HIV infection: the role of Vgamma9Vdelta2 T lymphocytes. Curr Mol Med. 2002;2:769–81. doi: 10.2174/1566524023361880. [DOI] [PubMed] [Google Scholar]
- 37.Ali Z, Yan L, Plagman N, et al. Gammadelta T cell immune manipulation during chronic phase of simian-human immunodeficiency virus infection [corrected] confers immunological benefits. J Immunol. 2009;183:5407–17. doi: 10.4049/jimmunol.0901760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauza CD, Riedel DJ, Gilliam BL, Redfield RR. Targeting gammadelta T cells for immunotherapy of HIV disease. Future Virol. 2011;6:73–84. doi: 10.2217/FVL.10.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–18. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 40.Keller RK, Fliesler SJ. Mechanism of aminobisphosphonate action: characterization of alendronate inhibition of the isoprenoid pathway. Biochem Biophys Res Commun. 1999;266:560–3. doi: 10.1006/bbrc.1999.1849. [DOI] [PubMed] [Google Scholar]
- 41.Meraviglia S, Eberl M, Vermijlen D, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–7. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–9. [PubMed] [Google Scholar]
- 43.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 45.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 46.Poccia F, Gioia C, Martini F, et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS. 2009;23:555–65. doi: 10.1097/QAD.0b013e3283244619. [DOI] [PubMed] [Google Scholar]