Abstract
Background. Elevated serum interleukin 7 (IL-7) levels are observed in lymphopenic conditions, including idiopathic CD4 lymphopenia (ICL), which is characterized by CD4 lymphopenia in the absence of human immunodeficiency virus infection or other known immunodeficiency.
Methods. To test whether defective IL-7 signaling could be an etiologic or contributing factor in ICL, peripheral blood mononuclear cells from patients with ICL (median CD4 T-cell count, 160 cells/μL) and healthy controls (median CD4 T-cell count, 582 cells/μL) were evaluated for expression of IL-7Rα chain (CD127) and intracellular phosphorylated STAT-5 (a marker of γc cytokine signaling) after cytokine stimulation. Gene expression was analyzed by real-time polymerase chain reaction following IL-7 stimulation.
Results. The percentage of CD4+CD127+ T cells was lower in patients with ICL, compared with controls (P < .001). Lower levels of STAT-5 phosphorylation after IL-7 stimulation were observed in both CD4 and CD8 T cells from patients with ICL, compared with controls (P < .001 and P = .017, respectively), that inversely correlated in CD4 T cells with serum IL-7 levels (r = −0.734, P = .013). Destabilization of p27kip1, a critical step for IL-7–induced T-cell cycling, was decreased in patients with ICL, compared with controls (P = .004), after IL-7 stimulation.
Conclusions. These data suggest that diminished responsiveness to IL-7 in CD4 and CD8 T cells during ICL may be contributing to the dysregulation of T-cell homeostasis.
Idiopathic CD4 lymphopenia (ICL) is a rare heterogeneous disorder of unknown etiology, defined by a total CD4 T-lymphocyte count of <300 cells/μL or <20% of total lymphocytes in the absence of human immunodeficiency virus (HIV) infection or other known cause of immunodeficiency. The clinical characteristics of ICL include susceptibility to opportunistic diseases and occasionally autoimmune disorders with an overall stable trajectory of CD4 T-cell counts over at least a period of 4–5 years [1].
Interleukin 7 (IL-7) is a homeostatic cytokine produced by bone marrow stromal and epithelial cells that is required for T-cell development and homeostasis [2]. IL-7 modulates development and selection of T cells in the thymus by enhancing survival of early thymocytes and expansion of T-cell precursors [3, 4]. IL-7 is also important in T-cell postthymic homeostatic proliferation and memory differentiation [5] and inhibits apoptosis via upregulation of BCL2 and MCL1. Further studies have demonstrated that the destabilization of p27Kip1 (CDKN1B) by IL-7 promotes T-cell proliferation [6]. IL-7 levels increase with T-cell depletion, possibly because of decreased use and receptor-mediated clearance [7] and high levels of IL-7 are thought to drive lymphopenia-induced T-cell proliferation [8, 9]. Despite high levels of IL-7 during lymphopenia, excess IL-7 can actually enhance lymphopenia-induced proliferation [10]. IL-7 has also recently been shown to promote T-cell proliferation and inhibit apoptosis in sepsis-induced immunosuppression [11].
The IL-7 receptor is a heterodimer composed of the IL7-Rα chain (CD127) and the common γ chain (γc, or CD132). IL-7Rα is expressed at higher levels on naive T cells, compared with memory T cells. On engagement of IL-7, the IL-7Rα receptor signals via JAK-STAT phosphorylation, permitting evaluation of IL-7 signaling by measurement of intracellular phosphorylated STAT-5 [12, 13]. The IL-7 receptor is endocytosed rapidly on IL-7 engagement, resulting in lower expression of IL-7Rα at high levels of IL-7 [12, 14].
Previous studies have shown an inverse relationship between CD4 T-cell counts and IL-7 levels in patients with lymphopenia due to HIV infection or chemotherapy [15, 16]. In ICL, the relationship between IL-7 levels and CD4 T-cell counts appears to be less consistent, with some patients exhibiting levels of IL-7 that are inappropriately low for the degree of CD4 T-cell depletion observed [17]. Increased turnover of T cells has been described in ICL [1, 18, 19]; however, heightened CD4 T-cell cycling does not restore CD4 T-cell counts. Patients with ICL also remain lymphopenic despite elevated serum IL-7 levels. Preliminary studies in patients with HIV infection or cancer have shown that IL-7 administration can lead to significant T-cell expansion [20–22], opening the possibility of use of IL-7 as a potential therapeutic modality in ICL. Therefore, we investigated the possibility of defective IL-7 signaling contributing to the pathogenesis of ICL. We evaluated the presence of anti-IL-7 antibodies, expression of IL-7 receptor (CD127), and signaling in response to IL-7 in patients with ICL and found that CD127 receptor expression on CD4 T cells is lower than expression on CD8 T cells in ICL and that both CD4 and CD8 T cells had decreased responsiveness to IL-7, as measured by STAT-5 phosphorylation. These findings were further confirmed by decreased upregulation of IL-7–induced genes in peripheral blood mononuclear cells (PBMCs) and blunted IL-7–induced BCL2 expression and cycling of T cells in vitro.
MATERIALS AND METHODS
Patients
Cryopreserved PBMCs from 21 patients with ICL and 17 healthy controls were analyzed. All study participants provided written informed consent, and the human experimentation guidelines of the US Department of Health and Human Services were followed in the conduct of this research. An additional 13 healthy controls without available demographic and lymphocyte count data were used in some experiments. The patients with ICL represent a subset of the originally described cohort by Zonios et al [1] and 2 newly recruited subjects on the basis of specimen availability. Study participants with ICL were not acutely ill at the time of blood collection. Seven patients had a history of severe persistent warts from human papillomavirus infection, 5 had cryptococcal disease, 2 had disseminated Mycobacterium avium complex infection, 2 had esophageal candidiasis, 1 had disseminated histoplasmosis, 1 had Mycobacterium chelonae infection, and 6 had no evidence or previous history of infection. CD4 T-cell counts were measured in a Clinical Laboratory Improvement Amendments–approved laboratory.
Measurement of Serum IL-7 Levels, Anti-IL-7 Antibodies, and Soluble CD127 (sCD127)
Serum IL-7 levels were measured in a subset of 11 patients with ICL by enzyme-linked immunosorbant assay (R&D Biosciences; lower limit of detection, 2 pg/mL) performed according to the manufacturer’s instructions. Anti-IL-7 antibody detection was performed on plasma samples from all patients with ICL. Antibody concentrations were measured using an enzyme-linked immunosorbant assay (MSD Multi-Array; lower limit of detection, 3 ng/mL). The sCD127 plasma concentrations were measured in a subset of 19 participants, using Meso Scale Discovery (MSD) technology as previously described [23]. MSD technology is based on the electrochemiluminescence detection principle and uses streptavidin-coated microplates with electrodes integrated into the bottom of the plate. The limit of detection was 0.23 pg/mL.
Lymphocyte Immunophenotyping
Immunophenotyping by flow cytometry was conducted on cryopreserved PBMCs from study participants. The fluorochrome-conjugated antibodies used were anti-CD3 Alexa-700 (clone SP34-2; BD-Pharmingen), anti-CD3 APC-Cy7 (clone Sk7; BD-Pharmingen), anti-CD3 Pacific Blue (clone UCHT1; BD-Pharmingen), anti-CD3 PerCP (clone SK7; BD-Pharmingen), anti-CD4 Cy5.5PE (clone OKT4; Invitrogen), anti-CD4 Pacific Blue (clone RPA-T4; BD-Pharmingen), anti-CD4 PerCP-Cy5.5 (clone SK3; BD-Pharmingen), anti-CD8 Pacific Blue (clone RPA-T8; BD-Pharmingen), anti-CD8 QDot 655 (clone 3B5; Invitrogen), anti-CD27 FITC (clone L128; BD-Pharmingen), anti-CD27 PC5 (clone 1A4CD27; Beckman Coulter), anti-BCL-2 PE (clone BCL-2/100; BD-Pharmingen), anti-CD45RO ECD (clone UCHL1; Beckman Coulter), anti-CD45RO PE (clone UCHL1; BD-Pharmingen), anti-CD127 PE (clone R34.34; Beckman Coulter), anti-TCR γδ FITC (clone B1; BD-Pharmingen), and anti-Ki67 FITC (clone B56; BD-Pharmingen). Stained samples were acquired using a FACSAria or LSR II flow cytometer with BD FACS DiVa software (BD Biosciences) and were analyzed using FlowJo software (version 8.8.6; Treestar).
Intracellular Phosphoprotein Analysis
Cryopreserved PBMCs were thawed, washed in media with Benzonase nuclease (1 μL/mL; Novagen), resuspended in Roswell Park Memorial Institute medium supplemented with 10% heat-inactivated human serum AB (Gemini Biotech), and incubated for 8–10 hours. Cells were then stimulated for 30 minutes with IL-7 (100 ng/mL and 1 μg/mL; R&D Biosciences) or interleukin 2 (IL-2; 500 international units (IU)/mL as a positive control; Peprotech) or interleukin 15 (IL-15) at 100 ng/mL (Peprotech) for a subset of experiments. Cells were subsequently fixed, permeabilized, and stained intracellularly with anti-phosphorylated STAT-5 Alexa 647 (clone 47; BD-Pharmingen) and analyzed by flow cytometry (Supplemental Figure 1A). Optimal concentrations of IL-7 were determined by performing a dose-response titration (Supplemental Figure 1B).
IL-7 Stimulation Assays
Cryopreserved PBMCs from a subset of participants were thawed as above and stimulated with IL-7 (100 ng/mL) for 18 hours. An additional experiment was done with IL-7 stimulation for 6, 12, and 18 hours to assess the kinetics of gene induction. Aliquots were washed with phosphate-buffered saline and centrifuged at 20 000 g for 5 minutes to create dry cell pellets after supernatant aspiration for RNA isolation and polymerase chain reaction (PCR). For analysis of BCL2 expression and cycling by intracellular Ki67, PBMCs were stimulated with IL-7 (100 ng/mL) for 3 (BCL2) or 6 (Ki67) days. Immunophenotypic analysis of CD4 and CD8 T lymphocytes was performed at baseline and 3 or 6 days after baseline to evaluate expression of BCL2 and Ki67.
RNA Isolation and Complementary DNA (cDNA) Synthesis
Total RNA was extracted from PBMC pellets, using the RNeasy kit (Qiagen) according to the manufacturer’s protocol. Total RNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific). cDNA was synthesized with random hexamers from the total RNA, using the Superscript First-Strand Synthesis System for real-time PCR (Invitrogen) according to the manufacturer’s protocol.
Real-Time PCR
Real-time PCR reactions were performed on the 7900HT Fast Real-Time PCR System (Applied Biosystems). PCR was performed in a final volume of 25 μL including 11.25 μL of cDNA, 12.50 μL of TaqMan Universal PCR master Mix (Applied Biosystems), and 1.25 μL of PCR primers/probes from TaqMan Gene Expression Assays (Applied Biosystems). Real-time PCR cycling conditions consisted of initial incubations of 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Reactions were replicated twice per experiment to verify results. The primer/probe sets used were Beta-Actin (ACTB) gene (Hs99999903_m1), B-cell lymphoma 2 (BCL2) gene (Hs00153350_m1), Cyclin D2 (CCND2) gene (Hs00277041_m1), Cyclin D3 (CCDN3) gene (Hs00426901_m1), Janus kinase 1 (JAK1) gene (Hs01026983_m1), Janus kinase 3 (JAK3) gene (Hs00169663_m1), p27Kip1 (CDKN1B) gene (Hs00153277_m1), signal transducer and activator of transcription 5A (STAT5A) gene (Hs00559643_m1), and signal transducer and activator of transcription 5B (STAT5B) gene (Hs00560035_m1) from TaqMan Gene Expression Assays (Applied Biosystems). β-actin was used for normalization.
Statistical Analysis
Median values were compared by nonparametric methods (Mann–Whitney U test), using SAS (version 9.2; SAS Institute) and Prism software (version 5.0a; GraphPad). Associations were assessed by Spearman correlation. Because of the exploratory nature of the study, there was no correction for multiple comparisons, and calculated P values are reported on the basis of the Mann–Whitney U test.
RESULTS
Study Participants
The characteristics of study participants are shown in Table 1. CD4 T-cell counts were significantly lower in patients with ICL, compared with controls (160 vs 582 cells/μL [P < .001]; Table 1). CD8 T-cell counts did not differ significantly between the 2 groups (284 vs 397 cells/μL [P = .411]; Table 1). The median serum IL-7 level measured in a subset of 11 patients with ICL was 19.9 pg/mL, which is higher than historical values previously reported in healthy adults [7, 24–26]. None of the 21 plasma samples from patients with ICL were found to have detectable anti-IL-7 antibodies.
Table 1.
Characteristics of Health Control Subjects and Patients With Idiopathic CD4 Lymphopenia (ICL)
| Characteristic | Control Group (n = 17) | ICL Group (n = 21) | P |
| Males/females | 15/2 | 10/11 | .015 |
| Age, years | 51 (40–56) | 54 (45–58) | .252 |
| CD4 T-cell count, cells/μL | 582 (458–816) | 160 (78–288) | <.001 |
| CD4 T cells, % | 47 (38–55) | 22 (8–38) | <.001 |
| CD8 T-cell count, cells/μL | 397 (204–502) | 284 (147–624) | .411 |
| CD8 T cells, % | 26 (15–35) | 39 (24–50) | .046 |
| Ratio of CD4 T cells to CD8 T cells | 1.8 (1.1–3.2) | 0.7 (0.3–1.1) | <.001 |
Data are no. of participants or median value (interquartile range).
T-Cell Immunophenotyping
Patients with ICL had a decreased proportion of naive (CD45RO-CD27+) CD4 T cells, compared with controls (10.6% vs 40.7% [P < .001]; Figure 1A). Naive CD8 T-cell populations did not differ significantly between the 2 groups (31.6% vs 43.2% [P = .254]; Figure 1A).
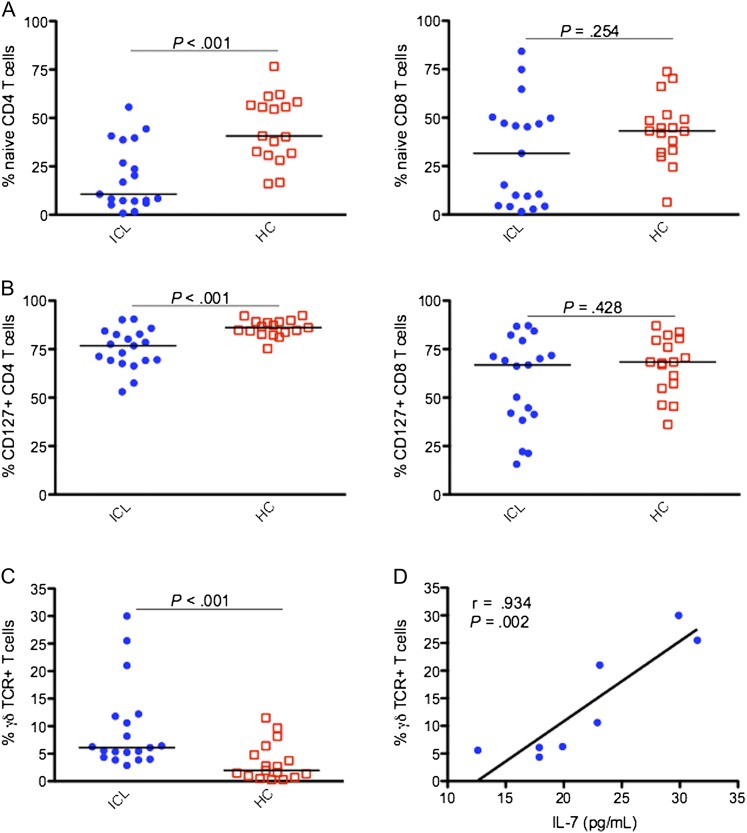
Figure 1.
Comparison of percentages of CD45RO-CD27+ (naive) T cells (A), T cells expressing CD127 (B), and T cells expressing the γδ T-cell receptor (TCR; C) between 19 patients with idiopathic CD4 lymphopenia (ICL) and 17 healthy controls (HCs). Serum level of interleukin 7 (IL-7) correlated strongly with γδ TCR expression (n = 8; D). Horizontal bars represent median values.
A significantly lower proportion of CD4 T cells expressed the IL-7R α-chain (CD127) in patients with ICL, compared with controls (76.8% vs 86.1% [P < .001]; Figure 1B). IL-7 receptor expression on CD8 T cells did not differ significantly between patients with ICL and controls (66.9% vs 68.4% [P = .428]; Figure 1B). The patients with ICL had significantly higher frequencies of T cells that expressed γδTCR (6.11% vs 1.98% [P < .001]; Figure 1C), but the absolute counts did not differ between the 2 groups (33 vs 19 cells/μL; P = .071). The frequency of γδ T cells did not correlate with total CD4 or CD8 T-cell counts (data not shown) but correlated with the serum level of IL-7 in a subset of patients with ICL (r = 0.934; P = .002; Figure 1D). A low proportion of γδ T cells expressed CD127 (5.5% [IQR, 1.8%–20.4%]; n = 9).
Decreased IL-7 Receptor Signaling
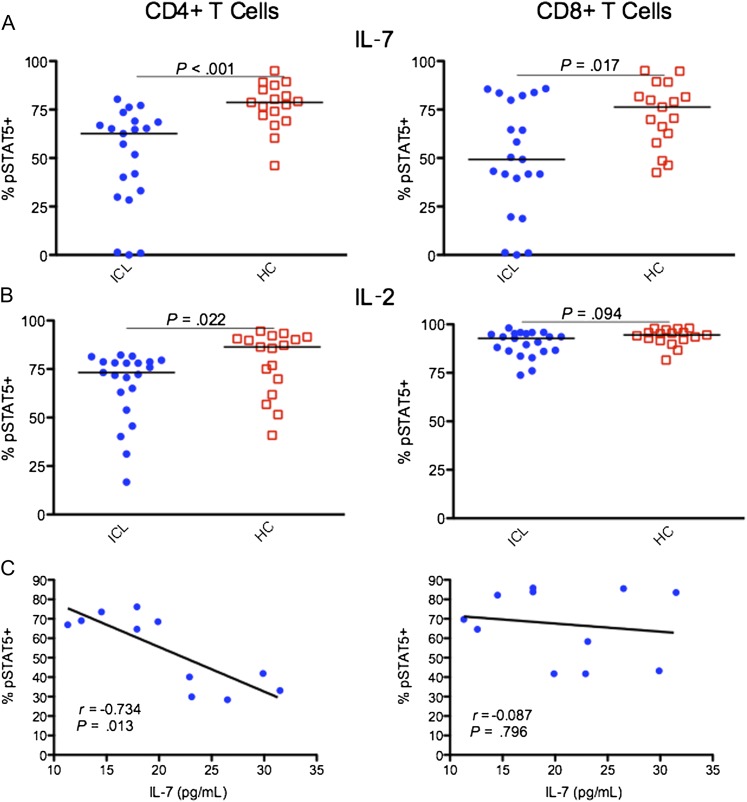
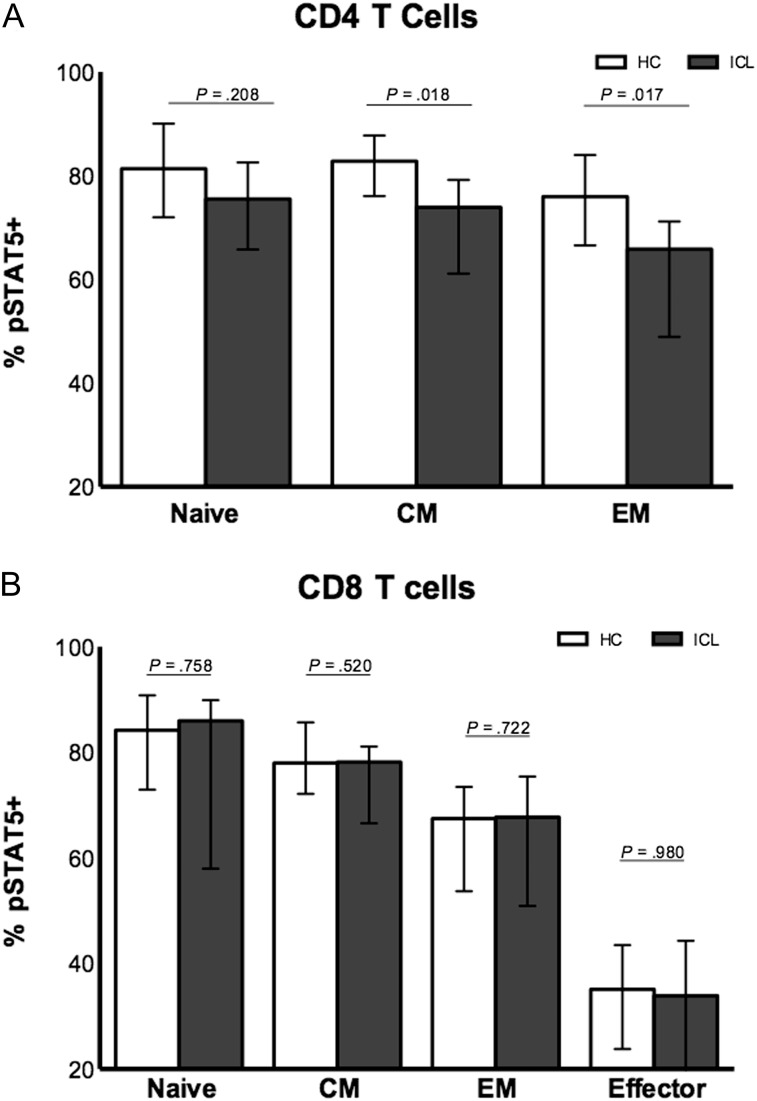
Significantly lower STAT-5 phosphorylation in response to IL-7 was observed in CD4 T cells and CD8 T cells from patients with ICL, compared with controls (62.6% vs 78.7% [P < .001] and 49.3% vs 76.3% [P = .017], respectively; Figure 2A). CD4 T cells from patients with ICL also displayed a small decrease in signaling in response to IL-2 (73.2% vs 86.4% [P = .022]; Figure 2B), while IL-2 signaling in CD8 T cells did not differ significantly between the groups (92.8% vs 94.5% [P = .094]; Figure 2B). Responses to IL-15 appeared intact in T cells from a subset of patients with ICL (Supplemental Figure 1C). No difference was observed in soluble (non–membrane bound) CD127 between patient and control groups (86.2 vs 71.2 pg/mL; P = .454). In central and effector memory subsets of CD4 T cells but not CD8 T cells, STAT-5 phosphorylation in response to IL-7 was significantly decreased in patients with ICL, compared with healthy controls (P = .018 and P = .017, respectively; Figure 3 and Supplemental Figure 1D). STAT-5 phosphorylation did not correlate with total CD4 T-cell or CD8 T-cell counts, IL-7Rα expression, or percentage of naive T cells. In a subset of 11 patients with ICL in whom serum levels of IL-7 were measured, IL-7 levels inversely correlated significantly with IL-7 signaling in CD4 T cells (r = −0.734; P = .013; Figure 2C). The proportion of CD4 T cells or CD8 T cells expressing CD127 did not correlate with serum IL-7 levels (data not shown).
Figure 2.
Comparison of STAT-5 phosphorylation in response to interleukin 7 (IL-7) stimulation at a concentration of 100 ng/mL (A) and interleukin 2 (IL-2) stimulation at a concentration of 500 U/mL (B). Serum concentration of IL-7 correlated inversely with IL-7–stimulated STAT-5 phosphorylation in CD4 T cells in 11 patients with idiopathic CD4 lymphopenia (ICL; C). pSTAT5+, STAT-5 phosphorylated. Horizontal bars represent median values.
Figure 3.
STAT-5 phosphorylation in response to interleukin 7 stimulation at a concentration of 100 ng/mL in naive and memory subsets of CD4 T cells (A) and CD8 T cells (B) in 14 patients with idiopathic CD4 lymphopenia (ICL) versus 12 healthy controls (HCs). Abbreviations: CM, central memory T-cell subset; EM, effector memory T-cell subset; pSTAT5+, STAT-5 phosphorylated. Median values with IQR are depicted on graphs.
Analysis of Gene Expression After IL-7 Stimulation
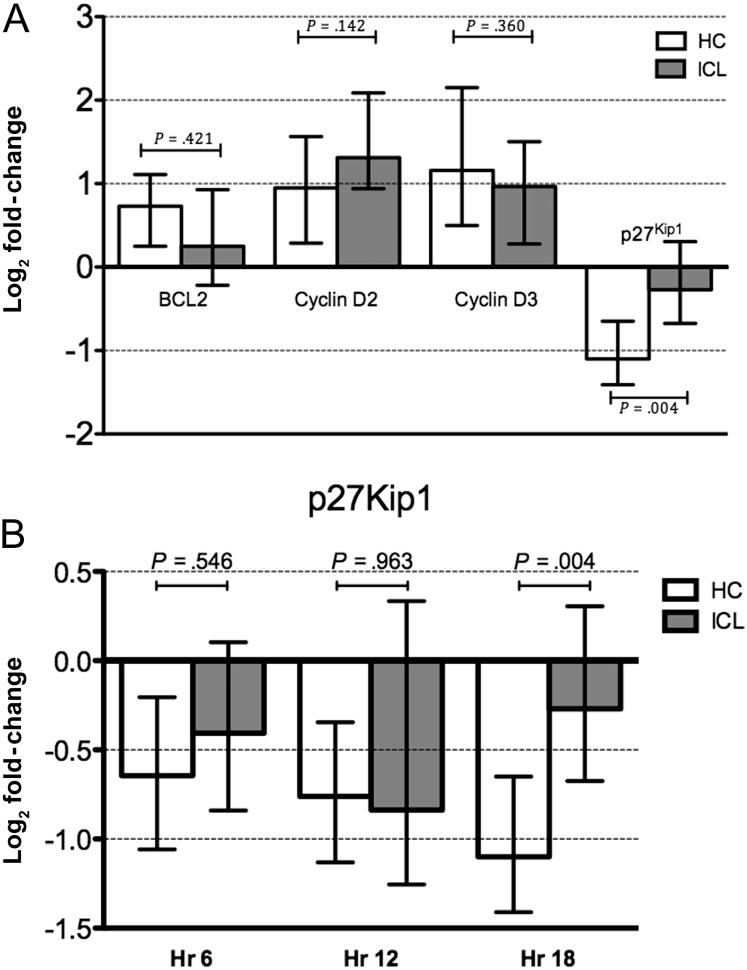
To further study the potential effects of decreased STAT-5 phosphorylation on the downstream events after IL-7 signaling, analysis of genes that are affected by IL-7 was done by real-time PCR after stimulation of PBMCs with IL-7. Upregulation of expression of BCL2 in IL-7–stimulated PBMCs was decreased in patients with ICL, compared with healthy controls (0.25-fold vs 0.73-fold increases from baseline), but did not reach statistical significance (P = .421). Expression of cyclin D2 and D3 did not vary significantly between patients with ICL and healthy controls (Figure 4A). In contrast, the decrease in expression of p27Kip1 in PBMCs from patients with ICL was significantly different from that for healthy controls (−0.68-fold vs −1.41-fold decreases from baseline [P = .002]; Figure 4A). A kinetic analysis showed that the difference was most evident at 18 hours of stimulation (Figure 4B). Furthermore, real-time quantitative PCR for measuring gene expression levels of JAK1, JAK3, STAT5A, and STAT5B showed no significant difference between cells from patients with ICL and cells from healthy control subjects.
Figure 4.
A, Fold-change in gene expression in interleukin 7 (IL-7)–stimulated peripheral blood mononuclear cells, relative to unstimulated control cells, in patients with idiopathic CD4 lymphopenia (ICL) and 15 healthy controls (HCs). B, Kinetic analysis of p27Kip1 after IL-7 stimulation in 9 patients with ICL and 9 healthy controls. Abbreviation: Hr, hours. Median values with IQR are depicted on graphs.
Analysis of BCL2 Expression and Cycling of T Cells in Response to IL-7
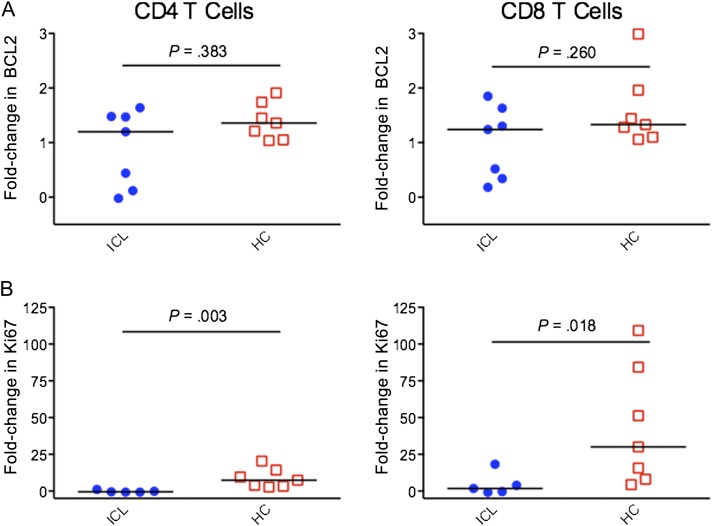
Expression of BCL2, an anti-apoptotic protein, was analyzed in CD4 T cells and CD8 T cells after 3 days of IL-7 stimulation by flow cytometry. In comparing unstimulated cells to cells stimulated with IL-7, expression of BCL2 in both CD4 T cells and CD8 T cells did not differ significantly between the ICL and control groups (P = .383 for CD4 T cells, and P = .260 for CD8 T cells; Figure 5A). Ki67 expression in CD4 T cells and CD8 T cells was analyzed by flow cytometry after stimulation with IL-7 for 6 days. In both CD4 T cells and CD8 T cells, patients with ICL showed a significantly lower increase in Ki67 expression in response to IL-7, compared with controls (−0.51 vs 7.45 for CD4 T cells [P = .003], and 1.75 vs 30.02 for CD8 T cells [P = .018]; Figure 5B).
Figure 5.
A, Fold-change in BCL2 expression in cells stimulated with interleukin 7 (IL-7) for 3 days, relative to unstimulated cells, in patients with idiopathic CD4 lymphopenia (ICL) and healthy controls (HCs) . B, Fold-change in Ki67 expression in cells stimulated with IL-7 for 6 days, relative to unstimulated cells, in patients with ICL and HCs. Horizontal bars represent median values.
DISCUSSION
In this study, CD4 and CD8 T cells from patients with ICL, compared with those from controls, showed decreased STAT-5 phosphorylation in response to IL-7 that did not correlate with CD127 expression on T cells but correlated inversely in CD4 T cells with serum IL-7 levels. This decreased responsiveness was observed in total CD4 T cells, as well as within both central and effector memory subsets. Decreased IL-7 responsiveness was further supported by evidence of diminished gene upregulation and cycling of T cells after IL-7 stimulation. These data suggest a possible role of defective IL-7 signaling in the perturbation of T-cell homeostasis during ICL.
In our analysis, patients with ICL showed decreased responsiveness to IL-7, compared with control subjects, in the presence of elevated serum IL-7 levels. This phenomenon may be partially related to decreased IL-7Rα expression in CD4 T cells during ICL, observed in this and prior studies [1, 15]. As previously observed [1], the present study confirmed that patients with ICL have a lower percentage of naive CD4 T cells, which express CD127 constitutively and could account for these differences. However, receptor expression and STAT-5 phosphorylation did not correlate in either CD4 T cells or CD8 T cells, although diminished responsiveness was observed predominantly in central and effector memory CD4 T cells. Because of limited sample availability, serum IL-7 analysis was only conducted in a subset of patients with ICL. In each of the patients with ICL we analyzed, serum IL-7 levels were markedly higher than reported levels in healthy adults, indicating that, at least among these patients, the defect did not seem to be limited IL-7 production or availability. The possibilities of anti-IL-7 antibodies or increased levels of sCD127 that could interfere by IL-7 binding were also excluded in our cohort. Alternatively, decreased IL-7 responsiveness ex vivo may be due to tachyphylaxis from exposure to high levels of IL-7 in vivo, which could also explain why both CD4 T cells and CD8 T cells are affected. Supporting this, serum levels of IL-7 correlated negatively with IL-7 responsiveness in CD4 T cells during ICL, although this correlation was not observed in CD8 T cells. This could be related to the overall lower levels of CD127 expression in CD8 T cells or to the small sample size. Previous studies have also found that, while IL-7 is essential for homeostatic proliferation of CD4 T cells, memory CD8 T cells also rely on IL-15 [27, 28], although this relationship could be altered in the presence of high IL-7 levels [29]. In addition, cytokine-mediated suppression of IL-7Rα transcription involves different molecular mechanisms in CD4 T cells and CD8 T cells [14], as does stimulation via T-cell receptor versus IL-7 [30].
To evaluate whether decreased responsiveness to IL-7 was due to decreased IL-7R expression, cells were also stimulated with IL-2, a γc chain cytokine that, like IL-7, signals via STAT-5 phosphorylation. CD4 T cells from patients with ICL showed only minimally decreased responsiveness to IL-2, indicating that although an overall defect in γc cytokine signaling may be contributing to decreased IL-7 responsiveness of CD4 T cells in ICL, the primary defect appears to lie in the IL-7 pathway itself, as this was not observed in CD8 T cells and responses to IL-15 appeared intact in a subset of our cohort.
The presence of increased proportions but not total counts of γδ T cells during ICL may simply be a function of depletion of CD4 T cells (which are αβ). However, elevated serum IL-7 levels may underlie expansion of γδ T cells, as previous studies have shown that IL-7 prevents T-cell precursors from differentiating into αβ T cells [31, 32]. Indeed, IL-7 levels were found to correlate strongly with the frequency of γδ T cells. Moreover, mice deficient in αβ T cells show increased homeostatic proliferation of γδ T cells, a process that was enhanced by both IL-15 and IL-7 [33], while IL-7 knockout mice do not make γδ T cells [34]. As many γδ T cells normally reside in the lymphoid tissue of the gastrointestinal tract, the increased frequencies we observed may also be a reflection of differential trafficking of these cells from gut mucosal tissue through peripheral blood. Lymph node, gut, and other tissue biopsy specimens from patients with ICL could clarify the question of possible CD4 T-cell redistribution in individuals with ICL.
A recent study of IL-7 signaling during HIV infection found that decreased IL-7 signaling correlated with total T-cell count, indicating the degree of IL-7 responsiveness may serve as a marker of the ability of lymphopenic patients to reconstitute T-cell counts [35]. Whether the lymphopenia in ICL is secondary to decreased production, increased death, or redistribution of CD4 T cells remains unclear. Our data support that the defective IL-7 responsiveness may be contributing to both increased death of T cells, which has been previously described [19], and inadequate cycling. Gene expression in PBMCs from patients with ICL, compared with those from healthy controls, showed overall lower increases in BCL2 induction after stimulation with IL-7, indicating that IL-7 may have less of an antiapoptotic effect during ICL. More importantly, there was clear evidence of decreased downregulation of p27kip1 in PBMCs from patients with ICL, a cyclin-dependent kinase inhibitor whose destabilization is the main mechanism of cycle progression after IL-7 stimulation. It thus appears that the T cells in patients with ICL may be unable to optimally respond to IL-7, despite the observed high serum levels.
Previous studies have shown increased turnover of CD4 T cells during ICL [1, 18, 36]. A recent primate study showed that IL-7 induced T-cell redistribution from peripheral blood to secondary lymphoid organs, resulting in a transient drop in peripheral CD4 T cell numbers [37]. This initial drop in CD4 T-cell count was also observed when IL-7 was administered to patients with HIV infection [20, 21]. In all studies, however, exogenous IL-7 resulted in a significant increase in peripheral T-cell counts in humans with lymphopenic conditions (HIV infection and cancer) without significant toxicity [20–22], even though IL-7 signaling during HIV infection is defective [35]. Further, the resulting T-cell expansions occurred primarily in the naive subset [22], which is disproportionately depleted in ICL, and included significant expansion of CD8 T cells [20–22], which may be beneficial given the association of very low CD8 T-cell counts with poor prognosis in ICL [1].
In conclusion, in this study we demonstrated that T cells from patients with ICL display blunted in vitro responses to IL-7, which may reflect in vivo phenomena. Defective IL-7 signaling could, therefore, be important in the pathogenesis and persistence of lymphopenia in this setting and may have implications in the potential use of IL-7 as immunotherapy during ICL, which is currently being investigated in a phase I study (ClinicalTrials.gov identifier: NCT00839436). On the basis of these findings, IL-7 remains a potentially promising agent to study in patients with ICL, although ascertaining the appropriate dosing needed to obtain the desired effects of improved cycling and survival of T cells may be challenging.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment.
We thank all study participants.
Disclaimer.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support.
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH). Additionally, this project has also been funded in part with federal funds from the National Cancer Institute, NIH (grant HHSN261200800001E). C. E. P. was a participant in the NIH Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer.
Potential conflicts of interest.
S. B. is an employee of Cytheris SA. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287–94. doi: 10.1182/blood-2007-12-127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–6. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–41. [PubMed] [Google Scholar]
- 4.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–7. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 6.Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled AR, Durum SK. IL-7 promotes T cell proliferation through destabilization of p27Kip1. J Exp Med. 2006;203:573–82. doi: 10.1084/jem.20051520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge JN, Srinivasula S, Hu Z, et al. Decreases in IL-7 levels during antiretroviral treatment of HIV infection suggest a primary mechanism of receptor-mediated clearance. Blood. 2011;118:3244–53. doi: 10.1182/blood-2010-12-323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–57. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosco N, Agenes F, Ceredig R. Effects of increasing IL-7 availability on lymphocytes during and after lymphopenia-induced proliferation. J Immunol. 2005;175:162–70. doi: 10.4049/jimmunol.175.1.162. [DOI] [PubMed] [Google Scholar]
- 11.Unsinger J, McGlynn M, Kasten KR, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–79. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 13.Sasson SC, Zaunders JJ, Kelleher AD. The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr Drug Targets. 2006;7:1571–82. doi: 10.2174/138945006779025365. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 16.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 17.Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–8. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PI, Ciccone EJ, Read SW, et al. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–70. doi: 10.1086/598953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurence J, Mitra D, Steiner M, Lynch DH, Siegal FP, Staiano-Coico L. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J Clin Invest. 1996;97:672–80. doi: 10.1172/JCI118464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sereti I, Dunham RM, Spritzler J, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janot-Sardet C, Assouline B, Cheynier R, Morre M, Beq S. A validated assay to measure soluble IL-7 receptor shows minimal impact of IL-7 treatment. J Immunol Methods. 2010;353:115–23. doi: 10.1016/j.jim.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 25.Read SW, Rupert A, Stevens R, O'Shea A, Sereti I. Delayed sample processing leads to marked decreases in measured plasma IL-7 levels. J Acquir Immune Defic Syndr. 2006;42:511–2. doi: 10.1097/01.qai.0000225741.16840.ac. [DOI] [PubMed] [Google Scholar]
- 26.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 29.Kieper WC, Tan JT, Bondi-Boyd B, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–9. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180:5201–10. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 31.Ikuta K, Lee HC, Ye SK. Role of the IL-7 receptor in gamma-delta T cell development. Chem Immunol. 2001;79:29–42. doi: 10.1159/000058830. [DOI] [PubMed] [Google Scholar]
- 32.Plum J, De Smedt M, Leclercq G. Exogenous IL-7 promotes the growth of CD3-CD4-CD8-CD44+CD25+/- precursor cells and blocks the differentiation pathway of TCR-alpha beta cells in fetal thymus organ culture. J Immunol. 1993;150:2706–16. [PubMed] [Google Scholar]
- 33.French JD, Roark CL, Born WK, O'Brien R L. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc Natl Acad Sci U S A. 2005;102:14741–6. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 35.Camargo JF, Kulkarni H, Agan BK, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–82. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurence J. T-cell subsets in health, infectious disease, and idiopathic CD4+ T lymphocytopenia. Ann Intern Med. 1993;119:55–62. doi: 10.7326/0003-4819-119-1-199307010-00010. [DOI] [PubMed] [Google Scholar]
- 37.Beq S, Rozlan S, Gautier D, et al. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114:816–25. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.