Abstract
Background
Treatment of HIV/malaria-coinfected patients with antiretroviral therapy (ART) and artemisinin-based combination therapy has potential for drug interactions. We investigated the pharmacokinetics of artemether, dihydroartemisinin and lumefantrine after administration of a single dose of 80/480 mg of artemether/lumefantrine to HIV-infected adults, taken with and without lopinavir/ritonavir.
Methods
A two-arm parallel study of 13 HIV-infected ART-naive adults and 16 HIV-infected adults stable on 400/100 mg of lopinavir/ritonavir plus two nucleoside reverse transcriptase inhibitors (ClinicalTrials.gov, NCT 00619944). Each participant received a single dose of 80/480 mg of artemether/lumefantrine under continuous cardiac function monitoring. Plasma concentrations of artemether, dihydroartemisinin and lumefantrine were measured.
Results
Co-administration of artemether/lumefantrine with lopinavir/ritonavir significantly reduced artemether maximum concentration (Cmax) and area under the concentration–time curve (AUC) [median (range): 112 (20–362) versus 56 (17–236) ng/mL, P = 0.03; and 264 (92–1129) versus 151 (38–606) ng · h/mL, P < 0.01]. Dihydroartemisinin Cmax and AUC were not affected [66 (10–111) versus 73 (31–224) ng/mL, P = 0.55; and 213 (68–343) versus 175 (118–262) ng · h/mL P = 0.27]. Lumefantrine Cmax and AUC increased during co-administration [2532 (1071–5957) versus 7097 (2396–9462) ng/mL, P < 0.01; and 41 119 (12 850–125 200) versus 199 678 (71 205–251 015) ng · h/mL, P < 0.01].
Conclusions
Co-administration of artemether/lumefantrine with lopinavir/ritonavir significantly increases lumefantrine exposure, but decreases artemether exposure. Population pharmacokinetic and pharmacodynamic trials will be highly valuable in evaluating the clinical significance of this interaction and determining whether dosage modifications are indicated.
Keywords: antiretrovirals, antimalarials, drug interactions
Introduction
Malaria and HIV are two infectious diseases causing significant morbidity and mortality worldwide. The two diseases have overlapping geographical distribution in sub-Saharan Africa, where over 90% of the world malaria burden and 67% of the global HIV burden occur.1,2 Significant interactions occur between the two diseases, with HIV increasing the risks for malaria frequency and severity.3,4 Infection with malaria stimulates immune mechanisms that activate HIV replication, causing a transient increase in HIV viral load.5,6
Major effort has been made to ensure universal access to antiretroviral therapy (ART), with significant improvement in quality of life and survival of people living with HIV. In 2009, 1.2 million people were initiated on ART, a 30% increase in ART coverage in one year.7 Successful treatment of infectious diseases such as HIV and malaria requires adequate drug concentrations at the target site to produce maximal efficacy with minimal toxicity. Drug pharmacokinetics might be influenced by drug–drug interactions. Antiretroviral drugs, specifically the non-nucleoside reverse transcriptase inhibitors and protease inhibitors, are potent inducers and/or inhibitors of cytochrome (CYP) enzymes and transporter proteins, with potential for drug–drug interactions when co-administered with other drugs.8,9
The WHO recommends artemisinin-based combination therapy (ACT) for the treatment of uncomplicated malaria.2 The combination of artemether and lumefantrine offers excellent efficacy against susceptible and multidrug-resistant Plasmodium falciparum. Both artemether and lumefantrine are metabolized predominantly by CYP3A4.10 Artemether is metabolized to dihydroartemisinin, predominantly by CYP3A4/5 and to a lesser extent by CYP2B6, CYP2C9, CYP2C19 and possibly CYP2A6.8,10–12 Dihydroartemisinin is rapidly converted into inactive metabolites primarily by glucuronidation via uridine diphosphoglucuronyltransferases (UGTs) UGT1A1, UGT1A8/9 and UGT2B7.10,12–14 Both artemether and dihydroartemisinin possess potent antimalarial properties, causing a rapid reduction in asexual parasite biomass, with prompt resolution of symptoms.15,16
Lumefantrine is slowly eliminated, mainly metabolized by CYP3A4 to desbutyl-lumefantrine.10–12 Lumefantrine eradicates residual malaria parasites thereby preventing recrudescence.10,13,14 Total exposure to lumefantrine predicts parasite eradication and is the principal pharmacokinetic correlate of artemether/lumefantrine treatment.16
Lopinavir and ritonavir are inhibitors of CYP3A4, so co-administration with artemether/lumefantrine may result in increased artemether and lumefantrine plasma concentrations. Elevated lumefantrine plasma concentrations are of particular concern because of the structural similarity to halofantrine, a drug associated with cardiac arrythmias and sudden death.17–19 In a previous study, co-administration of lopinavir/ritonavir with artemether/lumefantrine to healthy volunteers resulted in significantly increased lumefantrine exposure, decreased dihydroartemisinin exposure and a trend towards decreased artemether exposure.20
The aim of the present study was to investigate the pharmacokinetics of artemether, dihydroartemisinin and lumefantrine after administration of a single dose of 80/480 mg of artemether/lumefantrine to HIV-infected adults, taken with and without lopinavir/ritonavir-based ART. To avoid unknown adverse effects, we administered a single dose of artemether/lumefantrine to HIV-infected patients without malaria and vigilantly monitored their cardiac function.
Methods
Study site
The study was conducted between January 2008 and June 2009 at the Infectious Diseases Institute (IDI) and the Uganda Heart Institute, Mulago Hospital, Kampala, Uganda.
Study design and population
This was a two-arm parallel study to assess the pharmacokinetics of a single dose of artemether/lumefantrine co-administered with and without lopinavir/ritonavir-based ART to HIV-infected patients without malaria. Patients were eligible to participate if they were older than 18 years, with no evidence of systemic illness and no indication for medications with known potential for drug interactions with the study drugs. Patients with abnormal cardiac, liver or renal function, positive blood smear for malaria, pregnant mothers and those who reported use of any herbal medication were excluded.
Ethical considerations
The study was approved by the Uganda National HIV/AIDS Research Committee (ARC 056) and the Uganda National Council of Science and Technology (HS 195), and was registered with ClinicalTrials.gov (NCT 00619944). Study procedures were explained to participants in their local languages. Each participant received an information leaflet to take home. All participants provided written informed consent prior to study entry. Study procedures were conducted in accordance with the principles of Good Clinical Practice.
Study procedures
Patients were screened and enrolled consecutively from the cohort of patients attending the IDI. The artemether/lumefantrine plus lopinavir/ritonavir arm consisted of HIV-positive patients stable on 400/100 mg of lopinavir/ritonavir plus two nucleoside reverse transcriptase inhibitors (NRTIs) taken twice daily for at least 1 month. The artemether/lumefantrine arm consisted of HIV-positive ART-naive patients who had not started ART and were not yet eligible for ART according to national guidelines. Patients in both arms took co-trimoxazole daily for prophylaxis against opportunistic infections. Adherence to study drugs was assessed using self-report and pill count at each clinical visit. On the evening prior to the study day, participants were reminded of their study-day appointment and were given detailed instructions to eat food; those in the lopinavir/ritonavir arm were reminded to administer their ART by 8.00 pm, and arrive at the hospital by 7.00 am in a fasting state.
On the morning of the study day, patients were admitted to the Heart Institute. Blood smears for malaria parasites were performed, and patients found to have positive smears were given a standard six-dose course of artemether/lumefantrine and excluded from further study. A 12-lead electrocardiograph (ECG) monitor was attached for continuous cardiac function monitoring. An indwelling intravenous catheter was inserted following aseptic techniques, and blood samples were drawn for the determination of pre-dose concentrations of artemether, dihydroartemisinin and lumefantrine. A standardized breakfast with added fat to cater for the fat requirement for artemether/lumefantrine absorption was administered.21 The intake of breakfast and study drugs was directly observed by study staff.
All patients took a single dose of four tablets, equivalent to 80/480 mg of artemether/lumefantrine (Coartem®, Novartis Pharma AG, Basel, Switzerland; Batch number: F0660) with water immediately after breakfast. Patients in the lopinavir/ritonavir arm took 400/100 mg of lopinavir/ritonavir (Aluvia®, Abbott Laboratories, USA) plus two NRTIs with their study artemether/lumefantrine dose. The NRTI combination consisted of zidovudine plus didanosine, or tenofovir plus emtricitabine.
Sampling was performed at 1, 2, 4, 6, 8, 12, 24, 48 and 72 h post-artemether/lumefantrine dosing. An aliquot of 4 mL of blood was collected per sampling time in lithium–heparin tubes. Samples were centrifuged immediately for 10 min; plasma was separated and stored immediately at −70°C until shipment on dry ice to the Clinical Pharmacology Laboratory, Mahidol-Oxford Tropical Medicine Research Unit, Mahidol University, Bangkok, Thailand for measurement of artemether, dihydroartemisinin and lumefantrine plasma concentrations.
Safety assessment
Medical history, physical examination, routine clinical laboratory tests, ECG and urine screens for pregnancy were performed at screening. On the study day, medical history, physical examination and blood smears for malaria parasites were performed. Standard 12-lead ECGs were recorded at screening, immediately prior to dosing, then continuously for 12 h post-dose of artemether/lumefantrine and once daily for 3 days thereafter. Participants were monitored for adverse events until 2 weeks post-sampling; the onset, duration, severity and relationship to the trial drugs (if any) were noted.
Artemether, dihydroartemisinin and lumefantrine plasma concentration measurement
Artemether and dihydroartemisinin concentrations were measured using solid-phase extraction and liquid chromatography/mass spectrometry.22 Total-assay coefficients of variation for dihydroartemisinin and artemether during analysis were less than 5% at all quality control levels. The lower limit of quantification was 1.4 ng/mL and the limit of detection was 0.5 ng/mL for both drugs.22
Lumefantrine concentrations were determined using a solid-phase extraction/liquid chromatographic assay with ultraviolet detection.23 The coefficient of variation was less than 6% at all quality control levels. The lower limit of quantification was 25 ng/mL and the limit of detection was 15 ng/mL.23
Analytical and pharmacokinetic methods
Non-compartmental analysis was performed using WinNonlin Professional™ software, version 5.2 (Pharsight Corp., Mountain View, CA, USA). Pharmacokinetic parameters included the observed maximum concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration–time curve from zero to the last observation (AUC0-last), area under the plasma concentration-time curve from zero extrapolated to infinity (AUC0-∞), elimination clearance (CL/F), apparent volume of distribution (V/F), elimination half-life (t1/2) and absorption lag time (Tlag). The trapezoidal rule (linear-up/log-down) was used to estimate AUC. All parameters were calculated using actual blood sampling times. Drug concentrations below the lower limit of quantification of the bioanalytical assays were treated as missing data. The median values and ranges of the pharmacokinetic parameters were recorded for the two groups.
Statistical analysis
Data were analysed using STATA® version 10.0 (StataCorp, College Station, TX, USA). Baseline characteristics were summarized as mean with 95% CI and compared using the independent t-test. The Wilcoxon rank-sum test was used to compare pharmacokinetic parameters between the two groups. A P value of <0.05 was considered statistically significant.
Results
A total of 36 participants were enrolled, of whom 29 completed the 72 h sampling. Of the seven participants who did not complete sampling, two dropped out before sampling started, one participant had only the first three samples drawn due to difficulty with cannulation and four patients had positive blood smears for malaria on the sampling visit; the latter were given the standard six-dose regimen of artemether/lumefantrine and excluded from further study.
Analyses were performed on data from the 29 participants who completed sampling: 16 [9 (56%) female] in the artemether/lumefantrine plus lopinavir/ritonavir arm, and 13 [9 (69%) female] in the artemether/lumefantrine arm. All participants taking lopinavir/ritonavir-based ART had viral load below the level of detection (400 copies/mL). Mean (95% CI) of the log of viral load was 4.5 (4.0–5.0) copies/mL among the ART-naive patients. Participants in the two study arms were comparable for all other baseline characteristics measured except haemoglobin, which was significantly higher among patients taking lopinavir/ritonavir-based ART (Table 1). All participants tolerated study drugs very well, with no adverse events reported. ECG parameters for patients in both study arms remained well within normal limits throughout the 72 h follow-up period. These data have been published elsewhere.24
Table 1.
Baseline characteristics of study participants
| Mean (95% CI) |
|||
|---|---|---|---|
| Variable | artemether/lumefantrine arm | artemether/lumefantrineplus lopinavir/ritonavir arm | P value |
| Age (years) | 34.5 (29.9–39.0) | 37.6 (34.3–40.9) | 0.2 |
| Body weight (kg) | 63.8 (58.1–69.5) | 64.0 (57.6–70.5) | 0.9 |
| Height (cm) | 160.1 (155.2–165.0) | 165.8 (161.5–170.0) | 0.06 |
| Body mass index (kg/m2) | 25.1 (22.2–28.0) | 23.4 (21.0–25.8) | 0.3 |
| Haemoglobin (mg/dL) | 12.6 (11.4–13.8) | 14.3 (13.8–14.9) | 0.004a |
| QTc (ms) | 411.5 (402.3–420.6) | 416.8 (406.0–427.5) | 0.4 |
aStatistically significant.
Effect of lopinavir/ritonavir on artemether and dihydroartemisinin pharmacokinetics
Co-administration of artemether/lumefantrine with lopinavir/ritonavir significantly increased artemether CL/F and V/F, by 67% (P < 0.01) and 39% (P = 0.02), respectively. Artemether Cmax and AUC0-last were significantly reduced, by 50% (P = 0.03) and 43% (P < 0.01), respectively (Table 2 and Figure 1a). Dihydroartemisinin CL/F and V/F were not influenced by lopinavir/ritonavir co-administration. Similarly dihydroartemisinin Cmax and AUC0-last were unaffected (Table 2 and Figure 1b).
Table 2.
Comparison of pharmacokinetic parameters of artemether, dihydroartemisinin and lumefantrine
| Parameter | Artemether/lumefantrine(N = 13), median (range) | Artemether/lumefantrine plus lopinavir/ritonavir(N = 16), median (range) | P value |
|---|---|---|---|
| Artemether | |||
| Cmax (ng/mL) | 112 (20–362) | 56 (17–236) | 0.03 |
| Tmax (h) | 1 (1–4) | 2 (1–4) | 0.38 |
| CL/F (L/h) | 295 (69–817) | 492 (129–1805) | <0.01 |
| V/F (L) | 1072 (593–2651) | 1487 (762–3485) | 0.02 |
| t1/2 (h) | 2 (1–5) | 1 (1–6) | 0.04 |
| AUC0-last (ng · h/mL) | 264 (92–1129) | 151 (38–606) | <0.01 |
| AUC0-∞ (ng · h/mL) | 271 (97–1150) | 162 (44–618) | <0.01 |
| Dihydroartemisinin | |||
| Cmax (ng/mL) | 66 (10–111) | 73 (31–224) | 0.55 |
| Tmax (h) | 2 (1–4) | 2 (1–4) | 0.89 |
| CL/F (L/h) | 350 (210–942) | 424 (280–626) | 0.23 |
| V/F (L) | 922 (498–4779) | 876 (734–1315) | 1 |
| t1/2 (h) | 1 (1–3) | 1 (1–2) | 0.06 |
| AUC0-last (ng · h/mL) | 213 (68–343) | 175 (118–262) | 0.27 |
| AUC0-∞ (ng · h/mL) | 217 (81–363) | 180 (121–272) | 0.23 |
| Lumefantrine | |||
| Tlag (h) | 1 (0–4) | 1 (0–1) | 0.16 |
| Cmax (ng/mL) | 2532 (1071–5957) | 7097 (2396–9462) | <0.01 |
| Tmax (h) | 8 (3–12) | 8 (4–12) | 0.26 |
| CL/F (L/h) | 10 (3–32) | 1 (1–5) | <0.01 |
| V/F (L) | 179 (53–860) | 86 (59–219) | 0.01 |
| t1/2 (h) | 23 (6–51) | 31 (24–43) | <0.01 |
| AUC0-last (ng · h/mL) | 41 119 (12 850–125 200) | 199 678 (71 205–251 015) | <0.01 |
| AUC0-∞ (ng · h/mL) | 46 925 (14 559–136 297) | 267 386 (84 845–344 468) | <0.01 |
Figure 1.
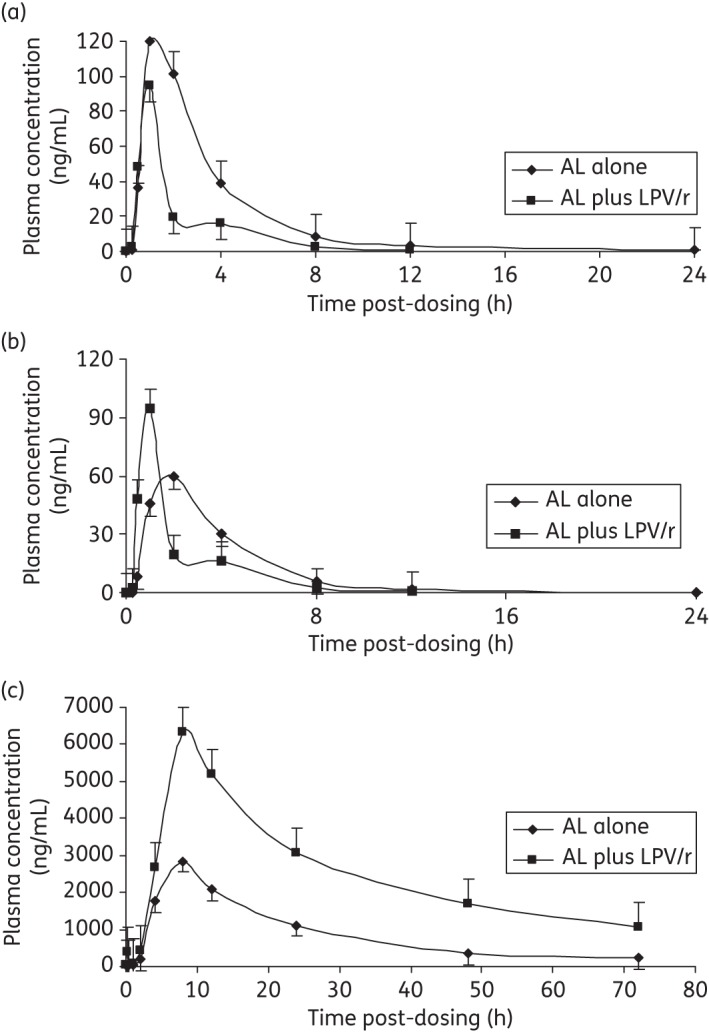
Mean (±SEM) plasma concentration versus time of (a) artemether, (b) dihydroartemisinin and (c) lumefantrine for participants taking artemether/lumefantrine alone (AL alone) and artemether/lumefantrine in combination with lopinavir/ritonavir (AL plus LPV/r).
Effect of lopinavir/ritonavir on lumefantrine pharmacokinetics
Co-administration of artemether/lumefantrine with lopinavir/ritonavir significantly reduced lumefantrine CL/F and V/F, by 90% (P < 0.01) and 52% (P = 0.01), respectively. Lumefantrine Cmax increased significantly by 180% (P < 0.01) and AUC0-last by 386% (P < 0.01) (Table 2 and Figure 1c).
Discussion
We investigated the pharmacokinetics of artemether, dihydroartemisinin and lumefantrine after administration of a single dose of 80/480 mg of artemether/lumefantrine to HIV-infected adults, taken with and without lopinavir/ritonavir-based ART. Co-administration of artemether/lumefantrine with lopinavir/ritonavir significantly increased artemether clearance with a consequently significant reduction in artemether exposure. Dihydroartemisinin pharmacokinetic parameters were not affected by lopinavir/ritonavir. Lumefantrine clearance significantly decreased with a consequently significant increase in exposure.
Our data for the direction of the interaction between lopinavir/ritonavir and artemether/lumefantrine show a similar trend to data from a previous study by German et al.;20 however, differences in the magnitude of the interaction as well as the effect on dihydroartemisinin were evident between the two studies. The previous study demonstrated a trend towards decreased artemether exposure, significant reduction in dihydroartemisinin exposure and significant increase in lumefantrine exposure following standard six-dose artemether/lumefantrine administration with lopinavir/ritonavir to 13 healthy HIV-seronegative adults.20 The differences in the results from the two studies possibly arise from differences in the study designs and population. German et al.20 conducted a sequential cross-over study in which artemether/lumefantrine parameters were compared within the same individuals with and without lopinavir/ritonavir, while we employed a parallel study design with comparison of parameters from different individuals with and without lopinavir/ritonavir. The parallel study design was adequate for the objectives of our study, but has a limitation due to the high inter-individual variability of artemether and dihydroartemisinin. Comparison of pharmacokinetic exposures in the same individuals using the sequential design was not feasible given that lopinavir/ritonavir is used for second-line HIV treatment in our study setting.
In addition, our population was composed of HIV-infected adults of African origin, unlike the HIV-uninfected healthy volunteers of primarily white origin in the study by German et al.20 Genetic variation may cause inter-individual pharmacokinetic variability due to polymorphisms of genes encoding drug-metabolizing enzymes.25–27 In addition, drug pharmacokinetics may differ in healthy volunteers compared with patients with disease.
Further differences in the magnitude of the effects of interaction between our data and the German et al.20 data could have arisen from the six-dose compared with the single-dose regimen of artemether/lumefantrine. We administered a single dose of artemether/lumefantrine to avoid any unknown adverse effects of co-administration of artemether/lumefantrine with lopinavir/ritonavir in HIV-infected participants. German et al.20 administered the standard six-dose artemether/lumefantrine regimen to healthy volunteers. Artemether undergoes auto-induction of its metabolism, and artemether/dihydroartemisinin ratios after 3 days of treatment with the standard dose are lower than those seen after a single dose.28
In both studies lumefantrine exposure was elevated during co-administration with lopinavir/ritonavir; however, despite the elevated lumefantrine exposure, participants tolerated the study drugs very well, with all reported adverse events consistent with what had previously been reported for artemether/lumefantrine and lopinavir/ritonavir. Our data did not demonstrate evidence of cardiac conduction abnormalities. However, caution and safety monitoring of HIV/malaria-coinfected patients receiving artemether/lumefantrine with lopinavir/ritonavir is advised. It will be important to determine if these effects are additive in the standard six-dose artemether/lumefantrine regimen in HIV/malaria-coinfected patients receiving lopinavir/ritonavir.
Ritonavir-boosted lopinavir influences the activity of several CYP enzymes and drug transporters such as the efflux transporter P-glycoprotein.29 Both lopinavir and ritonavir inhibit intestinal and hepatic CYP3A4 and P-glycoprotein.29,30 Inhibition of CYP3A4 or P-glycoprotein expression decreases biotransformation, resulting in an increase in bioavailability of co-administered substrates.9,31–33 Previous data demonstrated increased artemether, dihydroartemisinin and lumefantrine exposure in the presence of the CYP3A4 inhibitors ketoconazole and grapefruit juice.34,35 Inhibitions of CYP3A4 and P-glycoprotein are likely explanations for the increased lumefantrine exposure in our study.
The reduction in artemether exposure was unexpected, since CYP3A4 is suggested to be the predominant CYP enzyme in the metabolism of artemether.10 Although artemether is predominantly metabolized via CYP3A4/5, other CYP enzymes (CYP2B6, CYP2C9, CYP2C19 and possibly CYP2A6) are involved.10,13,14 Lopinavir/ritonavir was shown to induce CYP1A2, CYP2B6, CYP2C9, and CYP2C19.30,36 The observed increased clearance and decreased artemether exposure is likely due to induction of these CYP enzymes by lopinavir/ritonavir.
Dihydroartemisinin is converted into inactive metabolites by UGT1A1, UGT1A8/9 and UGT2B7.10,13,14 Induction and inhibition of UGTs by xenobiotics have been described previously, and lopinavir/ritonavir was shown to inhibit UGTs 1A1, 1A3, 1A4, 1A6, 1A9 and 2B7.37–39 However, we found no statistical difference in the pharmacokinetic parameters of dihydroartemisinin after lopinavir/ritonavir co-administration compared with administration alone. The reason for this is unclear, but might be due to the small numbers and large inter-individual variability.
Artemether and dihydroartemisinin have very short half-lives and rapidly clear parasites from circulation.11 Both are very potent antimalarial agents, although dihydroartemisinin is more potent.16 Lumefantrine has a much longer half-life and mainly clears residual parasites, preventing recrudescence. Higher artemether and dihydroartemisinin exposure decreases parasite clearance time,13 but the major determinant of radical cure is lumefantrine exposure.40 Given that HIV/malaria-coinfected patients present with higher parasite counts,3,41 which is an independent predictor of poor treatment response,42 reduction in artemether exposure may predispose patients to develop severe malaria due to slower parasite clearance. The clinical relevance of the present findings should be interpreted with caution given that we administered a single artemether/lumefantrine dose while a six-dose artemether/lumefantrine regimen is administered for malaria treatment.
The reduction in artemether exposure by lopinavir/ritonavir after the single artemether/lumefantrine dose may be offset by the increase in lumefantrine exposure. Previous data revealed that lumefantrine exposure is the key determinant for malaria cure,43 therefore the increase in lumefantrine exposure during lopinavir/ritonavir co-administration may be beneficial for malaria cure. However, rapid clearance of artemether and reduced clearance of lumefantrine may create longer periods of exposure to lumefantrine monotherapy with the risk of development of resistance.
Conclusions
Co-administration of a single dose of artemether/lumefantrine with lopinavir/ritonavir significantly reduced artemether exposure, with a significant increase in lumefantrine exposure. Population pharmacokinetic and pharmacodynamic trials will be highly valuable in evaluating the clinical significance of this interaction and determining whether dosage modifications are indicated.
Funding
Support for this study was provided by a Monument Fund grant to the University of Liverpool, UK. Additional support was provided by the Infectious Diseases Network for Treatment and Research in Africa and the HIV Research Trust.
W. H., J. T. and N. L. are part of the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme (077166/Z/05/Z) supported by the Wellcome Trust of Great Britain.
Transparency declarations
None to declare.
Author contributions
P. B.-K., M. L., S. K. and C. M. contributed to the design and conduct of the study. P. B.-K., M. L. and V. O.-K. participated in recruitment of patients and data collection. W. H. and N. L. performed the bioanalytical assays. P. B.-K., T. P. C. D., J. T., N. L. and P. J. d. V. analysed and interpreted the pharmacokinetic data. H. M.-K., E. K., N. P., P. J. d. V., D. B., S. K. and C. M., participated in training the study staff, and provided scientific support. P. B.-K. drafted the first version, and all authors reviewed and approved the manuscript for submission.
Acknowledgements
We thank the study participants and members of the clinical study team (Dr J. Mayito, Dr L. Nabukeera, D. Ekusai, J. Nakku, H. Tibakabikoba, R. Namakula and J. Magoola). We acknowledge training, staff and data management support from the Infectious Diseases Network for Treatment and Research in Africa and the Sewankambo Scholarship programme.
References
- 1.UNAIDS and WHO. 09 AIDS Epidemic Update. http://data.unaids.org/pub/report/2009/jc1700_epi_update_2009_en.pdf. (14 November 2011, date last accessed) [Google Scholar]
- 2.WHO. Guidelines for the Treatment of Malaria. Geneva: WHO; 2010. [Google Scholar]
- 3.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–6. doi: 10.1016/S0140-6736(00)02727-6. doi:10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 4.French N, Nakiyingi J, Lugada E, et al. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS. 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. doi:10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–40. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–6. doi: 10.1126/science.1132338. doi:10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS and WHO. UNAIDS Report on the Global AIDS Epidemic. 2010. http://www.unaids.org/documents/20101123_GlobalReport_em.pdf. (14 November 2011, date last accessed)
- 8.Khoo S, Back D, Winstanley P. The potential for interactions between antimalarial and antiretroviral drugs. AIDS. 2005;19:995–1005. doi: 10.1097/01.aids.0000174445.40379.e0. doi:10.1097/01.aids.0000174445.40379.e0. [DOI] [PubMed] [Google Scholar]
- 9.Barry M, Mulcahy F, Merry C, et al. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. doi:10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Novartis Pharmaceuticals Corporation. Coartem (Artemether and Lumefantrine) Tablet: Human Prescription Drug Label. http://www.pharma.us.novartis.com/product/pi/pdf/coartem.pdf. (9 August 2011 2011, date last accessed) [Google Scholar]
- 11.Kokwaro G, Mwai L, Nzila A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin Pharmacother. 2007;8:75–94. doi: 10.1517/14656566.8.1.75. doi:10.1517/14656566.8.1.75. [DOI] [PubMed] [Google Scholar]
- 12.German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47:91–102. doi: 10.2165/00003088-200847020-00002. doi:10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46:553–61. doi: 10.1046/j.1365-2125.1998.00830.x. doi:10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet. 1999;37:105–25. doi: 10.2165/00003088-199937020-00002. doi:10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 15.White N. Preventing antimalarial drug resistance through combinations. Drug Resist Updat. 1998;1:3–9. doi: 10.1016/s1368-7646(98)80208-2. doi:10.1016/S1368-7646(98)80208-2. [DOI] [PubMed] [Google Scholar]
- 16.Djimdé A, Lefèvre G. Understanding the pharmacokinetics of Coartem. Malar J. 2009;8(Suppl 1):S4. doi: 10.1186/1475-2875-8-S1-S4. doi:10.1186/1475-2875-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malvy D, Receveur MC, Ozon P, et al. Fatal cardiac incident after use of halofantrine. J Trav Med. 2000;7:215–6. doi: 10.2310/7060.2000.00065. doi:10.2310/7060.2000.00065. [DOI] [PubMed] [Google Scholar]
- 18.Touze JE, Bernard J, Keundjian A, et al. Electrocardiographic changes and halofantrine plasma level during acute falciparum malaria. American J Trop Med Hyg. 1996;54:225–8. doi: 10.4269/ajtmh.1996.54.225. [DOI] [PubMed] [Google Scholar]
- 19.Giudicelli CP, Touze JE, Bernard J. [Electrocardiographic changes due to halofantrine in the treatment of malaria: therapeutic implications] Bull Acad Natl Med. 1996;180:71–80. discussion 80–2. [PubMed] [Google Scholar]
- 20.German P, Parikh S, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–9. doi: 10.1097/QAI.0b013e3181acb4ff. doi:10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 21.Ashley EA, Stepniewska K, Lindegårdh N, et al. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health. 2007;12:195–200. doi: 10.1111/j.1365-3156.2006.01784.x. doi:10.1111/j.1365-3156.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanpithakpong W, Kamanikom B, Dondorp AM, et al. A liquid chromatographic-tandem mass spectrometric method for determination of artesunate and its metabolite dihydroartemisinin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:61–8. doi: 10.1016/j.jchromb.2008.10.018. doi:10.1016/j.jchromb.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Annerberg A, Singtoroj T, Tipmanee P, et al. High throughput assay for the determination of lumefantrine in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:330–3. doi: 10.1016/j.jchromb.2005.06.022. doi:10.1016/j.jchromb.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Byakika-Kibwika P, Lamorde M, Lwabi P, et al. Cardiac conduction safety during coadministration of artemether-lumefantrine and lopinavir/ritonavir in HIV-infected Ugandan adults. Chemother Res Practice. 2011 doi: 10.1155/2011/393976. doi:10.1155/2011/393976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubomirov R, di Iulio J, Fayet A, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20:217–30. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 26.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 27.Piedade R, Gil JP. The pharmacogenetics of antimalaria artemisinin combination therapy. Expert Opin Drug Metab Toxicol. 2011;7:1185–200. doi: 10.1517/17425255.2011.608660. doi:10.1517/17425255.2011.608660. [DOI] [PubMed] [Google Scholar]
- 28.van Agtmael MA, Cheng-Qi S, Qing JX, et al. Multiple dose pharmacokinetics of artemether in Chinese patients with uncomplicated falciparum malaria. Int J Antimicrob Agents. 1999;12:151–8. doi: 10.1016/s0924-8579(99)00063-1. doi:10.1016/S0924-8579(99)00063-1. [DOI] [PubMed] [Google Scholar]
- 29.Wyen C, Fuhr U, Frank D, et al. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther. 2008;84:75–82. doi: 10.1038/sj.clpt.6100452. doi:10.1038/sj.clpt.6100452. [DOI] [PubMed] [Google Scholar]
- 30.Yeh RF, Gaver VE, Patterson KB, et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 31.Corbett AH, Lim ML, Kashuba AD. Kaletra (lopinavir/ritonavir) Ann Pharmacother. 2002;36:1193–203. doi: 10.1345/aph.1A363. doi:10.1345/aph.1A363. [DOI] [PubMed] [Google Scholar]
- 32.Cooper CL, van Heeswijk RP, Gallicano K, et al. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin Infect Dis. 2003;36:1585–92. doi: 10.1086/375233. doi:10.1086/375233. [DOI] [PubMed] [Google Scholar]
- 33.Weemhoff JL, von Moltke LL, Richert C, et al. Apparent mechanism-based inhibition of human CYP3A in-vitro by lopinavir. J Pharm Pharmacol. 2003;55:381–6. doi: 10.1211/002235702739. doi:10.1211/002235702739. [DOI] [PubMed] [Google Scholar]
- 34.Lefèvre G, Carpenter P, Souppart C, et al. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br J Clin Pharmacol. 2002;54:485–92. doi: 10.1046/j.1365-2125.2002.01696.x. doi:10.1046/j.1365-2125.2002.01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Agtmael M, Gupta V, van der Wösten TH, et al. Grapefruit juice increases the bioavailability of artemether. Eur J Clin Pharmacol. 1999;55:405–10. doi: 10.1007/s002280050648. doi:10.1007/s002280050648. [DOI] [PubMed] [Google Scholar]
- 36.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59. doi: 10.1345/aph.1K615. doi:10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 37.Faucette SR, Zhang TC, Moore R, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. doi:10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie W, Yeuh MF, Radominska-Pandya A, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA. 2003;100:4150–5. doi: 10.1073/pnas.0438010100. doi:10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D, Chando TJ, Everett DW, et al. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–39. doi: 10.1124/dmd.105.005447. doi:10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 40.Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–7. doi: 10.1086/503423. doi:10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Geertruyden JP, Menten J, Colebunders R, et al. The impact of HIV-1 on the malaria parasite biomass in adults in sub-Saharan Africa contributes to the emergence of antimalarial drug resistance. Malar J. 2008;7:134. doi: 10.1186/1475-2875-7-134. doi:10.1186/1475-2875-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ittarrat W, Pickard AL, Rattanasinganchan P, et al. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am J Trop Med Hyg. 2003;68:147–52. [PubMed] [Google Scholar]
- 43.Ezzet F, van Vugt M, Nosten F, et al. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44:697–704. doi: 10.1128/aac.44.3.697-704.2000. doi:10.1128/AAC.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


