Abstract
The introduction of highly active antiretroviral therapy (HAART) in 1996 has transformed a lethal disease to a chronic pathology with a dramatic decrease in mortality and morbidity of AIDS-related symptoms in infected patients. However, HAART has not allowed the cure of HIV infection, the main obstacle to HIV eradication being the existence of quiescent reservoirs. Several other problems have been encountered with HAART (such as side effects, adherence to medication, emergence of resistance and cost of treatment), and these motivate the search for new ways to treat these patients. Recent advances hold promise for the ultimate cure of HIV infection, which is the topic of this review. Besides these new strategies aiming to eliminate the virus, efforts must be made to improve current HAART. We believe that the cure of HIV infection will not be attained in the short term and that a strategy based on purging the reservoirs has to be associated with an aggressive HAART strategy.
Keywords: CCR5, reservoirs, latency, purge, HAART
Introduction
Human immunodeficiency virus 1 (HIV-1), identified 28 years ago,1 remains a global health threat responsible for a worldwide pandemic with an estimated 33 million people infected.2 More than 7000 new HIV infections occur each day, and the number of newly diagnosed infections remains far greater than the number of people (around 50%) who have access to highly active antiretroviral therapy (HAART). Advances have been made in treating AIDS since the introduction of HAART in 1996. This has transformed a lethal disease into a chronic pathology, with a dramatic decrease of mortality and morbidity of AIDS-related symptoms in infected patients.3,4
Why is achieving a cure important?
To date, the only way to treat patients infected with HIV relies on a combination of drugs that acts at different stages of the viral life cycle, preventing the virus from replicating. These molecules target four stages of the cycle: viral entry, reverse transcription of the viral genome, integration into the genome of the host cell and maturation of viral proteins. This therapy can reduce plasma virus levels below detection limits (≤50 copies/mL). However, with very sensitive but expensive and technically challenging methods, a residual viraemia is still detected in patients on HAART.5–8 Moreover, HIV RNA typically returns to a measurable plasma level in less than 2 weeks when HAART is interrupted, suggesting that even long-term suppression of HIV-1 replication by HAART fails to totally eliminate HIV-1. These two latter phenomena are mainly due to the existence of HIV reservoirs.6,9–13 The existence of integrated latent viruses or virus replicating at a very low level in different cellular reservoirs is an obstacle to the eradication of the virus, and thus the total recovery of patients, and requires strict adherence to lifelong treatment.14–21 In addition, these cellular reservoirs are often found in tissue sanctuaries, such as the brain, where drug penetration may be several orders of magnitude lower than in other tissues.16,18 Viral clearance from other reservoirs, such as from chronically infected macrophages, is also difficult since reverse transcriptase inhibitors are usually ineffective and protease inhibitors have significantly lower activities in these cells than in lymphocytes.22,23 Moreover, emergence of many side effects may require the cessation of treatment.24 Furthermore, the development of many types of resistance, related to the extreme mutability of the virus and in part to treatment interruptions, has been described in the literature.25–28 Another major concern is related to non-AIDS events and non-AIDS mortality in patients having a residual viraemia and a normal CD4+ count, a situation also described in some HIV non-progressors. Owing to the residual viraemia, patients develop chronic inflammation that leads to several complications, for instance, cardiovascular disease, nephropathy, faster evolution of viral hepatitis and cancer.29–33
Last but not least, a major problem related to HAART is the cost of the treatment. Even the cost associated with the cheaper generic forms of the drugs far exceeds the abilities of many resource-limited countries in providing treatment. The cost of this treatment will be increasingly important in the future, with an overall global budget requirement to address this problem from today to 2031 being estimated at US$397–727 billion.34 Since, to date, no effective HIV-1 vaccine is available,35–38 it appears crucial to improve HAART and to develop new strategies to cure HIV.39,40
Which cure is needed: a functional or a sterilizing cure?
A sterilizing cure requires the total eradication of all HIV-infected cells, including quiescent reservoirs. On the other hand, a functional cure aims to mimic a situation encountered in some special patients called ‘elite controllers’ who are able to control viral replication and have less than 50 copies/mL of the virus without any treatment. Although a sterilizing cure would be the most appropriate and desirable, it may be difficult or impossible to really achieve. Only one reported case, the German case, is known in the literature that suggests a possible eradication of the virus.41 A functional cure appears more feasible since it seems impossible to get rid of HIV from latent cells and from sanctuaries. We have to keep in mind however that the chronic inflammation described in patients under HAART has also been described in some elite controllers who have presented with residual viraemia and higher immune activation compared with healthy patients.42–44 It is very likely that these patients will develop more non-AIDS events compared with those who are uninfected or actually cured.
How might we achieve a cure?
The best scenario would be to eradicate the virus from all infected cells. Even though this appears very difficult, we should be able to drastically decrease the HIV reservoirs by identifying and then eliminating them. Residual on-going viral replication, whatever its origin, also has to be reduced to preclude non-AIDS events.
In this article we will discuss new strategies under investigation that aim to eradicate HIV from infected patients. First we will discuss a recently described case that showed a possible eradication of HIV following transplantation of CCR5-deficient haematopoietic stem cells. This strategy may open new avenues to cure HIV-infected patients. We will also discuss novel strategies based on purging reservoirs followed by aggressive HAART. This approach has already been used in several clinical trials. Finally, we believe that HAART has to be improved and/or intensified; however, we have to keep in mind that HAART alone will not allow for a cure.
The critical role of CCR5 in maintaining HIV-1 infection
A proof of concept
A report of a German patient being transplanted with stem cells from a donor who carried the Δ32 CCR5 mutation and then controlled his HIV infection has highlighted the critical role of CCR5 in maintaining HIV infection.45 It is well known that HIV-1 enters cells by using CD4 receptors and CCR5 or CXCR4 coreceptors and persons homozygotic for a 32 bp deletion in the gene coding for CCR5 are resistant to HIV-1 infection.46,47 It is noteworthy that the origin of the CCR5-Δ32-containing ancestral haplotype is recent (estimated range of 275–1875 years) and might be related to a historic strong selective event such as an epidemic of a pathogen that, like HIV-1, utilizes CCR5. This hypothetical epidemic has increased the frequency of this mutation in ancestral Caucasian populations.48 Hutter understood the significance of the CCR5 mutation and suggested that transplantation of stem cells originating from a donor homozygotic for the mutation could effectively eradicate the virus. After the relapse of leukaemia in the German patient with HIV there was no other choice but to transplant allogeneic stem cells to this person. The patient, as suggested by Hutter, received Δ32 CCR5 mutant stem cells. Following the medical intervention, the patient has stopped HAART and HIV RNA has remained below 1 copy/mL for now over 4 years. In a recent paper this group showed evidence even for a possible cure of HIV-1 infection in this patient. Indeed, they demonstrated reconstitution of both circulating and mucosal CD4+ T cells that do not express CCR5 while the patient remained free of the virus. Moreover, they also found evidence that long-lived cells such as macrophages became Δ32 CCR5. Since these cells are reservoirs for the virus along with CD4+ T memory cells, it appears that the size of the viral reservoir has decreased. This result was unexpected since the patient's CD4+ memory cells are still susceptible to productive infection by lymphotropic (CXCR4-tropic) HIV. The combination of radiotherapy and chemotherapy has allowed the eradication of long-lived reservoirs, which has prevented HIV rebound during the process of immune reconstitution following stem cell transplantation. Although this specific case is a real success, stem cell transplantation as a general strategy to cure infected patients is not yet feasible due to the high mortality of this treatment (20%–30%). This report constitutes a proof of concept and opens the development of new strategies targeting the CCR5 coreceptor.
CCR5 gene therapy
Among new treatments, CCR5 gene therapy could be a potential treatment to cure HIV (Figure 1). In preclinical trials, HIV-1-infected mice engrafted with zinc finger nuclease (ZFN)-modified CD4+ T cells had lower viral loads and higher CD4+ T cell counts than mice engrafted with wild-type CD4+ T cells, consistent with the potential to reconstitute immune function in individuals with HIV/AIDS by the maintenance of an HIV-resistant CD4+ T cell population.49,50 Preliminary results of two Phase 1 clinical trials using this attractive approach were presented at the 2011 Conference on Retroviruses and Opportunistic Infections (CROI).51,52 Lalezari presented data on transformed CD4+ T cells. The wild-type CD4+ T cells were obtained from six patients who had been living with HIV infection for >20 years. Participants chosen had continued low CD4+ T cell counts (ranging from 200 to 500 cells/mm3), despite receiving antiretroviral therapy, which reduced HIV viral load to an undetectable level. Both studies showed a successful and tolerated engraftment of the transformed CD4+ T cells. At the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), data from another clinical trial was also presented in which six subjects having initially >450 CD4+ T cells/mm3 under HAART53 were followed for 12 weeks after infusion of ex vivo transformed CD4+ T cells. Only one patient in this clinical trial became undetectable for the virus. However, this patient entered the clinical study with one Δ32 CCR5 mutation. Therefore a functional cure with this gene therapy was not attained. As explained during this conference, only 5% of the total CD4+ T cells were transformed, in contrast to the 100% in the German patient who benefited from stem cell transplantation. There is hope however that this small fraction of cells will rise in the body, since it is expected that the CCR5+ cells infected by HIV-1 will die over time. It is possible that CCR5− mutants will be selected and will replace the normal CCR5+ cells, since the release of virus from these CCR5+ cells will not be able to infect the transfused population of CCR5− mutants. A much longer follow-up is needed to confirm these expectations.
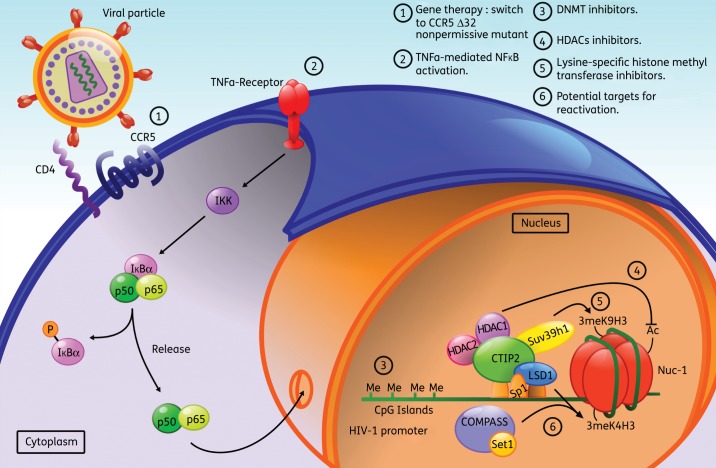
Figure 1.
Promising new approaches to cure patients of HIV-1: molecular mechanisms at the macrophage level. Beside increasing the pool of new molecules and improving the currently used ones in HAART, new approaches are required to reach a full recovery from HIV-1 infection. To date, HAART can only control and prevent viral replication, but fails to achieve total viral clearance. New potential strategies include virus eradication through gene therapy and clearance of the viral reservoirs. The first strategy derived from the observation of the Δ32 CCR5 bone marrow transplanted German patient, who seems to be free of HIV-1 infection. Owing to the high risk associated with surgery and the impossibility of using this method in a large number of patients, gene therapy could be a way to disrupt the CCR5-mediated infection in order to mimic the previous results of the German patient (1). The second strategy relies on associating the current HAART with molecules activating the viral transcription and/or targeting host proteins favouring HIV-1 latency. On the one hand, the early stage of viral replication requires the transcription activator NF-κB, thus cytokines such as TNF-α may allow the recovery of full viral transcription in latent reservoirs (2). On the other hand, chromatin-modifying enzymes have been associated with HIV-1 transcription extinction through fine modifications of the epigenetic code on the viral promoter. Limiting DNA methylation of the CpG islands (3), increasing activation marks, such as acetylation of histones from Nuc-1 (4), and/or avoiding marks associated with heterochromatin, such as simultaneous trimethylation of lysine 4 and lysine 9 (5,6) of histone H3 in Nuc-1 may revert the latently infected state back to productively infected macrophages. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The long-term control of HIV by the German patient who received a transplant of CCR5-deficient haematopoietic stem cells holds promise for a real cure,54 but due to its toxicity, it is not a realistic one as claimed by Lewin and Rouzioux.55 Further investigations in order to understand the mechanism by which HIV was eradicated have to be performed. It would also be interesting to repeat this approach in other patients, which will help us to make further conclusions.56 It even raises questions such as why there is no HIV rebound from long-lived viral reservoirs producing CXCR4-tropic viruses or what the role is of the transplantation procedure itself45,55 in the eradication of the virus. The debate, regarding whether the treatment of the German patient represents a sterilizing cure or not, is far from over.57 Gene therapy (including CCR5 gene therapy) is indeed a very attractive approach to cure HIV-1 infection, but it is not for immediate use, even though considerable progress in gene delivery has been made.58,59 Moreover, the debate as to whether or not this gene therapy will lead to a sterilizing cure is still open.60
Purging viral reservoirs
The main drawback of HAART is that it is unable to purge the virus from quiescent reservoirs, i.e. truly latent cells,61–63 and/or from cells with cryptic on-going HIV replication,7,64,65 or from sanctuaries such as the brain.66–68 Resting memory CD4+ T cells are the major cellular and the best characterized reservoirs in the natural host.6,7,69–71 The presence of latent proviral HIV-1 DNA in this cell population has definitely been proven.69
Other reservoirs than resting CD4+ T cells have also been proposed.16,18–20 Genetic studies showed that during rebound viraemia (due to HAART interruption) the virus could be detected from reservoirs other than CD4+ T cells.62,72,73 It has been proposed that peripheral blood monocytes, dendritic cells and macrophages in the lymph nodes and haematopoietic stem cells in the bone marrow can be infected latently and therefore contribute to viral persistence.14–17,19,61,74 It is still debated whether or not viral persistence in these latter reservoirs is due to true latency or to a low-level on-going replication.75,76
Deciphering the molecular mechanisms underlying HIV persistence is a prerequisite to devise novel treatments aiming to purge these reservoirs. Several recent reviews describe in more detail the mechanisms of HIV persistence with implications for the development of new therapeutic strategies.18,20,40,77–79 Before using strategies that aim at purging the reservoir in combination with an intensified HAART, we need: (i) to identify and characterize the molecular actors involved in the persistence of latency, which relies on the chromatin environment; and (ii) to understand the mechanisms of reactivation in order to prevent it.
Persistence of latency
Once HIV-1 DNA has integrated into the host genome, and latency has been established, maintenance of HIV-1 latency depends on the chromatin environment. The chromatin organization of the HIV-1 promoter with precisely positioned nucleosomes80,81 has been well described. Nuc-1, a nucleosome located immediately downstream of the transcription initiation site, impedes long terminal repeat (LTR) activity. Epigenetic modifications and disruption of Nuc-1 are a prerequisite of activation of LTR-driven transcription and viral expression.82 It was recently found that recruitment of deacetylases and methylases on the LTR was associated with epigenetic modifications (deacetylation of H3K9 followed by H3K9 trimethylation and recruitment of HP1 proteins) in CD4+ T cells. In these experiments, the methylase Suv39H1 and the HP1γ proteins were knocked down by small interfering RNA (siRNA). The depletion of these factors increased the level of HIV-1 expression.83
Epigenetic modifications of the LTR have also been described in microglial cells, the CNS-resident macrophages. These cells are major targets for HIV-1 and constitute latently infected cellular reservoirs in the brain.84 Previous work from our laboratory has shown that a COUP-TF interacting protein 2 (CTIP2), a recently cloned transcriptional repressor that can associate with members of the COUP-TF family,85 inhibits HIV-1 replication in human microglial cells.86,87 Subsequently we showed that CTIP2 inhibited HIV-1 gene transcription in these cells by recruiting a chromatin-modifying complex.88 As demonstrated in T lymphocytes, our work suggests a concomitant recruitment of histone deacetylases HDAC1, HDAC2 and methylase SUV39H1 to the viral promoter by CTIP2. Ordered histone modifications would allow HP1 binding, heterochromatin formation and, as a consequence, HIV silencing. The heterochromatin formation at the HIV-1 promoter has been linked to post-integration latency, and this suggests that transcriptional repressors such as CTIP2 are involved in the establishment and maintenance of viral persistence and post-integration latency in the brain.
The corepressor CTIP2 has an even more pleiotropic action by regulating the expression of genes of infected cells. Recruited to the cellular cyclin-dependent kinase inhibitor CDKN1A/p21waf (p21) promoter, CTIP2 silences p21 gene transcription by inducing epigenetic modifications, as described above, for the HIV-1 promoter. This effect indirectly favours HIV-1 latency since activation of the p21 gene stimulates viral expression in macrophages.89 Moreover, CTIP2 counteracts HIV-1 Vpr, which is required for p21 expression. We suggest that all these factors contribute together to HIV-1 transcriptional latency in microglial cells.90 The picture regarding the importance of p21 in the replicative cycle of HIV-1 is far more complicated since p21 has been described as a restriction factor in macrophages and in resting CD4 T cells.91,92 The protein p21 might have different effects on HIV-1 infection of macrophages depending on the targeted viral life cycle step, and therefore on the time since infection.93
We have also identified a new actor involved in the maintenance of HIV-1 latency in microglial cells, the lysine-specific demethylase (LSD1).94 We notably showed that LSD1 repressed HIV-1 transcription and viral expression in a synergistic manner with CTIP2 and reported that recruitment of LSD1 at the HIV-1 proximal promoter is associated with both H3K4me3 and H3K9me3 epigenetic marks. Association of both H3K4me3 and H3K9me3 epigenetic marks with LSD1 recruitment may thus constitute a new level of eukaryotic gene regulation. These observations are consistent with the discovery that H3K4 methylation at certain chromatin loci may prevent gene expression.95 Interestingly, such a gene repression linked to H3K4me3 has been proposed to prevent the expression of cryptic promoters.95,96 This is strengthened by the finding that HIV-1 preferentially integrates into active genes and therefore could be considered as a cryptic gene.
Surprisingly, LSD1 has been associated with activation of HIV transcription in CD4+ T cells through demethylation of K51 Tat.97 However, in microglial cells the mechanisms underlying LSD1-mediated increase of H3K4 trimethylation is different and might rely on the ability of LSD1 to anchor other factors at the promoter rather than its own enzymatic activity. Indeed, H3K4 trimethylation was associated with the recruitment of LSD1, hSET1 and WDR5 at the Sp1 binding sites of the HIV-1 LTR. Moreover, reactivation of HIV-1 proviruses correlated with the release of LSD1, hSET1 and WDR5 from the viral promoter and with a reduced H3K4 trimethylation. In contrast to CD4+ T cells, LSD1 is involved in the maintenance of HIV-1 latency in microglial cells by favouring a local heterochromatin structure. These two studies reporting a dual role of LSD1 through different mechanisms in two main HIV-1 targets point to the complexity of HIV latency and raise the question of how effective the use of inhibitors of LSD1 would be for full HIV-1 reactivation. Indeed, targeting LSD1 for full reactivation in microglial cells might not work in lymphocytes. Instead, in the latter cells an induction of HIV latency is expected.98 Further investigation of the epigenetic regulation of HIV latency is therefore needed in order to design efficient drugs targeting viral reservoirs.
Another field of interest is DNA methylation, which has been involved in DNA silencing and latency.99 It is now well established that DNA CpG methylation plays an important role in maintaining HIV-1 latency,100,101 despite previous controversies.102 Therefore DNA methylase inhibitors, such as 5-azacytidine, could be useful in strategies aiming to reactivate reservoirs. It is noteworthy that only a few percent of the latent viruses are methylated on their DNA, but these reservoirs of latent viruses are highly resistant to reactivation. Achieving a cure would probably require the treatment of many different types of latency simultaneously by a combination therapy approach.
Preventing reactivation
Several mechanisms acting at the transcriptional and post-transcriptional level are at work in order to preclude HIV reactivation in latent reservoirs. Affecting these mechanisms may open new ways to purge reservoirs. Sequestration of nuclear factor κB (NF-κB) in the cytoplasm of latent cells is one of these mechanisms.103 T cell activation with tumour necrosis factor α (TNF-α) allows translocation to the nucleus of NF-κB, which then binds to the LTR and activates the early phase (Tat independent) virus transcription (Figure 1). Besides TNF-α, many other factors have been involved in HIV reactivation, including interleukins (IL) IL-1β, IL-2, IL-6, IL-7, interferon γ (IFN-γ) and CD154,104–109 and could be used to purge the reservoirs. Among mechanisms acting at the post-transcriptional level, regulation of the exportation of viral RNAs by the poly track binding protein (PTB) seems to be important in memory CD4+ T cells.110 Another important mechanism that acts at the post-transcriptional level involves microRNAs (miRNAs). These are single-stranded RNAs of 19–25 nucleotides involved in various biological processes in eukaryotic cells.111,112 miRNAs interact with a complementary sequence in the 3′-untranscribed region (UTR) of target mRNAs by partial sequence matching, which leads either to mRNA degradation or, more often, to translational inhibition.112 miRNAs are involved in the regulation of virus expression as well.113 Recently it was shown that miRNAs regulate the expression of the histone acetyltransferase Tat cofactor PCAF and HIV replication.114 In a recent paper, Huang et al. reported an enrichment of miRNAs in clusters, which has been observed only in resting CD4+ T cells and not in active CD4+ T cells.115 They found that several of the miRNA clusters inhibited HIV replication, and suggested that miRNAs contribute to HIV latency in resting primary CD4+ T cells. They proposed to use specific antagomirs (anti-miRNA antisense) raised against these miRNA in order to reactivate latent CD4+ T cells.116 However, as discussed by Sun and Rossi, the use of antagomirs to reactivate latently infected cells could be toxic for uninfected cells.117 The feasibility of using miRNAs for HIV treatment is premature and will need far more investigation.
Implications for therapies based on purging reservoirs
Original strategies based on the combination of a purge of the reservoirs and intensifying HAART aim to eradicate the virus from infected patients. Understanding the molecular mechanisms involved in latency will allow us to devise new strategies that will facilitate the reactivation of all the reservoirs.
One strategy, known as ‘Immune Activation Therapy’, aims to activate T cells118–121 (Figure 1). Many physiological stimuli that effectively activated T cells passed preclinical studies, but all failed in clinical studies.122 IL-7 held promise since this cytokine is known to be essential for the maintenance of T cell homeostasis. Indeed, there are two subsets of memory T cells:10 central memory T cells (Tcm), which are maintained through T cell survival and low-level driven proliferation and can persist for decades, and transitional memory T cells (Ttm), which persist, in contrast, by homeostatic proliferation of infected cells and could be reduced by using drugs preventing memory T cells from dividing. Interestingly, an IL-7-driven proliferation of Ttm cells can induce HIV expression from quiescent resting cells without the death of the infected cells. This cytokine might therefore be tested for its ability to reactivate expression of latent HIV in order to purge this quiescent HIV reservoir.123–125 A clinical trial using IL-7 in order to reduce the size of the latent reservoir is currently running (ERAMUNE led by C. Katlama; http://www.clinicaltrials.gov). Another profound therapeutic implication, put forward by Chomont et al.,10 is that the size of the pool of CD4+ Tcm cells infected by HIV-1 should decrease with early treatment interventions.126 Indeed, these memory Tcm cells (and the CD8+ T cells) are thought to be very important in the control of HIV infection, as shown in elite controllers.127,128 Since IL-7 is also involved in CD8+ T cell function and T cell survival,129–131 an early treatment that combines HAART and IL-7 will certainly help patients to control their HIV-1 infection (i.e. to get a functional cure), but might not allow the eradication of the virus (i.e. to get a sterilizing cure).
A second strategy aiming to develop rational therapeutics to flush out HIV from latency relies on the knowledge of its epigenetic regulation132 (Figure 1). Several potential interesting candidates have emerged, such as the histone deacetylase (HDAC),88 the histone methyltransferase,83,88,133 DNA methyltransferases (DNMTs)100,101 and proteins from the SWI/SNF chromatin complexes.134,135 A switch from latent to active transcription has been described following treatment with several HDAC inhibitors such as trichostatin, trapoxin, valproic acid and sodium butyrate.136–141 Valproic acid has been described to effectively reactivate latent HIV reservoirs in a first clinical trial,142,143 but two other clinical trials did not confirm this.144,145 Failure of this first clinical trial might be due to the ineffectiveness of valproic acid in inhibiting HDAC3 activity in CD4 T cells.146 Indeed, several other HDACs, including HDAC3, contribute to the repression of HIV-1 LTR expression.147–149 Further investigations are needed using inhibitors against newly identified epigenetic regulators of HIV latency such as chaetocine (a histone methyl transferase inhibitor) or the DNA methyltransferase inhibitors, including well-characterized nucleotide analogue methylation inhibitors (5-azacytidine, 5-aza-2′-deoxycytidine, 5-fluoro-2′-deoxycytidine and zebularidine) and non-nucleoside DNA methylation inhibitors (procaine, procainamide, hydralazine and RG108).78,150 Purging of latent reservoirs could also be achieved by inhibiting regulatory processes that prevent reactivation.151 The p-TEFb activator HMBA is a promising molecule currently under study. In pilot studies it was able to reactivate latently infected cells and prevent re-infection by down-regulating CD4 receptor expression.152
There are several encouraging new directions in the purge of reservoirs that are based on a combination therapy approach,153 as already used in clinical trials to treat cancer.154–156 Such an approach has been found to be promising since the association of an HDAC inhibitor or a DNA methylation inhibitor with prostratin has a synergistic effect on the activation of HIV-1 expression.101,157 The main benefit of this synergistic effect is that we might use drugs at suboptimal concentrations that would be sufficient to reactivate the virus but would have fewer side effects. We believe that the most promising strategy to purge the reservoirs relies on combinations of such drugs, which would be able to force viral gene expression at both the transcriptional and post-transcriptional levels.
Finally, an alternative option has been proposed, which is not based on virus reactivation, but on rendering the virus unable to replicate in latent cells without inducing cell death.158 This original ‘genome editing therapy’ is based on the recognition of essential sequences within HIV-1, such as the pol gene by zinc finger endonuclease. Such a therapy has already been proposed to disrupt the CCR5 gene, as described previously.159
Improving HAART
Why is it important to improve HAART?
There are several reasons why HAART should be improved. One is the existence of a residual viraemia in patients undergoing HAART. The origin of this viraemia is still debated. There are two theories explaining this residual viraemia: (i) long-lived cells containing latent HIV provirus that can produce HIV at low levels following reactivation; and (ii) low-level cryptic on-going replication despite therapy. Latency is best described as a lack of proviral gene expression. In contrast, on-going replication requires continuous viral gene expression without cytopathic effects. Ineffective treatment in cells supporting on-going replication could result from poor drug penetration into sanctuaries such as the brain, where infected microglial cells are located,160 or from cell-to-cell transfer of the virus.161 It is important to distinguish between these two theories, since the therapeutic approaches they suggest are essentially different. The theory of on-going replication suggests that drug resistance to treatments might develop. In this case treatment intensification and the design of new anti HIV-1 molecules are needed in the long term. On the other hand, if viruses are released in bursts from stable reservoirs, multidrug resistance does not develop, however, HAART alone is ineffective as well. Several studies have looked at the efficiency of such intensification of HAART on residual viraemia and only one failed to reduce it.55,162 The second reason to improve HAART is related to the ‘shock and kill’ strategy discussed above. HAART by itself is not able to achieve a cure, but is still needed (to kill) in association with HIV reactivation from quiescent cells (to shock). Finally, emergence of drug resistances, toxicity and compliance with treatment are all obstacles to the current management of HIV-1 infection and therefore need improvement of HAART.163
How can we improve HAART?
Current management of HIV-1 treatment is based on seven classes of antiretrovirals: nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), nucleotide reverse transcriptase inhibitors (NtRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), entry/fusion inhibitors (EIs), coreceptor inhibitors (CRIs) and integrase inhibitors (INIs).164 The therapy of HIV-1-infected patients is based on a combination of three or more drugs from two or more classes.165
We believe that new drugs should target other steps of the HIV-1 cycle such as transcription, since there is no drug currently available targeting this step. An increasing number of studies suggest that inhibitors of cellular LTR-binding factors, such as NF-κB and Sp1, repress LTR-driven transcription.166–170 Recently it has been shown that proteins of the DING family are good candidates to repress HIV-1 gene transcription.171–173 Indeed, the inhibitory effect of the human DING protein named HPBP (human phosphate binding protein) on HIV-1 replication is very strong,173 even compared with other canonical drugs currently used in HAART.174 HPBP is also a potent anti-HIV-1 drug in peripheral blood lymphocytes and in primary macrophages, which is not true for several other anti-HIV-1 drugs. Very interestingly, HPBP, which targets transcription, is as effective against drug-resistant HIV strains as wild-type strains, highlighting the potential therapeutic advantage of HPBP. Moreover, such drugs could also be used to cope with chronic inflammation, which leads to non-AIDS events.175 We believe that this protein or its derivatives are potentially interesting molecules and deserve further study. As suggested for X-DING-CD4,172 proteins belonging to the DING protein family might have a role in the innate response to infections, including HIV-1.
Finally, the use of nanotechnology involving structures 1–100 nm in size is an exciting approach since it will make it possible to reduce toxicity and facilitate treatment adherence.176 Indeed, these nano-delivery systems will permit: (i) modulation of drug release; (ii) protection of drugs from metabolism; and (iii) specific targeting of infected cells, even those located in sanctuaries. In corollary, this approach will allow improved bioavailability and therefore reduce toxicity.177–179 Among new nanotechnology-based drug delivery systems are liposomes, polymeric micelles, dendrimers and nanosuspensions. Potential uses of these molecules have been reviewed.176 This elegant approach will surely improve gene therapy, immunotherapy, vaccinology and microbicides.180
When to start antiretroviral therapy?
Today there is no real consensus on when HAART should be started. Until now, generally HAART was started when the CD4+ T cell count was below 350 cells/mm3, however, several observations have pointed to a substantial benefit in reduced mortality if treatment is started at an earlier stage with no consideration of CD4+ T cell count.181,182 This is in agreement with the finding that starting treatment earlier reduced the size of the latently infected reservoirs, as discussed above. Another major concern with starting treatment earlier is that it should reduce the outcome of non-AIDS events and non-AIDS mortality.183 The cost of the treatment, drug toxicities and non-adherence to the treatment by healthy patients has led some regulatory organizations in Europe not to recommend initiation of HAART in asymptomatic patients or patients having more than 350 CD4+ T cells/mm3.
Conclusions
Are there reasons to be optimistic that a cure for HIV infection may be achieved? From our point of view the answer is ‘yes’, but this will not be achieved in the short term. Advances in some fields are very exciting and offer new opportunities to achieve a cure. For example, using gene therapy to confer HIV resistance (including the CCR5 gene therapy) is a valuable approach compared with chemotherapy, which has several drawbacks, including toxicities, development of resistance and cost. Several gene therapy trials are currently under way,58 but it is premature to make definitive conclusions regarding the feasibility of these therapies. The ‘holy grail’ for clinicians will be to achieve a sterilizing cure with total eradication of the virus from the body, but we might only get a functional cure, with few patients who control HIV-1 infection (the elite controllers). The major concern with a functional cure will be to drastically reduce the viraemia in order to prevent non-AIDS events. The ‘shock and kill’ strategy has also emerged as an exciting potential way to eliminate the virus. Here, too, we might be able to achieve only a functional cure. The German case is the only case where a possible sterilizing cure has occurred, incidentally indicating a weakness of HIV. Today, however, we are limited by a lack of technology to clearly demonstrate that this patient is definitively cured. The war against this virus is far from over and will need much more work. This review has focused on current therapeutic strategies that could lead in the long term to a cure. From a military point of view, this latter strategy constitutes the first front line. However, to win a war you usually need to open a second front line, and this one is research leading to the development of an HIV vaccine. Even if in practice this approach is not yet working, efforts in this direction must be made, but might require new avenues in HIV immunology research.36,184–186 Undoubtedly research aiming at a therapeutic cure will benefit from research aiming to develop a vaccine, and vice versa. Reasons to be optimistic come mainly from the intensive efforts made in different fields of research, i.e. a multidisciplinary approach, including immunologists, virologists, molecular biologists, clinicians, pharmacologists, chemists, physicists and mathematicians, who have already opened new ways and elaborated new concepts for therapies that are currently being tested in clinical trials.
Funding
This work was supported by grants from the Agence Nationale de Recherches sur le SIDA (ANRS) (to O. R., C. S. and R. R.), Sidaction Ligue contre le cancer (to O. R. and C. S.) and Institut Universitaire de France (to O. R.). A. J. is a fellow supported by the “Région Alsace”. V. L. D. is supported by a doctoral grant from the French Ministry of Research. S. A. is supported by a doctoral grant from the Pakistan Ministry of Research.
Transparency declarations
None to declare.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–71. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.UN/WHO. AIDS epidemic update 2010. http://wwwunaidsorg/en/ (01 December 2011, date last accessed)
- 3.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 4.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 5.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 7.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 8.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter CC, Onafuwa-Nuga A, McNamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–51. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai J, Agosto LM, Baytop C, et al. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J Virol. 2009;83:4528–37. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellery PJ, Tippett E, Chiu YL, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 13.Wightman F, Solomon A, Khoury G, et al. Both CD31(+) and CD31 naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis. 2010;202:1738–48. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- 14.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4:e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keele BF, Tazi L, Gartner S, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol. 2008;82:5548–61. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redel L, Le Douce V, Cherrier T, et al. HIV-1 regulation of latency in the monocyte-macrophage lineage and in CD4+ T lymphocytes. J Leukoc Biol. 2009 doi: 10.1189/jlb.0409264. [DOI] [PubMed] [Google Scholar]
- 19.Zhu T, Muthui D, Holte S, et al. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–16. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Douce V, Herbein G, Rohr O, et al. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7:32. doi: 10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trono D, Marzetta F. Profaning the ultimate sanctuary: HIV latency in hematopoietic stem cells. Cell Host Microbe. 2011;9:170–2. doi: 10.1016/j.chom.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Aquaro S, Bagnarelli P, Guenci T, et al. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J Med Virol. 2002;68:479–88. doi: 10.1002/jmv.10245. [DOI] [PubMed] [Google Scholar]
- 23.Perno CF, Newcomb FM, Davis DA, et al. Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J Infect Dis. 1998;178:413–22. doi: 10.1086/515642. [DOI] [PubMed] [Google Scholar]
- 24.Nolan D, Reiss P, Mallal S. Adverse effects of antiretroviral therapy for HIV infection: a review of selected topics. Expert Opin Drug Saf. 2005;4:201–18. doi: 10.1517/14740338.4.2.201. [DOI] [PubMed] [Google Scholar]
- 25.Vella S, Palmisano L. The global status of resistance to antiretroviral drugs. Clin Infect Dis. 2005;41(Suppl 4):S239–46. doi: 10.1086/430784. [DOI] [PubMed] [Google Scholar]
- 26.Kozal MJ. Drug-resistant human immunodefiency virus. Clin Microbiol Infect. 2009;15(Suppl 1):69–73. doi: 10.1111/j.1469-0691.2008.02687.x. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths PD. A perspective on antiviral resistance. J Clin Virol. 2009;46:3–8. doi: 10.1016/j.jcv.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Nijhuis M, van Maarseveen NM, Boucher CA. Antiviral resistance and impact on viral replication capacity: evolution of viruses under antiviral pressure occurs in three phases. Handb Exp Pharmacol. 2009;189:299–320. doi: 10.1007/978-3-540-79086-0_11. [DOI] [PubMed] [Google Scholar]
- 29.Achhra AC, Amin J, Law MG, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS. 2010;24:1877–86. doi: 10.1097/QAD.0b013e32833b1b26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 31.Ferry T, Raffi F, Collin-Filleul F, et al. Uncontrolled viral replication as a risk factor for non-AIDS severe clinical events in HIV-infected patients on long-term antiretroviral therapy: APROCO/COPILOTE (ANRS CO8) cohort study. J Acquir Immune Defic Syndr. 2009;51:407–15. doi: 10.1097/QAI.0b013e3181acb65f. [DOI] [PubMed] [Google Scholar]
- 32.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 33.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht R, Stover J, Bollinger L, et al. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009–31. Lancet. 2010;376:1254–60. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- 35.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–9. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medzhitov R, Littman D. HIV immunology needs a new direction. Nature. 2008;455:591. doi: 10.1038/455591a. [DOI] [PubMed] [Google Scholar]
- 37.Barouch DH, Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–67. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMichael AJ, Borrow P, Tomaras GD, et al. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richman DD, Margolis DM, Delaney M, et al. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 40.Trono D, Van Lint C, Rouzioux C, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–80. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 41.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 42.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereyra F, Palmer S, Miura T, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200:984–90. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutter G, Ganepola S. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. Sci World J. 2009;11:1068–76. doi: 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 47.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 48.Stephens JC, Reich DE, Goldstein DB, et al. Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes. Am J Hum Genet. 1998;62:1507–15. doi: 10.1086/301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lalezari J, Mitsuyasu R, Deeks S, et al. Successful and persistent engraftment of ZFN-M-R5-D autologous CD4 T cells (SB-728-T) in aviremic HIV-infected subjects on HAART. Program and Abstracts of the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2011; Abstract 46. [Google Scholar]
- 52.Tebas P, Levine B, Binder G, et al. Disruption of CCR5 in zinc finger nuclease-treated CD4 T cells: Phase I trials. Program and Abstracts from the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2011; Abstract 165. [Google Scholar]
- 53.Ando D, Tang WW, Stein D, et al. HAART treatment interruption following adoptive transfer of zinc finger nuclease (ZFN) modified autologous CD4 T-cells (SB-728-T) to HIV-infected subjects demonstrates durable engraftment and suppression of viral load. Abstracts of the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago IL, 2011; Washington, DC, USA: American Society for Microbiology; Abstract H2-794a. [Google Scholar]
- 54.Cohen J. The emerging race to cure HIV infections. Science. 2011;332:784–5. doi: 10.1126/science.332.6031.784. 7–9. [DOI] [PubMed] [Google Scholar]
- 55.Lewin SR, Rouzioux C. HIV cure and eradication: how will we get from the laboratory to effective clinical trials? AIDS. 2011;25:885–97. doi: 10.1097/QAD.0b013e3283467041. [DOI] [PubMed] [Google Scholar]
- 56.Hutter G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: an update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–4. doi: 10.1097/QAD.0b013e328340fe28. [DOI] [PubMed] [Google Scholar]
- 57.Levy JA. Not an HIV cure, but encouraging new directions. N Engl J Med. 2009;360:724–5. doi: 10.1056/NEJMe0810248. [DOI] [PubMed] [Google Scholar]
- 58.Cohen J. Building an HIV-proof immune system. Science. 2007;317:612–4. doi: 10.1126/science.317.5838.612. [DOI] [PubMed] [Google Scholar]
- 59.Kitchen SG, Zack JA. Stem cell-based approaches to treating HIV infection. Curr Opin HIV AIDS. 2011;6:68–73. doi: 10.1097/COH.0b013e3283412370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deeks SG, McCune JM. Can HIV be cured with stem cell therapy? Nat Biotechnol. 2010;28:807–10. doi: 10.1038/nbt0810-807. [DOI] [PubMed] [Google Scholar]
- 61.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–57. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chun TW, Davey RT, Jr, Ostrowski M, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–61. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 63.Nickle DC, Jensen MA, Shriner D, et al. Evolutionary indicators of human immunodeficiency virus type 1 reservoirs and compartments. J Virol. 2003;77:5540–6. doi: 10.1128/JVI.77.9.5540-5546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dornadula G, Zhang H, VanUitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–32. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 65.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7:142–53. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 67.Sawchuk RJ, Yang Z. Investigation of distribution, transport and uptake of anti-HIV drugs to the central nervous system. Adv Drug Deliv Rev. 1999;39:5–31. doi: 10.1016/s0169-409x(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 68.Solas C, Lafeuillade A, Halfon P, et al. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:238–43. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 70.Chun TW, Finzi D, Margolick J, et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–90. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 71.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 72.Ho DD, Zhang L. HIV-1 rebound after anti-retroviral therapy. Nat Med. 2000;6:736–7. doi: 10.1038/77447. [DOI] [PubMed] [Google Scholar]
- 73.Dybul M, Daucher M, Jensen MA, et al. Genetic characterization of rebounding human immunodeficiency virus type 1 in plasma during multiple interruptions of highly active antiretroviral therapy. J Virol. 2003;77:3229–37. doi: 10.1128/JVI.77.5.3229-3237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr Opin HIV AIDS. 2011;6:43–8. doi: 10.1097/COH.0b013e32834086b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–41. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 76.Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol. 2008;122:22–8. doi: 10.1016/j.jaci.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coiras M, Lopez-Huertas MR, Perez-Olmeda M, et al. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 78.Colin L, Van Lint C. Molecular control of HIV-1 postintegration latency: implications for the development of new therapeutic strategies. Retrovirology. 2009;6:111. doi: 10.1186/1742-4690-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Margolis DM. Mechanisms of HIV latency: an emerging picture of complexity. Curr HIV/AIDS Rep. 2010;7:37–43. doi: 10.1007/s11904-009-0033-9. [DOI] [PubMed] [Google Scholar]
- 80.Van Lint C, Emiliani S, Ott M, et al. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–20. [PMC free article] [PubMed] [Google Scholar]
- 81.Verdin E, Paras P, Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–59. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Lint C. Role of chromatin in HIV-1 transcriptional regulation. Adv Pharmacol. 2000;48:121–60. doi: 10.1016/s1054-3589(00)48005-1. [DOI] [PubMed] [Google Scholar]
- 83.du Chene I, Basyuk E, Lin YL, et al. Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 2007;26:424–35. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barber SA, Gama L, Dudaronek JM, et al. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis. 2006;193:963–70. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- 85.Avram D, Fields A, Pretty On Top K, et al. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–22. doi: 10.1074/jbc.275.14.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marban C, Redel L, Suzanne S, et al. COUP-TF interacting protein 2 represses the initial phase of HIV-1 gene transcription in human microglial cells. Nucleic Acids Res. 2005;33:2318–31. doi: 10.1093/nar/gki529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Rohr O, Lecestre D, Chasserot-Golaz S, et al. Recruitment of Tat to heterochromatin protein HP1 via interaction with CTIP2 inhibits human immunodeficiency virus type 1 replication in microglial cells. J Virol. 2003;77:5415–27. doi: 10.1128/JVI.77.9.5415-5427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marban C, Suzanne S, Dequiedt F, et al. Recruitment of chromatin-modifying enzymes by CTIP2 promotes HIV-1 transcriptional silencing. EMBO J. 2007;26:412–23. doi: 10.1038/sj.emboj.7601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vazquez N, Greenwell-Wild T, Marinos NJ, et al. Human immunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J Virol. 2005;79:4479–91. doi: 10.1128/JVI.79.7.4479-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cherrier T, Suzanne S, Redel L, et al. p21(WAF1) gene promoter is epigenetically silenced by CTIP2 and SUV39H1. Oncogene. 2009;28:3380–9. doi: 10.1038/onc.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergamaschi A, David A, Le Rouzic E, et al. The CDK inhibitor p21Cip1/WAF1 is induced by Fc{gamma}R activation and restricts the replication of HIV-1 and related primate lentiviruses in human macrophages. J Virol. 2009;83:12253–65. doi: 10.1128/JVI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen H, Li C, Huang J, et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest. 2011;121:1549–60. doi: 10.1172/JCI44539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bergamaschi A, Pancino G. Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology. 2010;7:31. doi: 10.1186/1742-4690-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Douce V, Colin L, Redel L, et al. LSD1 cooperates with CTIP2 to promote HIV-1 transcriptional silencing. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pinskaya M, Gourvennec S, Morillon A. H3 lysine 4 di- and tri-methylation deposited by cryptic transcription attenuates promoter activation. EMBO J. 2009;28:1697–707. doi: 10.1038/emboj.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pinskaya M, Morillon A. Histone H3 lysine 4 di-methylation: a novel mark for transcriptional fidelity? Epigenetics. 2009;4:302–6. doi: 10.4161/epi.4.5.9369. [DOI] [PubMed] [Google Scholar]
- 97.Sakane N, Kwon HS, Pagans S, et al. Activation of HIV transcription by the viral Tat protein requires a demethylation step mediated by lysine-specific demethylase 1 (LSD1/KDM1) PLoS Pathog. 2011;7:e1002184. doi: 10.1371/journal.ppat.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ott M, Geyer M, Zhou Q. The control of HIV transcription: keeping RNA polymerase II on track. Cell Host Microbe. 2011;10:426–35. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pierard V, Guiguen A, Colin L, et al. DNA cytosine methylation in the bovine leukemia virus promoter is associated with latency in a lymphoma-derived B-cell line: potential involvement of direct inhibition of cAMP-responsive element (CRE)-binding protein/CRE modulator/activation transcription factor binding. J Biol Chem. 2010;285:19434–49. doi: 10.1074/jbc.M110.107607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blazkova J, Trejbalova K, Gondois-Rey F, et al. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kauder SE, Bosque A, Lindqvist A, et al. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pion M, Jordan A, Biancotto A, et al. Transcriptional suppression of in vitro-integrated human immunodeficiency virus type 1 does not correlate with proviral DNA methylation. J Virol. 2003;77:4025–32. doi: 10.1128/JVI.77.7.4025-4032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ganesh L, Burstein E, Guha-Niyogi A, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–7. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 104.Biswas P, Poli G, Kinter AL, et al. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992;176:739–50. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butera ST, Perez VL, Wu BY, et al. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991;65:4645–53. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duh EJ, Maury WJ, Folks TM, et al. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–8. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Folks TM, Justement J, Kinter A, et al. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–2. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 108.Folks TM, Justement J, Kinter A, et al. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988;140:1117–22. [PubMed] [Google Scholar]
- 109.Poli G, Kinter AL, Fauci AS. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc Natl Acad Sci USA. 1994;91:108–12. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lassen KG, Ramyar KX, Bailey JR, et al. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ambros V, Chen X. The regulation of genes and genomes by small RNAs. Development. 2007;134:1635–41. doi: 10.1242/dev.002006. [DOI] [PubMed] [Google Scholar]
- 112.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 113.Yeung ML, Benkirane M, Jeang KT. Small non-coding RNAs, mammalian cells, and viruses: regulatory interactions? Retrovirology. 2007;4:74. doi: 10.1186/1742-4690-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Triboulet R, Mari B, Lin YL, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315:1579–82. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- 115.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–7. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H. Reversal of HIV-1 latency with anti-microRNA inhibitors. Int J Biochem Cell Biol. 2009;41:451–4. doi: 10.1016/j.biocel.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun G, Rossi JJ. MicroRNAs and their potential involvement in HIV infection. Trends Pharmacol Sci. 2011;32:675–81. doi: 10.1016/j.tips.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brooks DG, Arlen PA, Gao L, et al. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. Proc Natl Acad Sci USA. 2003;100:12955–60. doi: 10.1073/pnas.2233345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brooks DG, Hamer DH, Arlen PA, et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19:413–23. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 120.Arlen PA, Brooks DG, Gao LY, et al. Rapid expression of human immunodeficiency virus following activation of latently infected cells. J Virol. 2006;80:1599–603. doi: 10.1128/JVI.80.3.1599-1603.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kulkosky J, Nunnari G, Otero M, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186:1403–11. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 122.Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- 123.Lehrman G, Ylisastigui L, Bosch RJ, et al. Interleukin-7 induces HIV type 1 outgrowth from peripheral resting CD4+ T cells. J Acquir Immune Defic Syndr. 2004;36:1103–4. doi: 10.1097/00126334-200408150-00015. [DOI] [PubMed] [Google Scholar]
- 124.Sereti I, Dunham RM, Spritzler J, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–14. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang FX, Xu Y, Sullivan J, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005;115:128–37. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chomont N, DaFonseca S, Vandergeeten C, et al. Maintenance of CD4+ T-cell memory and HIV persistence: keeping memory, keeping HIV. Curr Opin HIV AIDS. 2011;6:30–6. doi: 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
- 127.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 128.van Grevenynghe J, Procopio FA, He Z, et al. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med. 2008;14:266–74. doi: 10.1038/nm1728. [DOI] [PubMed] [Google Scholar]
- 129.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 130.Beq S, Delfraissy JF, Theze J. Interleukin-7 (IL-7): immune function, involvement in the pathogenesis of HIV infection and therapeutic potential. Eur Cytokine Netw. 2004;15:279–89. [PubMed] [Google Scholar]
- 131.Schluns KS, Kieper WC, Jameson SC, et al. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 132.Hakre S, Chavez L, Shirakawa K, et al. Epigenetic regulation of HIV latency. Curr Opin HIV AIDS. 2011;6:19–24. doi: 10.1097/COH.0b013e3283412384. [DOI] [PubMed] [Google Scholar]
- 133.Imai K, Togami H, Okamoto T. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem. 2010;285:16538–45. doi: 10.1074/jbc.M110.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lusic M, Marcello A, Cereseto A, et al. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 2003;22:6550–61. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–74. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.El Kharroubi A, Piras G, Zensen R, et al. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–44. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–77. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kiernan RE, Vanhulle C, Schiltz L, et al. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 1999;18:6106–18. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quivy V, Adam E, Collette Y, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol. 2002;76:11091–103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sheridan PL, Mayall TP, Verdin E, et al. Histone acetyltransferases regulate HIV-1 enhancer activity in vitro. Genes Dev. 1997;11:3327–40. doi: 10.1101/gad.11.24.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steger DJ, Eberharter A, John S, et al. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc Natl Acad Sci USA. 1998;95:12924–9. doi: 10.1073/pnas.95.22.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mai A. The therapeutic uses of chromatin-modifying agents. Expert Opin Ther Targets. 2007;11:835–51. doi: 10.1517/14728222.11.6.835. [DOI] [PubMed] [Google Scholar]
- 144.Sagot-Lerolle N, Lamine A, Chaix ML, et al. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–9. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 145.Siliciano JD, Lai J, Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–6. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 146.Huber K, Doyon G, Plaks J, et al. Inhibitors of histone deacetylases: correlation between isoform specificity and reactivation of HIV type 1 (HIV-1) from latently infected cells. J Biol Chem. 2011;286:22211–8. doi: 10.1074/jbc.M110.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Keedy KS, Archin NM, Gates AT, et al. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol. 2009;83:4749–56. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malcolm T, Chen J, Chang C, et al. Induction of chromosomally integrated HIV-1 LTR requires RBF-2 (USF/TFII-I) and Ras/MAPK signaling. Virus Genes. 2007;35:215–23. doi: 10.1007/s11262-007-0109-9. [DOI] [PubMed] [Google Scholar]
- 149.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6:25–9. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu N, Wang M. Anticancer drug discovery targeting DNA hypermethylation. Curr Med Chem. 2008;15:1350–75. doi: 10.2174/092986708784567653. [DOI] [PubMed] [Google Scholar]
- 151.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr Opin HIV AIDS. 2011;6:4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Contreras X, Barboric M, Lenasi T, et al. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3:1459–69. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Savarino A, Mai A, Norelli S, et al. “Shock and kill” effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology. 2009;6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cameron EE, Bachman KE, Myohanen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 155.Chen M, Voeller D, Marquez VE, et al. Enhanced growth inhibition by combined DNA methylation/HDAC inhibitors in lung tumor cells with silenced CDKN2A. Int J Oncol. 2010;37:963–71. doi: 10.3892/ijo_00000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kawano T, Akiyama M, Agawa-Ohta M, et al. Histone deacetylase inhibitors valproic acid and depsipeptide sensitize retinoblastoma cells to radiotherapy by increasing H2AX phosphorylation and p53 acetylation-phosphorylation. Int J Oncol. 2010;37:787–95. doi: 10.3892/ijo_00000728. [DOI] [PubMed] [Google Scholar]
- 157.Reuse S, Calao M, Kabeya K, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PLoS One. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wayengera M. Proviral HIV-genome-wide and pol-gene specific zinc finger nucleases: usability for targeted HIV gene therapy. Theor Biol Med Model. 2011;8:26. doi: 10.1186/1742-4682-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Urnov FD, Rebar EJ, Holmes MC, et al. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–46. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 160.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sigal A, Kim JT, Balazs AB, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–8. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 162.Maldarelli F. Targeting viral reservoirs: ability of antiretroviral therapy to stop viral replication. Curr Opin HIV AIDS. 2010;6:49–56. doi: 10.1097/COH.0b013e32834134ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moreno S, Lopez Aldeguer J, Arribas JR, et al. The future of antiretroviral therapy: challenges and needs. J Antimicrob Chemother. 2010;65:827–35. doi: 10.1093/jac/dkq061. [DOI] [PubMed] [Google Scholar]
- 164.De Clercq E. Antiretroviral drugs. Curr Opin Pharmacol. 2010;10:507–15. doi: 10.1016/j.coph.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 165.Taiwo B, Murphy RL, Katlama C. Novel antiretroviral combinations in treatment-experienced patients with HIV infection: rationale and results. Drugs. 2010;70:1629–42. doi: 10.2165/11538020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 166.Baba M. Cellular factors as alternative targets for inhibition of HIV-1. Antiviral Res. 1997;33:141–52. doi: 10.1016/s0166-3542(96)01010-8. [DOI] [PubMed] [Google Scholar]
- 167.De Clercq E. New anti-HIV agents and targets. Med Res Rev. 2002;22:531–65. doi: 10.1002/med.10021. [DOI] [PubMed] [Google Scholar]
- 168.Baba M. Recent status of HIV-1 gene expression inhibitors. Antiviral Res. 2006;71:301–6. doi: 10.1016/j.antiviral.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 169.Daelemans D, Vandamme AM, De Clercq E. Human immunodeficiency virus gene regulation as a target for antiviral chemotherapy. Antivir Chem Chemother. 1999;10:1–14. doi: 10.1177/095632029901000101. [DOI] [PubMed] [Google Scholar]
- 170.Stevens M, De Clercq E, Balzarini J. The regulation of HIV-1 transcription: molecular targets for chemotherapeutic intervention. Med Res Rev. 2006;26:595–625. doi: 10.1002/med.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Darbinian-Sarkissian N, Darbinyan A, Otte J, et al. p27(SJ), a novel protein in St John's wort, that suppresses expression of HIV-1 genome. Gene Ther. 2006;13:288–95. doi: 10.1038/sj.gt.3302649. [DOI] [PubMed] [Google Scholar]
- 172.Lesner A, Shilpi R, Ivanova A, et al. Identification of X-DING-CD4, a new member of human DING protein family that is secreted by HIV-1 resistant CD4(+) T cells and has anti-viral activity. Biochem Biophys Res Commun. 2009;389:284–9. doi: 10.1016/j.bbrc.2009.08.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cherrier T, Elias M, Jeudy A, et al. Human-phosphate-binding-protein inhibits HIV-1 gene transcription and replication. Virol J. 2011;8:352. doi: 10.1186/1743-422X-8-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cihlar T, Birkus G, Greenwalt DE, et al. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 2002;54:37–45. doi: 10.1016/s0166-3542(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 175.Ivanova A, Shilpi RY, Sachdeva R, et al. Native X-DING-CD4 protein secreted by HIV-1 resistant CD4+ T cells blocks activity of IL-8 promoter in human endothelial cells infected with enteric bacteria. Innate Immun. 2011 doi: 10.1177/1753425911427065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim PS, Read SW. Nanotechnology and HIV: potential applications for treatment and prevention. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:693–702. doi: 10.1002/wnan.118. [DOI] [PubMed] [Google Scholar]
- 177.Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin Drug Deliv. 2006;3:613–28. doi: 10.1517/17425247.3.5.613. [DOI] [PubMed] [Google Scholar]
- 178.das Neves J, Amiji MM, Bahia MF, et al. Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Adv Drug Deliv Rev. 2010;62:458–77. doi: 10.1016/j.addr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 179.Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:491–502. doi: 10.1016/j.addr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 180.Mamo T, Moseman EA, Kolishetti N, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (Lond) 2010;5:269–85. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 183.Kitahata MM. When to start antiretroviral therapy. Top HIV Med. 2010;18:121–6. [PubMed] [Google Scholar]
- 184.Armitage AE, McMichael AJ, Drakesmith H. Reflecting on a quarter century of HIV research. Nat Immunol. 2008;9:823–6. doi: 10.1038/ni0808-823. [DOI] [PubMed] [Google Scholar]
- 185.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356:2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 186.Fauci AS, Johnston MI, Dieffenbach CW, et al. HIV vaccine research: the way forward. Science. 2008;321:530–2. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]